Abstract
Osteoarthritis (OA) is characterized by joint pain and stiffness with radiographic evidence of joint space narrowing, osteophytes, and subchondral bone sclerosis. Posttraumatic OA (PTOA) arises from joint trauma, which accounts for a fraction of all patients with OA. Articular cartilage breakdown can occur soon or for years after a joint injury. Even with the current care of joint injuries, such as anatomic reduction and rigid fixation of intra-articular fractures and reconstruction of ruptured ligaments with successful restoration of joint biomechanics, the risk of PTOA after joint injuries ranges from 20% to more than 50%. The time course for the progression of PTOA is highly variable and risk of PTOA increases with patient age at the time of joint injury, suggesting that biologic factors may be involved in the progression of PTOA. Therapeutic options are limited due largely to the lack of information on the mechanisms underlying the progression of PTOA. This review summarizes the current studies on the pathogenetic mechanisms of PTOA, with a main focus on the metabolic changes in articular cartilage in the acute posttraumatic phase and the early chronic phase, a clinically asymptomatic period. Recent studies have revealed that mechanical damage to the articular tissues may lead to changes in gene expression and cartilage metabolism, which could trigger a cascade of events leading to degradation of articular cartilage and pathologic changes in other joint tissues. Understanding the mechanobiologic, molecular and cellular changes that lead to continued cartilage degradation in the relatively early phases after joint injury may open up new opportunities for early clinical intervention.
Keywords: Osteoarthritis, posttraumatic osteoarthritis, joint injury, articular cartilage, meniscus, ligament
Introduction
Posttraumatic osteoarthritis (PTOA) is a common clinic entity in diarthrodial joints due to damage to the articular cartilage, subchondral bone, incongruity of the articular surface or joint instability caused by an acute injury. Intra-articular fractures, meniscal tears, ligamentous injuries and chondral injuries are common causes leading to PTOA [1]. Unlike idiopathic OA which tends to affect older adults in specific joints such as the knee, hip and shoulder, PTOA occurs in younger patients, often develops and progresses more quickly, and in accordance with joint injury [2, 3]. In the ankle, for instance, trauma is the most common cause of OA. PTOA represents approximately 12% of all OA in the hip, knee and ankle. This translated to an estimate of 5.6 million people in the United States [4]. Articular cartilage defects have limited healing potential and only minimal animal evidence exists for cartilage regeneration [5, 6]. Surgical treatments are commonly employed to prevent the progression to arthritis, including anatomic reduction of intra-articular fractures, ligament repair and reconstruction, joint stabilization procedures, autologous chondrocyte transplantation, and osteotomies [7, 8]. Data from various studies have shown mixed results in the ability of these procedures to prevent posttraumatic arthritis from developing [3, 9, 10]. Once severe PTOA has developed, the treatment arsenal is essentially the same as for idiopathic arthritis, with joint arthroplasty or fusion often the final treatment, which is not desirable for younger patients. Although recent animal studies have shown some progress in pharma-cologic therapies, no medical therapies have been shown to halt or reverse the progression of PTOA. Clearly, new therapies are desired as PTOA represents a significant clinical and financial burden.
In the past decade, much has been learned about the pathogenesis of PTOA from in vivo studies with animal models and human subjects as well as in vitro studies using animal and human joint tissue cells. This review summarizes the current studies on the pathogenetic mechanisms of PTOA, with a main focus on the metabolic changes in articular cartilage in the acute posttraumatic phase and the early chronic phase, a clinically asymptomatic period. Improved understanding of the mechanobiologic, molecular and cellular processes that lead to articular cartilage degradation in the relatively early phases after resolution of the traumatic insult or instability potentially opens up new opportunities for early clinical intervention.
General pathogenetic processes following joint injury
The pathogenetic processes after joint injury may vary depending on the severity of mechanical impact and tissue damage. Low-energy injuries, such as joint contusions, dislocations, and ligamentous and meniscal injuries, commonly cause articular surface damage without displaced bone fracture, although microfractures of calcified cartilage and/or subchondral bone may exist. Higher energy injuries often cause displaced intra-articular fractures. Pathogenetic processes after joint injury can temporally be separated into the acute post-traumatic phase with inflammation of joint tissues, and the chronic phase.
In the acute posttraumatic phase, effects of joint trauma include structural damage to joint tissues, hemarthrosis, and death of articular chondrocytes [11]. The lubricating properties of the synovial fluid is compromised as a result of the dilution of synovial fluid by intra-articular bleeding and plasma extravasation, leading to lower concentrations of hyaluronic acid and lubricant. Joint trauma may lead to suppression of collagen and proteoglycan synthesis in articular cartilage. Remaining viable cells in joint tissues may respond to the injury with enhanced synthetic activity and overexpression of matrix-degrading enzymes and inflammatory mediators [12-14]. Initial cell necrosis is followed by a subsequent spreading of cell death mediated by apoptotic mechanisms, which occurs beyond the initial area into surrounding unimpacted regions.
In the chronic phase, metabolic changes in articular cartilage and other joint tissues slowly progress through a long, clinically asymptomatic period to a symptomatic phase with joint pain and dysfunction as a result of joint destruction. The majority of patients with PTOA are not clinically diagnosed with arthritis until the symptomatic phase. In general, once severe articular destruction has developed, the remaining surgical options are joint arthroplasties, osteotomies, and joint fusions. In order to develop effective therapeutic strategies for early surgical and pharmacologic interventions, it is critical to understand the metabolic changes in joint tissues at the cellular and molecular levels in the acute posttraumatic phase and the asymptomatic period of the chronic phase with minimal osteoarthritic structural changes. The current understanding of mechanobiologic, cellular, and molecular biologic changes during the early stages of PTOA are described below.
Mechanobiologic mechanisms
The mechanical disruption of the extracellular matrix (ECM) may lead to release of glycosalated amino glycans (GAG) and collagen molecules, which are sensed through mechanoreceptors and molecular cell surface receptors by the surrounding chondrocytes [15-18]. This leads to changes in gene expression and cartilage metabolism, which could set up a cascade of events leading to degradation of the articular cartilage [19]. Chondrocytes sense mechanical load through integrin-cytoskelatal interactions as well as ion-stretch activated channels in the membrane [18, 20]. Mechanical stimulations activates α5β1 integrin-dependent intracellular signaling cascade. This triggers Interleukin 4 (IL-4) release through focal adhesion kinase and kinase C [21]. Repression in gene expression of cell adhesion molecules, intercellular adhesion molecule 3 (ICAM-3), neural cell adhesion molecule (NCAM) and vascular cell adhesion protein are involved in homophilic cell-adhesion. Ezrin, an actin binding protein, is also up-regulated. Ezrin works with other actins in mediating cytoskelatal interactions with CD44 regulating hyaluronic acid metabolism. Other signal transduction molecules SP110 nuclear body protein, high-mobility group box 2 (HMGB-2) and neurogranin are also up-regulated [22]. At least a subset of N-methyl-D-aspartic acid (NMDA) receptors also appears to be involved in mechanotransduction in articular chondrocytes [21].
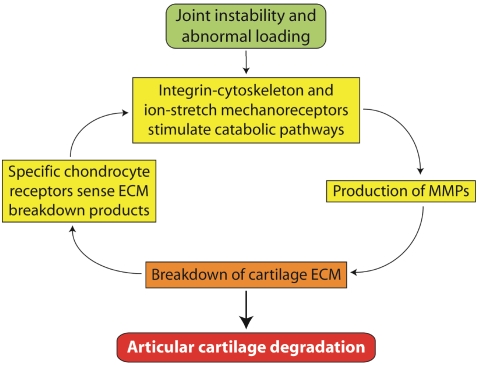
Mechanical compression activates pathways involving intracellular calcium release as well as activation of cyclic AMP-activated protein kinase A [23]. C-fos and c-jun mediated transcription factors are significantly up-regulated immediately following cartilage injury [14, 23]. Although much remains to be elucidated about how chondrocytes sense strain and damage to the matrix around them, it is clear that abnormal mechanical stimulations may cause dysfunction of articular chondrocytes and breakdown of cartilage ECM, leading to articular cartilage degradation (Figure 1).
Figure 1.
Abnormal loading activates chondrocyte mechanoreceptors and catabolic pathways, leading to articular cartilage degradation through a mechanoreceptor-MMP-ECM breakdown cycle.
Cellular mechanisms
Numerous in vitro as well as in vivo studies have identified chondrocyte death following impact to articular cartilage [15, 24-28]. This occurs both through cell necrosis as well as apoptosis [28]. Necrotic cell death can occur at the time of injury in areas of impaction greater than 15-20 MPa. This appears to be isolated to the cells directly under the compressive force [29]. The amount of necrosis increases predictably with the amount of force applied up to 35 MPa. At greater than 40 MPa of impaction, complete cell death is observed [15, 29]. Kurz et al. found that strain rate was also an important determinant of the chondrocyte in response to injury with higher strain rates associated with decreased cell anabolism and viability [30-33]. Thus, there is substantial evidence that a single traumatic insult with a threshold amount of force can cause chondrocyte necrosis.
As chondrocytes are the cells responsible for maintaining the function of articular cartilage, it seems logical that chondrocyte death, mostly through apoptotic mechanisms is a central feature to the development of PTOA [34]. Chondrocyte apoptosis may take place soon or more remotely after articular cartilage disruption [24, 26, 27, 35, 36]. This can occur with or without visible damage to the cartilage [36]. Apoptosis has been correlated with damage to the ECM and likely involves damage to the cell membrane [37, 38]. In both in vivo and in vitro models of cartilage damage, chondrocytes both in and peripheral to the zone of injury show continued biochemical and biomechanical changes over time [39]. With damage to the cartilage matrix the chondrocytes experience significant changes in expression of proteins involved in both anabolic as well as catabolic pathways. This increases the overall metabolic stress on the cell. There is continued mechanical stress on the cell through alterations in the cell environment caused by the initial trauma and/or continued abnormal loading of the matrix. There is evidence to suggest that some apoptotic chondrocyte death, at least in vitro models, is mediated through oxygen free radicals from the mitochondria [40-42]. A reduction in chondrocyte death has been shown with the use of anti-oxidants following cartilage injury [40, 43-45].
The Caspase pathway is likely the main mediator of apoptosis within the cell [18]. Extracellular signals such as tumor necrosis factor (TNF) and Fas can activate the cascade [46]. Intrinsic signals such as damage to DNA and signaling from the endoplasmic reticulum also activate caspases [47, 48]. As DNA damage accumulates, mitochondria are depolarized leading to further amplification of caspase 3 via caspase 9, which may result in continued DNA breakdown [18, 49]. As chondrocytes die they are no longer able to maintain the ECM around them. This puts greater mechanical and metabolic stress on the remaining chondrocytes. Therefore, it would seem that once a critical number of chondrocytes have undergone apoptosis, more chondrocyte death would continue until degeneration of the entire cartilage surface has occurred.
Instability of the joint may result in abnormal loading forces leading to changes in chondrocyte metabolism and cartilage degradation [50-52]. However, cell necrosis is unlikely to be the initial cause of alterations in chondrocyte metabolism in the animal model of PTOA induced by destabilization of the meniscus and transection of the ACL [24, 53, 54].
Molecular and metabolic mechanisms
Shortly after cartilage injury, articular cartilage displays significant changes in the expression of matrix-degrading enzymes. Matrix metalloproteinase 3 (MMP-3, stromelysin-1), a disintegrin and metalloproteinase with thrombospondin motifs 5 (ADAMTS-5), and tissue inhibitor of metalloproteinases 1 increased dramatically within 4 hours [14]. These enzymes lead to breakdown of the ECM of articular cartilage. ADAMTS-5 knockout showed less degeneration and less changes in their subchondral bone than their wild type littermates after destabilization of the medial meniscus (DMM) [55, 56]. A study by Polur et al. showed DMM led to an increased expression of high temperature requirement A 1 (HTRA1) protein in vivo [57]. This enzyme is involved in degradation of the pericellu-lar matrix. Release of collagen-2 and breakdown of proteoglycans occurs after joint injury [58]. Collagen-2 molecules then act on the discoidin domain receptor 2 (Ddr2) through ras/raf/MEK/ ERK and p38 signaling pathways, which induces the expression of MMP-13 [59, 60]. Klatt et al. also provide evidence that collagen II induces MMP1, 2, 13, 14 as well as Interleukin 1 beta (IL-1β), Interleukin 6 (IL-6) and Interleukin 8 (IL-8). This also involves Mitogen-activated protein kinase p38 (MAPKp38) and Nuclear factor kappa B (NFkB) signaling pathways [61]. This sets up positive feedback cycle in which the MMPs breakdown the ECM exposing the chondrocytes to more collagen II breakdown products, causing further production of degradative enzymes leading to continued degradation of the ECM.
Studies involving early gene expression after cartilage injury have shown increased transcription of molecules involved in both anabolic as well as catabolic pathways in articular chondrocytes [62]. PTOA cartilage may display loss of proteoglycans, increased water content and decreased stiffness of the ECM [6, 63]. There is also evidence for increases in anabolism, suggesting a reparative reaction to cartilage damage [39, 64-66]. Lorenz in a study of ACL transection in dogs showed significant and sustained increases in type II and type I collagen production [67]. In a rabbit lateral menisectomy model Hotta et al. showed increased synthesis of type II collagen as OA advances and this was highest in the areas with moderate degeneration [68]. In vitro studies have also shown an increase in type II collagen and aggrecan production after cartilage compression [69].
In adult mice, Runx2 is expressed in the articular cartilage of wild-type mice at the early stage of post-traumatic knee OA, induced by surgical transection of the medial collateral ligament and resection of the medial meniscus. In this mouse model of OA, Runx2 expression in osteoarthritic cartilage parallels collagen-10 expression but arises earlier than MMP13. After induction of post-traumatic knee OA by the same surgical procedure, Runx2+/- mice displayed decreased cartilage destruction and osteophyte formation, along with reduced expression of collagen-10 and MMP13, as compared with wild-type mice [70].
Adult articular cartilage is an avascular tissue. This is important for cartilage function, the environment of the articular chondrocytes and maintenance of the ECM. Vascular endothelial growth factor (VEGF) is induced by damage to articular cartilage [71-73]. Hayami et al. showed increased expression of VEGF was paralleled by decreased expression of chondromodulin-I, an anti-angiogenetic factor involved in maintaining the avascularity of articular cartilage. Pufe et al. showed that inhibition of VEGF suppressed mechanically induced MMP expression [27, 72].
In an ex vivo model at 24 hours after cartilage injury, Dell'accio showed up-regulation of the Wnt-16 pathways and up-regulation of Wnt target genes [74]. Nuclear localization of β-catenin was also seen. These genes were shown to be up-regulated in areas of degenerated cartilage, but showed normal expression in areas of normal cartilage within the same joint. These results implicate a reactivation of morphogenetic pathways by mechanical forces [75]. Adult mice forced to express β-catenin showed cartilage degeneration and increased expression of aggrecan, MMP-9 and MMP-13 [76]. In contrast to this, mice deficient in β-catenin signaling also show increased chondrocyte apoptosis and cartilage degeneration [77]. These results suggest that both excessive and insufficient β-catenin levels may impair the homeostasis of articular chondrocytes.
Inflammatory cytokines have also been shown to be elevated following joint injury [78, 79]. Lee et al. found increases in resistin, a macrophage derived proinflammatory mediator that stimulates inflammatory cytokines after joint injury. IL -I, IL-6 and tumor necrosis factor alpha (TNFa) have all been show to be increased after joint injury [78]. These inflammatory cytokines further the loss of proteoglycans in the ECM through activation of catabolic pathways and inhibition of anabolic activities [80-82]. IL-1 upregulates production of MMPs by increasing nitric oxide (NO) production in chondrocytes. Inoue et al. showed IL-1 induces expression of MMP-3 and VEGF by chondrocytes and synoviocytes. This may be inhibited by IL-1 receptor antagonist [73]. Inhibition of Nitric oxide synthase 2 (NOS2) has been shown to mitigate the effects of IL-1β [79]. Yorimitsu et al. showed, in destabilization of rat knees, that inhibition of NO production by IL-4 was protective to cartilage [83]. IL-6 and IL-7 also appear to be involved in degradation of cartilage and IL-1 may work partly through activation of IL-6 [82]. The catabolic effects of TNFa may also be mediated through endogenous IL-6 [84]. Interestingly Clements showed that IL-1β, IL-1β converting enzyme, stromelysin 1 and iNOS deficient mice all showed an increased progression of arthritic changes as well as increased levels of MMP, aggrecanase and collagenase in a mouse destabilization model. These results suggest that healthy articular cartilage demands a balance between anabolism and catabolism. Completely eliminating a catabolic cytokine will have unintended effects on the regulation of other cytokines and chondrocyte metabolism. This highlights the complex nature of the in vivo interactions of different signaling molecules and the likelihood of multiple regulatory effects on chondrocytes.
Pathogenetic processes in synovium
Regardless of the insult to a particular joint, trauma versus instability, changes in the synovium and synovial fluid are observed [42, 78, 85, 86]. Lubricin, a joint lubricating molecule produced by synoviocytes and superficial chondrocytes, is significantly lower in unstable knees and remains so at 12 months post injury [87]. Lowered levels of lubricin correlated with an increase in TNFa, and inhibition of TNFa resulted in increased lubricin levels [88]. The decreased concentrations of lubricin result in decreased boundary lubrication of the articular cartilage and this in turn is correlated with cartilage damage [89]. Concentrations of other joint lubricant, hyaluronan (HA), and proteoglycan 4 (PRG4), are also affected by factors such as TNFa, IL-1β, and TGF-β, known to be elevated after joint trauma [90].
Cytokines such as IL-1β, IL-6, and TNFa as well as MMP levels are significantly elevated in synovial fluid following injury such as ACL transection [91]. In a human study looking at synovial samples from patients with ACL and meniscal injuries, inflammatory patterns in the synovium were similar to those with clinical OA. Upstream factors such as Wnt signaling pathways are also up-regulated in synovial tissue [92]. Morisugi et al. isolated healthy synoviocytes and subjected them to mechanical stretch. Significant elevation of gene expression of COX-2, iNOS, NF-Kappaβ, and poly (ADP-ribose) (PAR) synthesis occurred. The PAR synthesis is thought to be representative of oxidative damage to the DNA. This study highlights that the synovium is independently capable of up-regulating cytokines. It is therefore possible that in some pathways the synovium is the primary tissue up-regulating destructive molecular pathways after trauma. For instance, in instability models, it could be that the increased stretch on the synovium is a primary event leading to up-regulation of inflammatory cytokines and ultimately OA development. Yang et al, found that synovial fluid taken from injured human knees could decrease GAG content and collagen II production in cultivated healthy cartilage [93]. This highlights how the synovial fluid can directly affect chondrocyte function.
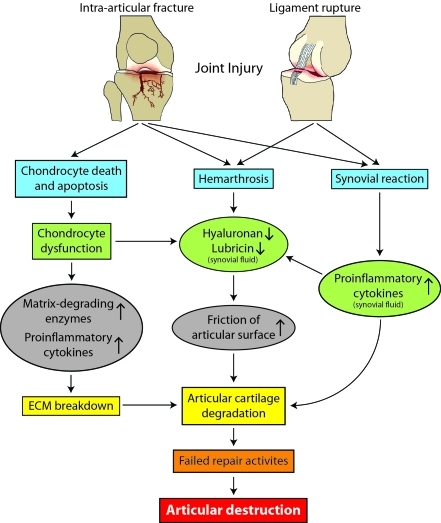
The possible pathogenetic mechanisms underlying the development of PTOA are illustrated in Figure 2.
Figure 2.
Possible pathogenetic mechanisms underlying the development of PTOA after joint injury. Although the initial pathological changes may vary depending on the damage to specific joint tissues, these changes eventually lead to articular cartilage degradation and joint destruction. The synovium and articular cartilage may interact with each other through specific mediators in synovial fluid, which are secreted by either chondrocytes or synoviocytes.
Pathologic changes in bone
As development of OA progresses, subchondral sclerosis and osteophyte formation are diagnostic markers of the disease [94]. In the case of an impact injury to a joint, such as an intraarticular fracture, there is obvious injury to the subchondral bone [95]. Most traumatic ligament injuries are also likely to have injury to the subchondral bone in form of bone bruises and microfractures [96, 97]. Histologic changes are seen in the subchondral bone of animal models of OA created by joint instability through ACL transection or DMM even though no direct injury to the subchondral bone has occurred [53, 54, 56]. Boyd et al. showed early architectural changes to the cancellous bone after surgical ACL transection in dogs [98]. The structure and function of the subchondral bone thus appears to be intimately connected with the health of the articular cartilage.
The early bone changes that were observed before visible damage to the articular cartilage could be appreciated [53]. Kawaguchi et al., using mechanical stress and induction of Runx2 in mice, suggested that endochondral ossification signals are likely important in the pathogenesis of OA [99]. Kamekura et al. also showed Runx+/- mice to have less osteophyte formation after surgical destabilization of the knee joint, which correlated with articular cartilage destruction [70]. These instability models highlight interplay between the subchondral bone and articular cartilage, demonstrating that subchondral bone changes are not simply later findings seen after cartilage destruction. Numerous and complex communication pathways exist between synovium, articular cartilage and bone. A greater understanding of the interactions of all joint tissues is needed.
Current therapeutic challenges
Current therapy for PTOA generally consists of surgery and pharmacological interventions. Surgical therapies, such as reduction and fixation of intra-articular fracture, repair of meniscal tear, and reconstruction of ligaments, currently remain the only means of preventing or slowing the progression of PTOA after injury. Surgical procedures with or without tissue engineering or gene therapy for regeneration of articular cartilage and menisci are mainly reserved for patients with an intra-articular injury or focal osteochondral lesions [100-102]. Surgery for PTOA aimed at reduction of intra-articular stress (e.g., osteotomy and distraction) has been performed for decades and remain reasonable treatment for patients whose PTOA is initiated from mechanical factors [103, 104]. Once severe joint destruction has developed, joint replacement and joint fusions remain the only surgical options. Unlike other forms of OA, which mostly affect middle-aged and older populations, PTOA often affects younger adults for whom joint replacement or joint fusion is not a desirable treatment.
Current pharmacologic management of OA pain (e.g., analgesics, non-steroidal antiinflammatory drugs (NSAIDs), and intra-articular injection of corticosteroids or hyaluronic acid derivatives) is only modestly effective and used for symptomatic control. NSAIDs can cause adverse effects on the gastrointestinal tract and cardiovascular system. Therefore, interest has been high in the development of structure or disease-modifying OA drugs (DMOADs) aimed at slowing, halting, or reversing the progression of structural damage of articular cartilage. However, the vast majority of research on DMOADs has focused on mid- to late-term OA. A large number of candidate DMOADs have been tested but none have been approved by American or European regulatory agencies [105].
Opportunities for early intervention
Surgical therapies remain the only means of correcting the structural and mechanical abnormalities caused by joint injury [6]. Early surgical intervention includes anatomic reduction and fixation of intra-articular fractures, repair or removal of torn menisci, repair or reconstruction of ruptured ligaments, and treatment of hemarthrosis if necessary. Surgical intervention along with technologic advances in fracture fixation and ligament reconstruction will undoubtedly play a role in improved treatment of joint injuries. However, a significant number of patients still progress to clinical OA even with the best surgical care of joint injuries [9].
It appears that greatest breakthroughs will likely be in early biologic therapies which could effectively block chondrocyte apoptosis and ECM destruction after appropriate surgical treatment. It has been recognized that chondrocyte apoptosis may be a common pathway in PTOA. Caspase inhibitors have been used in rabbits to reduce the severity of articular injury [106, 107]. Antioxidants including N-acetylcysteine, vitamin E, and superoxide dismutase have also shown promise to reduce chondrocyte apoptosis [40, 108, 109].
Altering the destruction of the ECM after injury appears to be a promising place for new therapies. However, inhibitors of MMPs have failed up to this point because of adverse events and/ or lack of efficacy [105]. Other targets involved in ECM breakdown such as Ddr2 receptor, ADAMTS-5, and MAPKp38 and NFkB signaling pathways may provide targets for intervention. Cytokine inhibitors have been shown to be effective in altering the PTOA in animal models. TNFa and IL-1β inhibition have shown promise in animal models. IL-10 administration may also alter metabolism, favoring more anabolism and have chondro-protective effects [110]. BMPs may up-regulate chondrocyte metabolism, with BMP-7 showing significant changes in anabolism of chondrocytes [111]. Calcitonin and parathyroid hormone 1-34 have also been used in animals to alter cartilage destruction after injury [112, 113].
Numerous attempts at disease modifying drugs for OA have failed [105]. Most candidate drugs have focused on inhibition of a specific enzyme or inflammatory mediator at the later stage of OA. In PTOA, the clear precipitating event presents a unique window of opportunity to intervene early in the acute post-traumatic period. Recent studies have revealed that inhibition of a single catabolic molecule may not be sufficient for the treatment of OA because multiple catabolic factors are involved in its pathogenesis. Furthermore, matrix turnover is a normal part of cartilage function and damaged ECM must be broken down before new matrix can take its place. Therefore, it is likely that potential therapeutic targets will need to be more upstream regulators that may regulate the expression of multiple cytokines, enzymes, and anabolic molecules, thereby maintaining the balance between anabolic and catabolic activity of articular chondrocytes and other joint tissue cells such as synoviocytes.
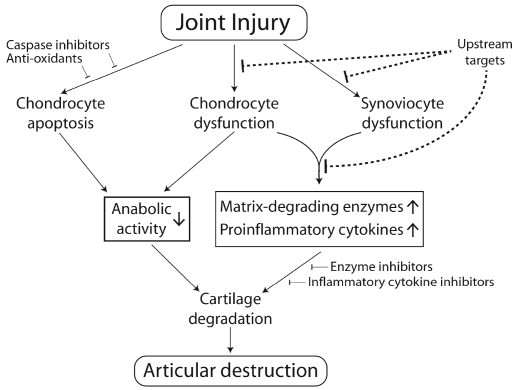
Recent studies have revealed that biological and mechanical abnormalities may affect transcriptional activity of specific transcription factors in articular chondrocytes. Transcription factor Sox9 is critical for the formation of cartilage, including articular cartilage, but its role in the maintenance of adult articular chondrocyte function remains to be elucidated. Transcription factor Nfat1 plays an important role in maintaining the physiological function of adult articular chondrocytes. Nfat1-deficient mice exhibit normal skeletal development but display most of the features of human OA in their adult stage, including chondrocyte hypertrophy with overexpression of specific matrix-degrading proteinases and proinflammatory cytokines in articular cartilage [114]. Transcription factor Runx2 [70] and β-catenin transcriptional signaling [76, 77] may also be involved in the pathogenesis of OA via regulating the expression of anabolic and catabolic molecules in articular chondrocytes. These specific transcriptional signaling molecules that regulate the expression of multiple catabolic and/or anabolic factors in articular chondrocytes may be potential upstream targets for the prevention and treatment of PTOA (Figure 3).
Figure 3.
Potential opportunities for early biologic/ pharmacologic interventions to prevent or halt the progression of PTOA. The therapeutic strategies proposed here emphasize the importance of early intervention by a combination of upstream targets (e.g., Nfatl and Runx2 inhibitors), caspase inhibitors, and anti-oxidants to block chondrocyte dysfunction, synoviocyte dysfunction, and chondrocyte apoptosis at the early stage of PTOA.
Conclusion
PTOA arises from joint trauma. Even with the best surgical care of joint injuries, the risk of PTOA after joint injuries ranges from 20% to more than 50%. The time course for the progression of PTOA is highly variable and risk of PTOA increases with age of patients at the time of joint injury, suggesting that biologic factors may be involved in the progression of PTOA. Current pharmacologic management of OA, including PTOA, is mainly for symptomatic control, which cannot halt or reverse the progression of PTOA. This review summarizes the recent studies on the pathogenetic mechanisms underlying the development of PTOA, which provides new insights into the potential opportunities for early biologic or pharmacologic intervention using more upstream regulators as targets for prevention and treatment of PTOA. Continued exploration of additional factors responsible for the homeostasis of articular cartilage and other joint tissues combined with appropriate surgical treatments may yield more effective therapies for PTOA.
Acknowledgments
This work was supported in part by US National Institutes of Health grant R01 AR059088 (to JW) and the Mary Alice & Paul R. Harrington Distinguished Professorship Endowment (to JW). The authors thank Brent Furomoto for his graphic design and editorial assistance.
References
- 1.Dirschl DR, Marsh JL, Buckwalter JA, Gelberman R, Olson SA, Brown TD, Llinias A. Articular fractures. J Am Acad Orthop Surg. 2004;12:416–423. doi: 10.5435/00124635-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Thambyah A. A hypothesis matrix for studying biomechanical factors associated with the initiation and progression of posttraumatic osteoarthritis. Med Hypotheses. 2005;64:1157–1161. doi: 10.1016/j.mehy.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 3.Buckwalter JA. Articular cartilage: injuries and potential for healing. J Orthop Sports Phys Ther. 1998;28:192–202. doi: 10.2519/jospt.1998.28.4.192. [DOI] [PubMed] [Google Scholar]
- 4.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 5.Nishida T, Kubota S, Kojima S, Kuboki T, Nakao K, Kushibiki T, Tabata Y, Takigawa M. Regeneration of defects in articular cartilage in rat knee joints by CCN2 (connective tissue growth factor) J Bone Miner Res. 2004;19:1308–1319. doi: 10.1359/JBMR.040322. [DOI] [PubMed] [Google Scholar]
- 6.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling: roles in post-traumatic osteoarthritis. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 7.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58:150–167. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 8.Schulze-Tanzil G. Activation and dedifferentiation of chondrocytes: implications in cartilage injury and repair. Ann Anat. 2009;191:325–338. doi: 10.1016/j.aanat.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Marsh JL, Buckwalter J, Gelberman R, Dirschl D, Olson S, Brown T, Llinias A. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84-A:1259–1271. [PubMed] [Google Scholar]
- 10.Nelson F, Billinghurst RC, Pidoux I, Reiner A, Langworthy M, McDermott M, Malogne T, Sitler DF, Kilambi NR, Lenczner E, Poole AR. Early post-traumatic osteoarthritis-like changes in human articular cartilage following rupture of the anterior cruciate ligament. Osteoarthritis Cartilage. 2006;14:114–119. doi: 10.1016/j.joca.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 11.Szczodry M, Coyle CH, Kramer SJ, Smolinski P, Chu CR. Progressive chondrocyte death after impact injury indicates a need for chondroprotective therapy. Am J Sports Med. 2009;37:2318–2322. doi: 10.1177/0363546509348840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DiMicco MA, Patwari P, Siparsky PN, Kumar S, Pratta MA, Lark MW, Kim YJ, Grodzinsky AJ. Mechanisms and kinetics of glycosaminoglycan release following in vitro cartilage injury. Arthritis Rheum. 2004;50:840–848. doi: 10.1002/art.20101. [DOI] [PubMed] [Google Scholar]
- 13.Patwari P, Kurz B, Sandy JD, Grodzinsky AJ. Mannosamine inhibits aggrecanasemediated changes in the physical properties and biochemical composition of articular cartilage. Arch Biochem Biophys. 2000;374:79–85. doi: 10.1006/abbi.1999.1538. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Fitzgerald JB, Dimicco MA, Grodzinsky AJ. Mechanical injury of cartilage explants causes specific time-dependent changes in chondrocyte gene expression. Arthritis Rheum. 2005;52:2386–2395. doi: 10.1002/art.21215. [DOI] [PubMed] [Google Scholar]
- 15.Milentijevic D, Rubel IF, Liew AS, Helfet DL, Torzilli PA. An in vivo rabbit model for cartilage trauma: a preliminary study of the influence of impact stress magnitude on chondrocyte death and matrix damage. J Orthop Trauma. 2005;19:466–473. doi: 10.1097/01.bot.0000162768.83772.18. [DOI] [PubMed] [Google Scholar]
- 16.Borrelli J, Jr, Torzilli PA, Grigiene R, Helfet DL. Effect of impact load on articular cartilage: development of an intra-articular fracture model. J Orthop Trauma. 1997;11:319–326. doi: 10.1097/00005131-199707000-00003. [DOI] [PubMed] [Google Scholar]
- 17.Natoli RM, Scott CC, Athanasiou KA. Temporal effects of impact on articular cartilage cell death, gene expression, matrix biochemistry, and biomechanics. Ann Biomed Eng. 2008;36:780–792. doi: 10.1007/s10439-008-9472-5. [DOI] [PubMed] [Google Scholar]
- 18.Natoli RM, Athanasiou KA. Traumatic loading of articular cartilage: Mechanical and biological responses and post-injury treatment. Biorheology. 2009;46:451–485. doi: 10.3233/BIR-2009-0554. [DOI] [PubMed] [Google Scholar]
- 19.Grodzinsky AJ, Levenston ME, Jin M, Frank EH. Cartilage tissue remodeling in response to mechanical forces. Annu Rev Biomed Eng. 2000;2:691–713. doi: 10.1146/annurev.bioeng.2.1.691. [DOI] [PubMed] [Google Scholar]
- 20.Smith RL, Carter DR, Schurman DJ. Pressure and shear differentially alter human articular chondrocyte metabolism: a review. Clin Orthop Relat Res. 2004;427S:S89–95. [PubMed] [Google Scholar]
- 21.Salter DM, Wright MO, Millward-Sadler SJ. NMDA receptor expression and roles in human articular chondrocyte mechanotransduction. Biorheology. 2004;41:273–281. [PubMed] [Google Scholar]
- 22.Chan PS, Schlueter AE, Coussens PM, Rosa GJ, Haut RC, Orth MW. Gene expression profile of mechanically impacted bovine articular cartilage explants. J Orthop Res. 2005;23:1146–1151. doi: 10.1016/j.orthres.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 23.Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279:19502–19511. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 24.Hembree WC, Ward BD, Furman BD, Zura RD, Nichols LA, Guilak F, Olson SA. Viability and apoptosis of human chondrocytes in osteochondral fragments following joint trauma. J Bone Joint Surg Br. 2007;89:1388–1395. doi: 10.1302/0301-620X.89B10.18907. [DOI] [PubMed] [Google Scholar]
- 25.Rundell SA, Baars DC, Phillips DM, Haut RC. The limitation of acute necrosis in retropatellar cartilage after a severe blunt impact to the in vivo rabbit patello-femoral joint. J Orthop Res. 2005;23:1363–1369. doi: 10.1016/j.orthres.2005.06.001.1100230618. [DOI] [PubMed] [Google Scholar]
- 26.Kim HT, Lo MY, Pillarisetty R. Chondrocyte apoptosis following intraarticular fracture in humans. Osteoarthritis Cartilage. 2002;10:747–749. doi: 10.1053/joca.2002.0828. [DOI] [PubMed] [Google Scholar]
- 27.Kurz B, Lemke AK, Fay J, Pufe T, Grodzinsky AJ, Schunke M. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat. 2005;187:473–485. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 28.D'Lima DD, Hashimoto S, Chen PC, Colwell CW., Jr and Lotz MK. Impact of mechanical trauma on matrix and cells. Clin Orthop Relat Res. 2001;391S:S90–99. doi: 10.1097/00003086-200110001-00009. [DOI] [PubMed] [Google Scholar]
- 29.Torzilli PA, Grigiene R, Borrelli J, Jr, Helfet DL. Effect of impact load on articular cartilage: cell metabolism and viability, and matrix water content. J Biomech Eng. 1999;121:433–441. doi: 10.1115/1.2835070. [DOI] [PubMed] [Google Scholar]
- 30.Kurz B, Jin M, Patwari P, Cheng DM, Lark MW, Grodzinsky AJ. Biosynthetic response and mechanical properties of articular cartilage after injurious compression. J Orthop Res. 2001;19:1140–1146. doi: 10.1016/S0736-0266(01)00033-X. [DOI] [PubMed] [Google Scholar]
- 31.Morel V, Berutto C, Quinn TM. Effects of damage in the articular surface on the cartilage response to injurious compression in vitro. J Biomech. 2006;39:924–930. doi: 10.1016/j.jbiomech.2005.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Monfort J, Garcia Giralt N, Lopez Armada MJ, Monllau JC, Bonilla A, Benito P, Blanco FJ. Decreased metalloproteinase production as a response to mechanical pressure in human cartilage: a mechanism for homeostatic regulation. Arthritis Res Ther. 2006;8:R149. doi: 10.1186/ar2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morel V, Quinn TM. Short-term changes in cell and matrix damage following mechanical injury of articular cartilage explants and modelling of microphysical mediators. Biorheology. 2004;41:509–519. [PubMed] [Google Scholar]
- 34.Buckwalter JA, Mankin HJ, Grodzinsky AJ. Articular cartilage and osteoarthritis. Instr Course Lect. 2005;54:465–480. [PubMed] [Google Scholar]
- 35.Robertson CM, Pennock AT, Harwood FL, Pomerleau AC, Allen RT, Amiel D. Characterization of pro-apoptotic and matrixdegradative gene expression following induction of osteoarthritis in mature and aged rabbits. Osteoarthritis Cartilage. 2006;14:471–476. doi: 10.1016/j.joca.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 36.Vrahas MS, Mithoefer K, Joseph D. The long-term effects of articular impaction. Clin Orthop Relat Res. 2004;423:40–43. doi: 10.1097/01.blo.0000133567.28491.7d. [DOI] [PubMed] [Google Scholar]
- 37.Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15:27–34. doi: 10.1016/j.joca.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 38.Baars DC, Rundell SA, Haut RC. Treatment with the non-ionic surfactant poloxamer P188 reduces DNA fragmentation in cells from bovine chondral explants exposed to injurious unconfined compression. Biomech Model Mechanobiol. 2006;5:133–139. doi: 10.1007/s10237-006-0024-3. [DOI] [PubMed] [Google Scholar]
- 39.Strauss EJ, Goodrich LR, Chen CT, Hidaka C, Nixon AJ. Biochemical and biomechanical properties of lesion and adjacent articular cartilage after chondral defect repair in an equine model. Am J Sports Med. 2005;33:1647–1653. doi: 10.1177/0363546505275487. [DOI] [PubMed] [Google Scholar]
- 40.Martin JA, Buckwalter JA. Post-traumatic osteoarthritis: the role of stress induced chondrocyte damage. Biorheology. 2006;43:517–521. [PubMed] [Google Scholar]
- 41.Martin JA, Brown T, Heiner A, Buckwalter JA. Post-traumatic osteoarthritis: the role of accelerated chondrocyte senescence. Biorheology. 2004;41:479–491. [PubMed] [Google Scholar]
- 42.Ostalowska A, Kasperczyk S, Kasperczyk A, Slowinska L, Marzec M, Stoltny T, Koczy B, Birkner E. Oxidant and anti-oxidant systems of synovial fluid from patients with knee posttraumatic arthritis. J Orthop Res. 2007;25:804–812. doi: 10.1002/jor.20357. [DOI] [PubMed] [Google Scholar]
- 43.Goodwin W, McCabe D, Sauter E, Reese E, Walter M, Buckwalter JA, Martin JA. Rotenone prevents impact-induced chondrocyte death. J Orthop Res. 28:1057–1063. doi: 10.1002/jor.21091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yudoh K, Shishido K, Murayama H, Yano M, Matsubayashi K, Takada H, Nakamura H, Masuko K, Kato T, Nishioka K. Water-soluble C60 fullerene prevents degeneration of articular cartilage in osteoarthritis via downregulation of chondrocyte catabolic activity and inhibition of cartilage degeneration during disease development. Arthritis Rheum. 2007;56:3307–3318. doi: 10.1002/art.22917. [DOI] [PubMed] [Google Scholar]
- 45.Ramakrishnan P, Hecht BA, Pedersen DR, Lavery MR, Maynard J, Buckwalter JA, Martin JA. Oxidant conditioning protects cartilage from mechanically induced damage. J Orthop Res. 2010;28:914–920. doi: 10.1002/jor.21072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Aizawa T, Kon T, Einhorn TA, Gerstenfeld LC. Induction of apoptosis in chondrocytes by tumor necrosis factor-alpha. J Orthop Res. 2001;19:785–796. doi: 10.1016/S0736-0266(00)00078-4. [DOI] [PubMed] [Google Scholar]
- 47.Harris SL, Levine AJ. The p53 pathway: positive and negative feedback loops. Oncogene. 2005;24:2899–2908. doi: 10.1038/sj.onc.1208615. [DOI] [PubMed] [Google Scholar]
- 48.Groenendyk J, Michalak M. Endoplasmic reticulum quality control and apoptosis. Acta Biochim Pol. 2005;52:381–395. [PubMed] [Google Scholar]
- 49.Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57:545–553. doi: 10.1007/s00280-005-0111-7. [DOI] [PubMed] [Google Scholar]
- 50.Buckwalter JA. Osteoarthritis and articular cartilage use, disuse, and abuse: experimental studies. J Rheumatol Suppl. 1995;43:13–15. [PubMed] [Google Scholar]
- 51.Chaudhari AM, Briant PL, Bevill SL, Koo S, Andriacchi TP. Knee kinematics, cartilage morphology, and osteoarthritis after ACL injury. Med Sci Sports Exerc. 2008;40:215–222. doi: 10.1249/mss.0b013e31815cbb0e. [DOI] [PubMed] [Google Scholar]
- 52.Salvarrey-Strati A, Watson L, Blanchet T, Lu N, Glasson SS. The influence of enrichment devices on development of osteoarthritis in a surgically induced murine model. ILAR J. 2008;49:23–30. doi: 10.1093/ilar.49.3.e23. [DOI] [PubMed] [Google Scholar]
- 53.Hayami T, Pickarski M, Zhuo Y, Wesolowski GA, Rodan GA, Duong le T. Characterization of articular cartilage and subchondral bone changes in the rat anterior cruciate ligament transection and meniscectomized models of osteoarthritis. Bone. 2006;38:234–243. doi: 10.1016/j.bone.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 54.Glasson SS, Blanchet TJ, Morris EA. The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthritis Cartilage. 2007;15:1061–1069. doi: 10.1016/j.joca.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 55.Glasson SS, Askew R, Sheppard B, Carito B, Blanchet T, Ma HL, Flannery CR, Peluso D, Kanki K, Yang Z, Majumdar MK, Morris EA. Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature. 2005;434:644–648. doi: 10.1038/nature03369. [DOI] [PubMed] [Google Scholar]
- 56.Botter SM, Glasson SS, Hopkins B, Clockaerts S, Weinans H, van Leeuwen JP, van Osch GJ. ADAMTS5-/- mice have less subchondral bone changes after induction of osteoarthritis through surgical instability: implications for a link between cartilage and subchondral bone changes. Osteoarthritis Cartilage. 2009;17:636–645. doi: 10.1016/j.joca.2008.09.018. [DOI] [PubMed] [Google Scholar]
- 57.Polur I, Lee PL, Servais JM, Xu L, Li Y. Role of HTRA1, a serine protease, in the progression of articular cartilage degeneration. Histol Histopathol. 2010;25:599–608. doi: 10.14670/hh-25.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nielsen RH, Stoop R, Leeming DJ, Stolina M, Qvist P, Christiansen C, Karsdal MA. Evaluation of cartilage damage by measuring collagen degradation products in joint extracts in a traumatic model of osteoarthritis. Biomarkers. 2008;13:79–87. doi: 10.1080/13547500701615108. [DOI] [PubMed] [Google Scholar]
- 59.Xu L, Servais J, Polur I, Kim D, Lee PL, Chung K, Li Y. Attenuation of osteoarthritis progression by reduction of the discoidin domain receptor 2 in mice. Arthritis Rheum. 2010;62:2736–2744. doi: 10.1002/art.27582. Sep. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Xu L, Peng H, Wu D, Hu K, Goldring MB, Olsen BR, Li Y. Activation of the discoidin domain receptor 2 induces expression of matrix metalloproteinase 13 associated with osteoarthritis in mice. J Biol Chem. 2005;280:548–555. doi: 10.1074/jbc.M411036200. [DOI] [PubMed] [Google Scholar]
- 61.Klatt AR, Paul-Klausch B, Klinger G, Kuhn G, Renno JH, Banerjee M, Malchau G, Wielckens K. A critical role for collagen II in cartilage matrix degradation: collagen II induces pro-inflammatory cytokines and MMPs in primary human chondrocytes. J Orthop Res. 2009;27:65–70. doi: 10.1002/jor.20716. [DOI] [PubMed] [Google Scholar]
- 62.Sandell LJ. Anabolic factors in degenerative joint disease. Curr Drug Targets. 2007;8:359–365. doi: 10.2174/138945007779940142. [DOI] [PubMed] [Google Scholar]
- 63.Otsuki S, Brinson DC, Creighton L, Kinoshita M, Sah RL, D'Lima D, Lotz M. The effect of glycosaminoglycan loss on chondrocyte viability: a study on porcine cartilage explants. Arthritis Rheum. 2008;58:1076–1085. doi: 10.1002/art.23381. [DOI] [PubMed] [Google Scholar]
- 64.Wildey GM, Billetz AC, Matyas JR, Adams ME, McDevitt CA. Absolute concentrations of mRNA for type I and type VI collagen in the canine meniscus in normal and ACL-deficient knee joints obtained by RNase protection assay. J Orthop Res. 2001;19:650–658. doi: 10.1016/S0736-0266(00)00053-X. [DOI] [PubMed] [Google Scholar]
- 65.Buckwalter JA. Articular cartilage injuries. Clin Orthop Relat Res. 2002:21–37. doi: 10.1097/00003086-200209000-00004. [DOI] [PubMed] [Google Scholar]
- 66.Frisbie DD, Oxford JT, Southwood L, Trotter GW, Rodkey WG, Steadman JR, Goodnight JL, McIlwraith CW. Early events in cartilage repair after subchondral bone microfracture. Clin Orthop Relat Res. 2003:215–227. doi: 10.1097/00003086-200302000-00031. [DOI] [PubMed] [Google Scholar]
- 67.Lorenz H, Wenz W, Ivancic M, Steck E, Richter W. Early and stable upregulation of collagen type II, collagen type I and YKL40 expression levels in cartilage during early experimental osteoarthritis occurs independent of joint location and histological grading. Arthritis Res Ther. 2005;7:R156–165. doi: 10.1186/ar1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hotta H, Yamada H, Takaishi H, Abe T, Morioka H, Kikuchi T, Fujikawa K, Toyama Y. Type II collagen synthesis in the articular cartilage of a rabbit model of osteoarthritis: expression of type II collagen C-propeptide and mRNA especially during early-stage osteoarthritis. J Orthop Sci. 2005;10:595–607. doi: 10.1007/s00776-005-0947-z. [DOI] [PubMed] [Google Scholar]
- 69.De Croos JN, Dhaliwal SS, Grynpas MD, Pilliar RM, Kandel RA. Cyclic compressive mechanical stimulation induces sequential catabolic and anabolic gene changes in chondrocytes resulting in increased extracellular matrix accumulation. Matrix Biol. 2006;25:323–331. doi: 10.1016/j.matbio.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 70.Kamekura S, Kawasaki Y, Hoshi K, Shimoaka T, Chikuda H, Maruyama Z, Komori T, Sato S, Takeda S, Karsenty G, Nakamura K, Chung UI, Kawaguchi H. Contribution of runt-related transcription factor 2 to the pathogenesis of osteoarthritis in mice after induction of knee joint instability. Arthritis Rheum. 2006;54:2462–2470. doi: 10.1002/art.22041. [DOI] [PubMed] [Google Scholar]
- 71.Hayami T, Funaki H, Yaoeda K, Mitui K, Yamagiwa H, Tokunaga K, Hatano H, Kondo J, Hiraki Y, Yamamoto T, Duong le T, Endo N. Expression of the cartilage derived antiangiogenic factor chondromodulin-I decreases in the early stage of experimental osteoarthritis. J Rheumatol. 2003;30:2207–2217. [PubMed] [Google Scholar]
- 72.Pufe T, Lemke A, Kurz B, Petersen W, Tillmann B, Grodzinsky AJ, Mentlein R. Mechanical overload induces VEGF in cartilage discs via hypoxia-inducible factor. Am J Pathol. 2004;164:185–192. doi: 10.1016/S0002-9440(10)63109-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Inoue K, Masuko Hongo K, Okamoto M, Nishioka K. Induction of vascular endothelial growth factor and matrix metalloproteinase-3 (stromelysin) by interleukin-1 in human articular chondrocytes and synoviocytes. Rheumatol Int. 2005;26:93–98. doi: 10.1007/s00296-004-0513-6. [DOI] [PubMed] [Google Scholar]
- 74.Dell'Accio F, De Bari C, El Tawil NM, Barone F, Mitsiadis TA, O'Dowd J, Pitzalis C. Activation of WNT and BMP signaling in adult human articular cartilage following mechanical injury. Arthritis Res Ther. 2006;8:R139. doi: 10.1186/ar2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dell'accio F, De Bari C, Eltawil NM, Vanhummelen P, Pitzalis C. Identification of the molecular response of articular cartilage to injury, by microarray screening: Wnt-16 expression and signaling after injury and in osteoarthritis. Arthritis Rheum. 2008;58:1410–1421. doi: 10.1002/art.23444. [DOI] [PubMed] [Google Scholar]
- 76.Zhu M, Tang D, Wu Q, Hao S, Chen M, Xie C, Rosier RN, O'Keefe RJ, Zuscik M, Chen D. Activation of beta-catenin signaling in articular chondrocytes leads to osteoarthritis-like phenotype in adult beta-catenin conditional activation mice. J Bone Miner Res. 2009;24:12–21. doi: 10.1359/JBMR.080901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu M, Chen M, Zuscik M, Wu Q, Wang YJ, Rosier RN, O'Keefe RJ, Chen D. Inhibition of beta-catenin signaling in articular chondrocytes results in articular cartilage destruction. Arthritis Rheum. 2008;58:2053–2064. doi: 10.1002/art.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lee JH, Ort T, Ma K, Picha K, Carton J, Marsters PA, Lohmander LS, Baribaud F, Song XY, Blake S. Resistin is elevated following traumatic joint injury and causes matrix degradation and release of inflammatory cytokines from articular cartilage in vitro. Osteoarthritis Cartilage. 2009;17:613–620. doi: 10.1016/j.joca.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 79.Abramson SB, Attur M, Amin AR, Clancy R. Nitric oxide and inflammatory mediators in the perpetuation of osteoarthritis. Curr Rheumatol Rep. 2001;3:535–541. doi: 10.1007/s11926-001-0069-3. [DOI] [PubMed] [Google Scholar]
- 80.Flannery CR, Little CB, Hughes CE, Curtis CL, Caterson B, Jones SA. IL-6 and its soluble receptor augment aggrecanase-mediated proteoglycan catabolism in articular cartilage. Matrix Biol. 2000;19:549–553. doi: 10.1016/s0945-053x(00)00111-6. [DOI] [PubMed] [Google Scholar]
- 81.Stevens AL, Wishnok JS, Chai DH, Grodzinsky AJ, Tannenbaum SR. A sodium dodecyl sulfate-polyacrylamide gel electrophoresisliquid chromatography tandem mass spectrometry analysis of bovine cartilage tissue response to mechanical compression injury and the inflammatory cytokines tumor necrosis factor alpha and interleukin-1beta. Arthritis Rheum. 2008;58:489–500. doi: 10.1002/art.23120. [DOI] [PubMed] [Google Scholar]
- 82.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 83.Yorimitsu M, Nishida K, Shimizu A, Doi H, Miyazawa S, Komiyama T, Nasu Y, Yoshida A, Watanabe S, Ozaki T. Intra-articular injection of interleukin-4 decreases nitric oxide production by chondrocytes and ameliorates subsequent destruction of cartilage in instability-induced osteoarthritis in rat knee joints. Osteoarthritis Cartilage. 2008;16:764–771. doi: 10.1016/j.joca.2007.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Sui Y, Lee JH, DiMicco MA, Vanderploeg EJ, Blake SM, Hung HH, Plaas AH, James IE, Song XY, Lark MW, Grodzinsky AJ. Mechanical injury potentiates proteoglycan catabolism induced by interleukin-6 with soluble interleukin-6 receptor and tumor necrosis factor alpha in immature bovine and adult human articular cartilage. Arthritis Rheum. 2009;60:2985–2996. doi: 10.1002/art.24857. [DOI] [PubMed] [Google Scholar]
- 85.Lohmander LS, Saxne T, Heinegard D. Increased concentrations of bone sialoprotein in joint fluid after knee injury. Ann Rheum Dis. 1996;55:622–626. doi: 10.1136/ard.55.9.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wassilew GI, Lehnigk U, Duda GN, Taylor WR, Matziolis G, Dynybil C. The expression of proinflammatory cytokines and matrix metalloproteinases in the synovial membranes of patients with osteoarthritis compared with traumatic knee disorders. Arthroscopy. 2010;26:1096–1104. doi: 10.1016/j.arthro.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 87.Wei L, Fleming BC, Sun X, Teeple E, Wu W, Jay GD, Elsaid KA, Luo J, Machan JT, Chen Q. Comparison of differential biomarkers of osteoarthritis with and without posttraumatic injury in the Hartley guinea pig model. J Orthop Res. 2010;28:900–906. doi: 10.1002/jor.21093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Elsaid KA, Machan JT, Waller K, Fleming BC, Jay GD. The impact of anterior cruciate ligament injury on lubricin metabolism and the effect of inhibiting tumor necrosis factor alpha on chondroprotection in an animal model. Arthritis Rheum. 2009;60:2997–3006. doi: 10.1002/art.24800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Elsaid KA, Jay GD, Warman ML, Rhee DK, Chichester CO. Association of articular cartilage degradation and loss of boundarylubricating ability of synovial fluid following injury and inflammatory arthritis. Arthritis Rheum. 2005;52:1746–1755. doi: 10.1002/art.21038. [DOI] [PubMed] [Google Scholar]
- 90.Blewis ME, Lao BJ, Schumacher BL, Bugbee WD, Sah RL, Firestein GS. Interactive cytokine regulation of synoviocyte lubricant secretion. Tissue Eng Part A. 2010;16:1329–1337. doi: 10.1089/ten.tea.2009.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Z, Yang L, Wang Y, Xue R, Zhang J, Huang W, Chen PC, Sung KL. Contributions of different intraarticular tissues to the acute phase elevation of synovial fluid MMP-2 following rat ACL rupture. J Orthop Res. 2009;27:243–248. doi: 10.1002/jor.20763. [DOI] [PubMed] [Google Scholar]
- 92.Blom AB, van Lent PL, van der Kraan PM, van den Berg WB. To seek shelter from the WNT in osteoarthritis? WNT-signaling as a target for osteoarthritis therapy. Curr Drug Targets. 2010;11:620–629. doi: 10.2174/138945010791011901. [DOI] [PubMed] [Google Scholar]
- 93.Yang KG, Saris DB, Verbout AJ, Creemers LB, Dhert WJ. The effect of synovial fluid from injured knee joints on in vitro chondrogenesis. Tissue Eng. 2006;12:2957–2964. doi: 10.1089/ten.2006.12.2957. [DOI] [PubMed] [Google Scholar]
- 94.Guilak F, Fermor B, Keefe FJ, Kraus VB, Olson SA, Pisetsky DS, Setton LA, Weinberg JB. The role of biomechanics and inflammation in cartilage injury and repair. Clin Orthop Relat Res. 2004;423:17–26. doi: 10.1097/01.blo.0000131233.83640.91. [DOI] [PubMed] [Google Scholar]
- 95.Furman BD, Strand J, Hembree WC, Ward BD, Guilak F, Olson SA. Joint degeneration following closed intraarticular fracture in the mouse knee: a model of posttraumatic arthritis. J Orthop Res. 2007;25:578–592. doi: 10.1002/jor.20331. [DOI] [PubMed] [Google Scholar]
- 96.Meyer EG, Baumer TG, Slade JM, Smith WE, Haut RC. Tibiofemoral contact pressures and osteochondral microtrauma during anterior cruciate ligament rupture due to excessive compressive loading and internal torque of the human knee. Am J Sports Med. 2008;36:1966–1977. doi: 10.1177/0363546508318046. [DOI] [PubMed] [Google Scholar]
- 97.Mrosek EH, Lahm A, Erggelet C, Uhl M, Kurz H, Eissner B, Schagemann JC. Subchondral bone trauma causes cartilage matrix degeneration: an immunohistochemical analysis in a canine model. Osteoarthritis Cartilage. 2006;14:171–178. doi: 10.1016/j.joca.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 98.Boyd SK, Muller R, Zernicke RF. Mechanical and architectural bone adaptation in early stage experimental osteoarthritis. J Bone Miner Res. 2002;17:687–694. doi: 10.1359/jbmr.2002.17.4.687. [DOI] [PubMed] [Google Scholar]
- 99.Payne KA, Didiano DM, Chu CR. Donor sex and age influence the chondrogenic potential of human femoral bone marrow stem cells. Osteoarthritis Cartilage. 2010;18:705–713. doi: 10.1016/j.joca.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Evans CH, Ghivizzani SC, Robbins PD. Progress and Prospects: genetic treatments for disorders of bones and joints. Gene Ther. 2009;16:944–952. doi: 10.1038/gt.2009.73. [DOI] [PubMed] [Google Scholar]
- 101.Evans CH, Ghivizzani SC, Herndon JH, Robbins PD. Gene therapy for the treatment of musculoskeletal diseases. J Am Acad Orthop Surg. 2005;13:230–242. doi: 10.5435/00124635-200507000-00003. [DOI] [PubMed] [Google Scholar]
- 102.Vangsness CT, Jr, Kurzweil PR, Lieberman JR. Restoring articular cartilage in the knee. Am J Orthop (Belle Mead NJ) 2004;33:29–34. [PubMed] [Google Scholar]
- 103.Lopez MJ, Kunz D, Vanderby R, Jr, Heisey D, Bogdanske J, Markel MD. A comparison of joint stability between anterior cruciate intact and deficient knees: a new canine model of anterior cruciate ligament disruption. J Orthop Res. 2003;21:224–230. doi: 10.1016/S0736-0266(02)00132-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brandt KD, Dieppe P, Radin EL. Etiopathogenesis of osteoarthritis. Rheum Dis Clin North Am. 2008;34:531–559. doi: 10.1016/j.rdc.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 105.Hellio Le Graverand Gastineau MP. OA clinical trials: current targets and trials for OA. Choosing molecular targets: what have we learned and where we are headed? Osteoarthritis Cartilage. 2009;17:1393–1401. doi: 10.1016/j.joca.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 106.D'Lima D, Hermida J, Hashimoto S, Colwell C, Lotz M. Caspase inhibitors reduce severity of cartilage lesions in experimental osteoarthritis. Arthritis Rheum. 2006;54:1814–1821. doi: 10.1002/art.21874. [DOI] [PubMed] [Google Scholar]
- 107.Huser CA, Peacock M, Davies ME. Inhibition of caspase-9 reduces chondrocyte apop-tosis and proteogiycan loss following mechanical trauma. Osteoarthritis Cartilage. 2006;14:1002–1010. doi: 10.1016/j.joca.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 108.Martin JA, McCabe D, Walter M, Buckwalter JA, McKinley TO. N-acetylcysteine inhibits post-impact chondrocyte death in osteo-chondral explants. J Bone Joint Surg Am. 2009;91:1890–1897. doi: 10.2106/JBJS.H.00545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Beecher BR, Martin JA, Pedersen DR, Heiner AD, Buckwalter JA. Antioxidants block cyclic loading induced chondrocyte death. Iowa Orthop J. 2007;27:1–8. [PMC free article] [PubMed] [Google Scholar]
- 110.Schulze Tanzil G, Zreiqat H, Sabat R, Kohl B, Haider A, Muller RD, John T. lnterleukin-10 and articular cartilage: experimental therapeutical approaches in cartilage disorders. Curr Gene Ther. 2009;9:306–315. doi: 10.2174/156652309788921044. [DOI] [PubMed] [Google Scholar]
- 111.Chubinskaya S, Hurtig M, Rueger DC. OP-1/BMP-7 in cartilage repair. Int Orthop. 2007;31:773–781. doi: 10.1007/s00264-007-0423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chang JK, Chang LH, Hung SH, Wu SC, Lee HY, Lin YS, Chen CH, Fu YC, Wang GJ, Ho ML. Parathyroid hormone 1-34 inhibits terminal differentiation of human articular chondro-cytes and osteoarthritis progression in rats. Arthritis Rheum. 2009;60:3049–3060. doi: 10.1002/art.24843. [DOI] [PubMed] [Google Scholar]
- 113.El Hajjaji H, Williams JM, Devogelaer JP, Lenz ME, Thonar EJ, Manicourt DH. Treatment with calcitonin prevents the net loss of collagen, hyaluronan and proteogiycan aggregates from cartilage in the early stages of canine experimental osteoarthritis. Osteoarthritis Cartilage. 2004;12:904–911. doi: 10.1016/j.joca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Rodova M, Lu Q, Li Y, Woodbury BG, Crist JD, Gardner BM, Yost JG, Zhong XB, Anderson HC, Wang J. Nfatl regulates adult articular chondrocyte function through its age-dependent expression mediated by epigenetic histone methylation. J Bone Miner Res. 2011;26:1974–1986. doi: 10.1002/jbmr.397. [DOI] [PMC free article] [PubMed] [Google Scholar]