Abstract
Inflammatory Bowel Disease (IBD) is a chronic and frequently disabling inflammatory disorder of the intestine. New developments in IBD therapy are primarily focused on biologic treatments; however, they are both expensive and associated with significant side effects. Here, we provide the first preclinical evidence that YunNan BaiYao (YNBY), a well-known traditional Chinese herbal remedy frequently used for treating hemorrhages and wounds, can effectively alleviate experimental colitis. Oral administration of YNBY in drinking water significantly reduced the disease activities of both DSS- and TNBS-induced experimental colitis. Mechanistic studies revealed that the effectiveness of YNBY was not due to an anti-bacterial function since YNBY had no effect on E. coli growth. Rather, it exhibited an anti-inflammatory or immunosuppressive function: In the DSS colitis model, YNBY treatment decreased the levels of several pro-inflammatory cytokines in colonic mucosa, including TNFα, IL-12p40, and IL-17. Similar cytokine changes were also observed in mouse serum, suggesting that systemic changes in general reflect the changes in the affected colon. Significant down-regulation of IL-12p40 and IL-17, in addition to IFNγ, was also seen in TNBS-colitis model. Another potential mechanism for the anti-inflammatory effects of YNBY involves the selective suppression of pro-inflammatory immune cells: YNBY effectively suppressed the growth of multiple T- and B-lymphocytes, including Molt-4, Jurkat, and EBV-transformed human B-lymphocytes, more potently than 6-mecaptopurine (6-MP) and 5-aminosalicylic acid (5-ASA), two of the most commonly used first-line drugs in IBD therapy. In sharp contrast, YNBY exhibited no cytotoxicity to colonic epithelial cells (Caco-2 cells), even at the concentration 10-fold higher than that used in the lymphocyte model; and instead promoted cell spreading and wound healing. These results strongly suggest that YNBY not only has effective anti-inflammatory properties through suppressing lymphocyte growth and pro-inflammatory cytokine expression, but also can promote intestinal epithelial wound-healing and repair. Therefore, YNBY demonstrates strong potential as an alternative herbal therapy for IBD.
Keywords: IBD, Crohn's disease, herbal medicine, complementary and alternative medicine (CAM), YunNan BaoYao (YNBY), DSS-colitis, TNBS-colitis, cytokines, wound healing, immunesuppression
Introduction
Inflammatory bowel disease (IBD) is an idio-pathic, chronic and frequently disabling inflammatory disorder of the intestine, characterized by the infiltration of pro-inflammatory cells into the intestinal mucosa [1-3]. IBD is generally classified into two subtypes: Crohn's disease (CD) and ulcerative colitis (UC) [1, 2, 4]. In the United States alone, there are more than one-million diagnosed IBD patients [5], resulting in enormous suffering and health-care costs. The precise etiology of IBD is unknown, but it is increasingly clear that IBD is a complex multifac-torial disease with both genetic and environmental contributions [1, 3, 4].
Although there is no cure for IBD, a host of current therapeutics reduce the chronic inflammation in the intestinal mucosa, including aminosalicylates, corticosteroids, thiopurines, methotrexate, cyclosporine, and biologics such as anti-TNFα therapies [6-8]. The specific therapy or combination of therapies prescribed for a patient depends on disease severity and activity, anatomical location, and the nature and extent of inflammation [8, 9]. Despite increasing therapeutic options available for disease management, current IBD therapies have significant limitations with regards to safety, efficacy, and applicability [6, 10, 11]. First, only approximately 60% of IBD patients respond to a particular IBD therapy currently on the market, as IBD manifests itself in a heterogenic myriad of forms that can vary greatly from patient to patient [12, 13]. Secondly, the majority of current IBD therapies are associated with severe side effects - sometimes rendering the treatment more deleterious than the disease itself. For instance, thiopurine drugs have been associated in subpopulations of patients with nausea, bone-marrow suppression, hepatitis, allergic reactions, infections, and possibly malignancy [14, 15]. Anti-TNF-α treatments have been demonstrated to be associated with fatal blood disorders, infections, liver injury, drug-induced lupus, demyelinating central nervous system disorders, allergic reactions, and lymphoma and other tissue cancers [10, 11, 13, 16]. Finally, the expenses of IBD therapy are exorbitantly high, particularly that of biologic therapies. For example, anti-TNF-α biologic treatments ranged typically from $3,900 to $6,000 per year for episodic therapy, accumulating up to $36,000 per year for systemic therapy [17]. Caps on most medical insurance plans permit coverage for an only a subset of treatments per year, conferring the rest of the payment to the patients themselves.
Complementary and alternative medicine (CAM) modalities have been categorized into several classes, including herbal/nutritional, mind-body, body-based, and energetic [18]. Once considered an ancient remedy exclusively used in the “old world", CAM is a rapidly growing area of public interest in the Western countries [19]. In the United States alone, out-of-pocket yearly expenses on CAM were estimated to exceed $34 billion in 2001 [20, 21] and growing. Driven by increasing public interest, health care practitioners and the medical research community are also demonstrating great interest in CAM, in search of not only more natural resources for patient care, but also elucidating its biology to justify its use in clinical applications, or as a health-management alternative to improve wellness [19]. Herbal medicine, constituting a major part of CAM [22], has attracted particular attention with its hypothesized ability to modulate the immune system, for management and prevention of various autoimmune diseases [23]. A total of 11,146 different species of medicinal plants have been recorded in CAM, spanning 383 families and 2309 genera [24]. Most importantly, herbal therapeutics have traditionally been associated with minimal side effects, as they usually are purely composed of natural components [25-27].
Despite the great potential of these natural remedies, there have been significant shortcomings in herbal therapeutic research, including inconsistent criteria in clinical application, insufficient preclinical studies using animal models, ambiguous and often secretive bioactive ingredients, and unclear pharmacological targets [28, 29]. As a result, a large portion of available clinical data involving herbal medicines is questionable, and their molecular mechanisms remain largely unknown, thereby significantly limiting the clinical applications of CAM as medical alternatives. Quite similarly, in terms of CAM with regards to IBD, although widely used by patients worldwide, there are to date limited high quality research work at the levels of both basic science and clinical application [30].
YunNan BaiYao (YNBY), also called Yunnan Paiyao in the past, formulated in either powder or capsule forms, is an amalgamation of various herbs and plants derived from southern China [31]. It is considered “a must for first aid” lowcost therapy in China for the treatment of both internal and external hemorrhage and open and closed wounds due to its well-known antihemorrhagic haemostatic functions [32-34]. From a historical perspective, the Western world was introduced to YNBY when the United States military discovered North Vietnamese soldiers carrying the drug for life-saving treatment of wounds or bleeding in the Vietnam War [31]. However, like many herbal medicines, the exact ingredients of YNBY are not known, and protected as one of the major intellectual properties in medicine by the Chinese government. Although there is a significant body of literature (both preclinical and clinical study) on YNBY, most of the studies were published in non-English language journals that were not evaluated by Thomson ISI, making it difficult to judge their clinical or scientific merit. Here we report for the first time that YNBY can effectively reduce the severity of murine experimental colitis by both immune-suppressing and wound-healing mechanisms.
Materials and methods
Experimental animals
Male and female C57BL/6 and BALB/c mice (6-8 wk, 18-22 g) (Jackson Laboratories, Bar Harbor, ME) were group-housed at the Johns Hopkins Animal Facility under controlled temperatures (25°C) and photoperiods (12:12-hour light-dark cycle), and allowed unrestricted access to a standard diet and tap water. For each group of experiments, mice were matched by age, sex, and body weight. Care and experimentation of mice were performed in accordance with institutional guidelines under protocols approved by the Institutional Animal Care and Use Committee at Johns Hopkins University.
Cell lines
Human T-lymphocytes, Molt-4 and Jurkat cells were obtained from ATCC (Rockville, MD). EBV-transformed human lymphocytes (B-lymphocytes) were generated from a patient with Crohn's disease at Johns Hopkins Tissue Culture Facility, under IRB protocols approved by at the Johns Hopkins University. Caco-2/BBE epithelial cells were described previously [35].
Antibodies
Primary antibodies used include goat anti-TNFα and anti-IL-17 (Santa Cruz Biotechnology, Inc, Santa Cruz, CA), rabbit anti-IFNγ and anti-IL-12/ p40 (Biosource Intl, Camarillo, CA), and rabbit anti-actin (Sigma-Aldrich, St. Louis, MO). Fluo-rescently labeled secondary IRDye antibodies were purchased from Rockland Immunochemicals, Inc (Gilbertsville, PA).
SDS-PAGE and western blot analysis
Protein extraction and western blots were performed as previously described [36]. Briefly, total colonic mucosal proteins were extracted, separated by SDS-PAGE, and transferred onto nitrocellulose membrane. SDS-PAGE (using 10% or 12.5 % SDS polyacrylamide gels) and protein transfer were performed using Bio-Rad systems (Bio-Rad, Hercules, CA). Western blot analyses were performed using the Odyssey Infrared Imaging system (LI-COR, Lincoln, NE) as we described previously [36, 37].
Induction and evaluation of experimental murine colitis
DSS- and TNBS-colitis models were established in C57BL/6 and BALB/c mice, respectively, and disease scores (disease activity index) were evaluated as we previously described in details [36].
Treatment of mice with DSS- and TNBS-colitis using YNBY
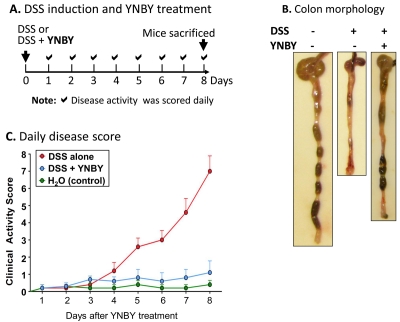
In the treatment of both DSS- and TNBS colitis mice, YNBY was dissolved in drinking water at a final concentration of 1.5 mg/ml. For DSS-colitis, YNBY was administered concurrently with DSS at day 1 and disease activity was obtained daily (Figure 1A). For TNBS-colitis mice, YNBY was supplied after TNBS induction and disease scores were calculated at day 8 (Figure 2A). For each colitis model, one control group was induced with colitis without YNBY administration, and another was neither induced with colitis nor treated with YNBY. All mice were sacrificed at day 8, and the colon was isolated and cleaned for histology and for mucosa protein isolation and analyses by SDS-PAGE and Western blot, as we previously described [36, 37].
Figure 1.
YNBY greatly improves the disease activity of DSS- induced UC-like colitis. A. A schematic illustration of experimental design: C57BL/6 mice (6-8 weeks old) were given drinking water containing either DSS alone or DSS and YNBY (1.5 mg/ml) at day 0. Clinical activity was scored daily. Mice were sacrificed at day 8 for tissue collection. Mice that received neither DSS nor YNBY were used as negative controls. B. A representative view of the colon morphology at the end of the YNBY treatment. Without YNBY, DSS induced severe colitis with bloody stools (or no stool due to diarrhea) and colon-shortening. Both symptoms were drastically improved by YNBY treatment, and the colon exhibited some well-formed stool pellets and no visible blood. C. Disease activity score of controls (green), DSS alone (red), and DSS +YNBY (blue) mice. At least 10 mice were used in each group.
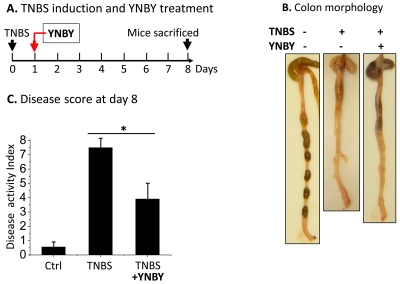
Figure 2.
YNBY effectively suppress TNBS-induced colitis. A. A schematic diagram to illustrate the timing of each treatment. BALB/c mice (6-8 weeks old) were induced for colitis using TNBS (0.5 mg in 0.1 ml 50% ethanol). Control (Ctrl) mice received 50% ethanol. Herbal formula YNBY (1.5 mg/ml) was given to the treatment group in drinking water 1 day following TNBS treatment. Disease activities were scored on day 8, immediately prior to sacrifice. B. A representative view of the colon morphology at the end of the YNBY treatment. TNBS induced severe colitis with bloody stools, and muco-sal thickening and shortening, all of which were markedly improved by YNBY treatment. No blood was visible in the colon. C. Disease activity assessment at day 8. Statistical analysis was done using Origin software (*P< 0.01). At least 12 mice were analyzed in each group.
Determination of anti-bacterial effect of YNBY
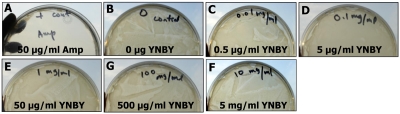
E. coli K12, a commensal bacterium in the mammalian gut, was used as a model bacterium in this study. E. coli K12 (50 μl; OD600=0.6) was plated evenly on LB agar plates, and incubated overnight in the absence (negative control) or presence of various concentrations of YNBY (0.0, 0.5, 5.0, 50, 500, and 5,000 μg/ml; final concentration). Ampicillin (50 μg/ml) was used as a positive control. Antibiotic activity was judged by the growth of the bacteria on the LB plates (the presence of bacterial colonies or lawns). At least four independent experiments were performed.
Assessment of killing of lymphocytes-killing or suppressing by YNBY
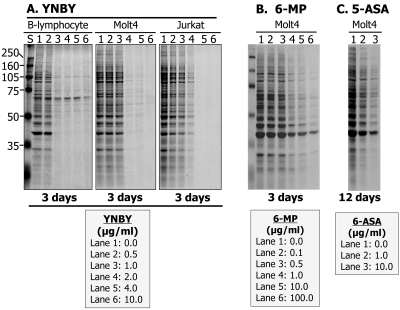
Three different lymphocyte cell lines were tested: EBV-immortalized B-lymphocytes and two T-lymphocytes (Molt-4 and Jurkat cells). Equal numbers of cells (1×106) were incubated for 72 hours with various concentrations of YNBY (0, 0.5, 1.0, 2.0, 4.0, and 10.0 μg/ml). Lymphocytes were also incubated with various concentrations of 6-mercaptopurine (6-MP) (0, 0.1, 0.5, 1.0, 10.0, 100.0 μg/ml) and 5-aminosalicylic acid (5-ASA) (0, 1.0, 10 μg/ml), two of the most widely used first-line drugs in current IBD therapy [6], for comparative purposes. Cells were washed three times with PBS buffer, and total lysates of each cell preparation were extracted in equal volumes of lysis buffer. Equal volumes of each lysate (∼50 μg proteins in the control cells) were separated by SDS-PAGE and transferred onto nitrocellulose membrane. Total proteins were stained with Ponceau S for visualization. Between the treatment groups, fewer proteins and lower staining intensity reflect a decrease in cell numbers. At least three independent experiments were performed for each treatment.
Effects of YNBY on the growth of colonic epithelial cells
To examine the specificity of YNBY's cytotoxic properties, cytotoxicity assays were preformed on the colonic epithelial cell line Caco-2/ BBE, one of the most commonly used intestinal epithelial cell models. Caco-2/ BBE cells were plated at 50% confluence in 6-well plates, and incubated with various concentrations of YNBY (0, 0.5, 1.0, 2.5, 5.0, 10.0 μg/ml) for 96 hours. Cells were harvested and total cellular proteins were separated by SDS-PAGE. Proteins were electrically transferred to nitrocellulose membrane, and visualized by Ponceau-S staining.
Wound healing assays
YNBY was further tested for its ability to promote intestinal mucosal healing - another potential independent mechanism for its therapeutic effects. Caco-2/BBE cells were incubated on 6-well plates in the presence of 1 μg/mL YNBY (with no YNBY as control). The cells were allowed to form a monolayer (3 days post-confluence). The wound healing assay was performed as described by Seltana et al, 2009 [38]. Briefly, wounds (scratches) were then gently created in the monolayer using a 200 μl pipette tip (∼0.6 mm in diameter), and subsequent wound healing (restitution of the denuded epithelial cell areas) was monitored and photographed every 12 hours for 72 hours using a Zeiss Axio Inverted Microscope (Zeiss, Thorn-wood, NY) equipped with an Olympus DP72 Digital Camera (Olympus America Inc., Center Valley, PA). The percentage of wound healing was calculated using the MetaMorph imaging system (Molecular Devices, Sunnyvale, CA) as previously described [38].
Analysis of major pro-inflammatory cytokines in the colon or serum
Colonic mucosa was isolated from DSS- and TNBS-colitis mice treated or not with YNBY, and total mucosal proteins were extracted for cytokine expression analyses by SDS-PAGE and Western blot, as described previously [36]. The protein levels of four major pro-inflammatory cytokines, TNFα, IFNγ, IL-12/p40, and IL-17, were analyzed using corresponding antibodies described in Antibody section above. Actin was used as a loading control.
Serum level cytokines were measured by multiplex ELISA as we previously described [36] (See details in Supplement Data).
Results
YNBY significantly reduced disease activity of DSS- and TNBS-colitis mice
YNBY exhibited potent inhibition of both DSS-induced (Figure 1) and TNBS-induced (Figure 2) colitis. DSS alone induced severe colitis in control mice, with a time-dependent increase of clinical disease activity, including bloody stools, diarrhea (resulting in little stool left in the colon), colon-shortening, and loss of body weight [36]. YNBY treatment resulted in drastically decreased disease activity, and healthier colon appearance (more stool and less shortening) (Figure 1B and 1C). By day 8, average disease activity index for YNBY-treated DSS-colitis mice maintained at ∼1.0, comparable to that for the healthy control mice without DSS induction (0.5) (Figure 1C).
Similar beneficial effects of YNBY were also observed in the TNBS-colitis model (Figure 2), although less potently compared to the DSS model. YNBY treatment resulted in ∼50% reduction of disease activity in TNBS colitis (Figure 2C). These data provided the impetus to search for a mechanistic basis of YNBY therapy in murine models of colitis.
YNBY is not anti-bacterial
Antibiotics have been known to be therapeutic in IBD due to the involvement of gut commensal bacteria in IBD pathogenesis [1, 6, 12, 39, 40]. Since topical application of YNBY alone (without any antibiotic) is known to be effective in controlling infections from open wounds and hemorrhages, it was conceivable that YNBY may possess antibiotic properties that account for its therapeutic efficacy in the murine colitis we observed. Therefore, we first tested a potential anti-bacterial mechanism for YNBY. As shown in Figure 3, when equal numbers of E. coli K12 bacteria were incubated with either ampicillin (A; positive control), or various concentrations (B-F) of YNBY, there was no bacterial growth in the positive control, (Figure 3A, as indicated by translucence of the LB plate). On the other hand, bacteria lawns were formed in all plates containing YNBY, irrespective of YNBY concentration (Figure 3B-F), even at a concentration of 5 mg/ml, which is >1,000-fold higher than the concentration that potently suppresses lymphocytes (see Figure 4). This suggests that YNBY has no anti-bacterial function, and that the therapeutic effect of YNBY on DSS- and TNBS-colitis is not due to its influence on commensal bacteria.
Figure 3.
YNBY does not have anti-bacterial capabilities against E. coli K12. 50 μL of E. coli K12 culture (OD=0.6) was incubated on plates with various concentrations of YNBY (B-F) overnight, at 37°C on LB agar plates. E. coli was also incubated with 50 μg/ml of antibiotic ampicillin (Amp) as a positive control (A), in which no bacteria grew (indicated by translucence). In contrast, bacterial lawns formed at all doses of YNBY (B-F). The hand-written indication at the top of each plate denotes the concentrations of the original YNBY stocks for our reference during the experiments. This suggests that YNBY is not anti-bacterial. At least five independent experiments were performed.
Figure 4.
YNBY exhibits dose-dependent cytotoxic-ity to both T- and B- lymphocytes, more potently than 6-mercaptopurine (6-MP) and 5-aminosalicylic acid (5-ASA), two of the most commonly used IBD medications. Total protein expression in cell lysates was used as an indicator of change in cell numbers, as analyzed bySDS-PAGE. A. As the concentration of YNBY treatment increased, the total proteins reflected on the gel decreased dramatically for both B-cells and T-cells (Molt4 and Jurkat), indicating a drastic decrease in cell numbers. B and C. Cytotoxicity of 6-MP (B) or 5 -ASA (C) to Molt4 cells was shown for comparison purpose with YNBY (A). These studies demonstrate that YNBY exhibited more cytoxicity to lymphocytes than 6-MP and 5-ASA. At least four independent experiments were performed.
YNBY exhibits dose-dependent suppression of lymphocytes
Given that the purported anti-bacterial mechanism was not responsible for the efficacy of YNBY in treating murine colitis, we hypothesized that YNBY may exert an anti-inflammatory function through potential cytoxicity to lymphocytes, a bioactive property we observed in IBD drug 6-MP (azathioprine). As shown in Figure 4A, YNBY exhibited potent dose-dependent suppressive effects on all three lines of lymphocytes. As the concentration of YNBY increased, total proteins decreased (first gradually up to 1.0 μg/ml, then dramatically afterward). At YNBY concentrations of 2 μg/ml, essentially all lymphocytes tested were suppressed, as indicated by the lack of visible proteins. Surprisingly, YNBY suppressed Molt4 cells more potently than the current IBD drugs 6-MP (Figure 4B) and 5-ASA (Figure 4C). 5 -ASA was far less potent than either YNBY or 6-MP, as it took as it took 4 times longer than YNBY and 6-MP (12 days instead of 3 days) to achieve similar cell-suppressing effects (Figure 4, compare C with A and B). These data suggest that YNBY exhibits dose-dependent cytotoxicity to lymphocytes, more potently than current IBD drugs 6-MP or 5-ASA.
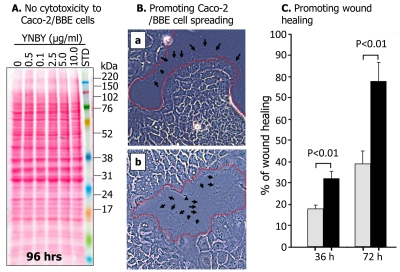
YNBY exhibits no cytotoxicity to intestinal epithelial cells
Although YNBY exhibited potent lymphocyte suppression, its viability as an IBD drug is heavily contingent upon its nontoxicity to noninflammatory cells, such as those in the intestinal epithelium. As shown in Figure 5A, YNBY has no influence on Caco-2/BBE cell growth, as evidenced by the constant total cellular proteins at every concentration of YNBY. In comparison to Figure 4, in which YNBY dose-dependently suppressed lymphocytes, little or no change in total protein concentration was observed in Caco-2/ BBE cells, even with longer incubation times (96 vs 72 hours) and higher concentrations of YNBY (10 μg/ml). Thus, the lack of intestinal epithelial cell suppression suggests that YNBY's cytotoxic effects are specific to lymphocytes.
Figure 5.
YNBY shows no cytotoxicity to Caco-2/BBE cells and promotes cell spreading. A. Caco-2/BBE cells were plated at 50% confluence in 6-well plates and incubated with various concentrations of YNBYfor 72 hrs. Cells were harvested and total proteins were analyzed by SDS-PAGE and visualized by Ponceau S staining. No change in protein profiles suggests no cytotoxicity of the drug to intestinal epithelial cells. B. YNBY promotes formation of lamellipodia and wound healing of Caco-2/BBE cells. Compared with the control (B-a; no YNBY), wounds on the cell layer closed more rapidly in cells incubated with YNBY at 1 Mg/ml (B-b). Cells incubated with YNBY exhibited larger and more extensive lamellipodia (edges marked by arrows) protruding from the wounded edges (marked by red dotted lines) compared to these of untreated cells (B-a). Cells images were taken with an Olympus DP72 Digital Camera on a Zeiss Axio Inverted Microscope. C. The rate of wound healing is almost doubled in Caco-/BBE cells treated with YNBY when compared to those untreated. At least five independent experiments were performed.
YNBY promotes promotes wound healing through cell spreading
Not only did YNBY exhibit no cytotoxicity to Caco -2/BBE cells, the drug enhanced wound healing (faster closing of wounds) by promoting formation of lamellipodia (indicated by arrows) and cell spreading and growth, as shown in Figure 5B. Specifically, in cells treated with YNBY (Figure 5B-b), larger and more extensive lamellipodia (edges indicated by black arrows) are visibly protruding from the wounded edges (compared to those of the untreated cells, Figure 5B-a). More extensive lamellipodia protrusions signify more rapid cell spreading across the wound compared to non-treated control cells. We therefore performed wound-healing assays. As shown in Figure 5C, in the presence of YNBY the wounds of Caco-2/BBE cell monolayer were closed much faster (approximately 2-fold) than on those with no YNBY.
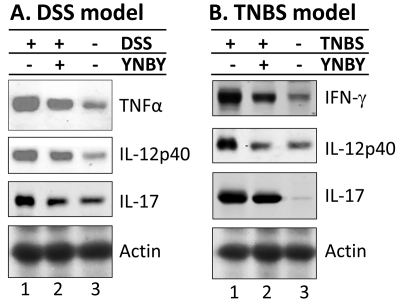
YNBY effectively suppresses proinflammatory cytokines in colon of experimental colitis mice
Since cytokines play an essential role in the pathogenesis of both human IBD and experimental colitis [36, 41, 42], we hypothesized that the therapeutic effect of YNBY may be partially attributed to its ability to suppress the expression of pro-inflammatory cytokines developed during the induction of colitis. To test this hypothesis, we analyzed, by SDS-PAGE and Western blot, the colonic expression of multiple proinflammatory cytokines that are known to be involved in the pathogenesis of IBD, including TNFα, IFNγ, IL-17, and IL-12p40. Consistent with our previous report [36], DSS induced a dramatic elevation of TNFα, IL -17, and IL-12p40 in the colonic mucosa compared to control (Figure 6A; lane 1 vs lane 3). YNBY effectively reduced the expression of all three pro-inflammatory cytokines, and IL-17 levels were essentially reduced to the control levels (Figure 6A; comparing lanes 1, 2 and 3). Interestingly, systemic (serum) levels of these cytokines (TNFα, IFNγ, IL-17, and IL-12) were similarly down-regulated after YNBY treatment (see Supplement Data), indicating that systemic levels of cytokines generally correlate well with those in the colonic mucosa where disease occurs.
Figure 6.
YNBY significantly decreases the TNFα and IL-12p40 in mice with DSS-induced colitis. Colonic mucosa was isolated from DSS-induced (A) or TNBS-induced (B) mice. Total mucosal proteins were extracted and analyzed with SDS-PAGE Western-blots using primary antibodies for TNFα, IFNγ, IL-17, IL-12p40, and actin (as a protein loading control). While DSS- and TNBS-colitis both had significantly higher IL-12p40 and IL-17 expression in the colon with additional elevations of TNFα in DSS-colitis and IFNγ in TNBS-colitis, YNBY treatment significantly decreased all four proinflammatory cytokines examined. At least 3 mice were analyzed in each group.
Similarly, in the TNBS model, YNBY also greatly suppressed the expression of IFNγ, IL-17, and IL -12p40, which were heavily upregulated by TNBS compared to controls (Figure 6B; comparing lanes 1, 2, and 3). Thus, YNBY treatment of both DSS- or TNBS-induced mice effectively decreased the colonic expression of all four proinflammatory cytokines examined, suggesting another potential therapeutic mechanism for YNBY.
Discussions
We present the first preclinical evidence that YNBY has potent suppressive functions in murine models of experimental colitis, modulated by several independent molecular and cellular mechanisms of action. Our data suggest at least three distinct yet functionally relevant mechanisms that could potentially contribute to the therapeutic effects of YNBY on colitis.
First, YNBY exhibits cytotoxicity to both B- and T - lymphocytes in a dose-dependent manner, with potency greater than the well-established IBD drugs 6-MP and 5-ASA. Thus, potent cytoxicity to lymphocytes suggest a potential mechanism of 6-MP functioning as an immunomodulator in IBD therapy. The cytotoxicity of YNBY is highly selective toward lymphocytes, exhibiting no detectable toxic effect on epithelial cells, including intestinal epithelial cells (Caco-2; Figure 5) and kidney proximal tubule epithelial cells (OK-cells; data not shown). Thus, our data suggest a strong and specific immunomodulatory function of YNBY that may be potentially exploited for treatment of not only IBD, but also other chronic inflammatory conditions.
Second, we demonstrated that YNBY could effectively promote cell spreading and wound healing of Caco-2/BBE cells, as evidenced by more extensive lamellipodia at the leading edges of the wounds and faster wound closing compared to the control. Inflammatory bowel disease is commonly characterized by mucosal damages such as lesions and ulcers, with alternating periods of remission and exacerbation [1, 2]. Thus mucosal healing is one of the most significant parameters that defines and/or predicts clinical remission, and remains as an important goal of IBD management [43-45]. This unique functional effect of YNBY on intestinal epithelial cells suggests that YNBY could accelerate mucosal healing, thus shortening exacerbation periods and prolonging remission. It is necessary to note that a recent study identified unique nanofibers in YNBY [46], leading to the speculation that these nanofibers may play an important role in blood-clotting (cessation of bleeding) and wound-sealing, the best-known effects of YNBY. Therefore, further investigation into the bio-properties of these nanofibers could provide insight into the wound-healing mechanism of YNBY in IBD therapy.
Finally, the immunomodulatory properties of YNBY were demonstrated in vivo in colonic mucosa of DSS- and TNBS-colitis mice. Administration of YNBY effectively suppressed the expression of four major pro-inflammatory cytokines: TNFα, IFNγ, IL-17, and IL-12p40 (Figure 5). These cytokines are among the most studied and characterized cytokines in IBD and have been shown in both animal models and humans to play a major role in IBD pathogenesis [2, 42, 47]. They are also among the most targeted proinflammatory cytokines in the development of biologic therapies for IBD [48, 49]. Currently, the most effective and extensively evaluated biologic drugs in treatment of IBD are TNFα-neutralizing agents, including infliximab, adalinumab and cetolizumab pegol [48, 50]. Furthermore, Ustekinumab, a humanized monoclonal IgG1 antibody against IL-12/p40 subunit, which is shared by both IL-12 and IL-23 signaling, simultaneously targets two major disease-causing inflammatory pathways, Th1 and Th17 [51]. Therefore, YNBY's ability to inhibit major pro-inflammatory cytokines, including TNF-α and IL-12/p40, provides another mechanistic explanation for its anti-inflammatory function in treating experimental murine colitis, and further suggests its significant therapeutic potential in the safe and effective management of IBD.
In summary, we present both in vitro and in vivo evidence to demonstrate that herbal medicine YNBY has promising potential for future development into an IBD treatment. The therapeutic effects of YNBY can be attributed to its “triple action", including 1) selective cytotoxicity toward lymphocytes, 2) promotion of wound healing in of colonic epithelial cells, and 3) immunomodulation through effective inhibition of pro-inflammatory cytokines. This synergistic immunomodulatory and wound-healing effects render YNBY more unique than any other known drug in IBD therapy.
Acknowledgments
The authors were primarily supported by the Student Fellowship Award Grant from the American Gastroenterological Association (AGA), Broad Medical Research Program (IBD-0119R), NIH-NIDDK R21 DK077064, NIH Ruth L Kirschstein National Research Service Award, and in part by NIH/NIDDK P30DK089502. R Li would also like to thank Ms. Barbara Jewett for her unconditional support and guidance.
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Strober W, Fuss I, Mannon P. The fundamental basis of inflammatory bowel disease. J Clin Invest. 2007;117:514–521. doi: 10.1172/JCI30587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Packey CD, Sartor RB. Interplay of commensal and pathogenic bacteria, genetic mutations, and immunoregulatory defects in the pathogenesis of inflammatory bowel diseases. J Intern Med. 2008;263:597–606. doi: 10.1111/j.1365-2796.2008.01962.x. [DOI] [PubMed] [Google Scholar]
- 4.Sartor RB. Mechanisms of disease: pathogenesis of Crohn's disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407. doi: 10.1038/ncpgasthep0528. [DOI] [PubMed] [Google Scholar]
- 5.Kappelman MD, Rifas Shiman SL, Kleinman K, Ollendorf D, Bousvaros A, Grand RJ, Finkelstein JA. The prevalence and geographic distribution of Crohn's disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol. 2007;5:1424–1429. doi: 10.1016/j.cgh.2007.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Baumgart DC, Sandborn WJ. Inflammatory bowel disease: clinical aspects and established and evolving therapies. Lancet. 2007;369:1641–1657. doi: 10.1016/S0140-6736(07)60751-X. [DOI] [PubMed] [Google Scholar]
- 7.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho GT, Satsangi J, Bloom S. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 8.Plevy SE, Targan SR. Future therapeutic approaches for inflammatory bowel diseases. Gastroenterology. 2011;140:1838–1846. doi: 10.1053/j.gastro.2011.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Diamond RH, Wagner CL, Fasanmade AA, Olson AD, Marano CW, Johanns J, Lang Y, Sandborn WJ. Clinical trial: benefits and risks of immunomodulators and maintenance infliximab for IBD-subgroup analyses across four randomized trials. Aliment Pharmacol Ther. 2009;30:210–226. doi: 10.1111/j.1365-2036.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 10.Siegel CA, Hur C, Korzenik JR, Gazelle GS, Sands BE. Risks and benefits of infliximab for the treatment of Crohn's disease. Clin Gastroenterol Hepatol. 2006;4:1017–1024. doi: 10.1016/j.cgh.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Blonski W, Lichtenstein GR. Safety of biologic therapy. Inflamm Bowel Dis. 2007;13:769–796. doi: 10.1002/ibd.20027. [DOI] [PubMed] [Google Scholar]
- 12.Sands BE. Therapy of inflammatory bowel disease. Gastroenterology. 2000;118:S68–S82. doi: 10.1016/s0016-5085(00)70007-2. [DOI] [PubMed] [Google Scholar]
- 13.Sands BE. New therapies for the treatment of inflammatory bowel disease. Surg Clin North Am. 2006;86:1045–1064. doi: 10.1016/j.suc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 14.Fraser AG, Orchard TR, Jewell DP. The efficacy of azathioprine for the treatment of inflammatory bowel disease: a 30 year review. Gut. 2002;50:485–489. doi: 10.1136/gut.50.4.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fraser AG, Orchard TR, Robinson EM, Jewell DP. Long-term risk of malignancy after treatment of inflammatory bowel disease with azathioprine. Aliment Pharmacol Ther. 2002;16:1225–1232. doi: 10.1046/j.1365-2036.2002.01297.x. [DOI] [PubMed] [Google Scholar]
- 16.Caviglia R, Boskoski I, Cicala M. Long-term treatment with infliximab in inflammatory bowel disease: safety and tolerability issues. Expert Opin Drug Saf. 2008;7:617–632. doi: 10.1517/14740338.7.5.617. [DOI] [PubMed] [Google Scholar]
- 17.Rutgeerts P, Van AG, Vermeire S. Optimizing anti-TNF treatment in inflammatory bowel disease. Gastroenterology. 2004;126:1593–1610. doi: 10.1053/j.gastro.2004.02.070. [DOI] [PubMed] [Google Scholar]
- 18.Wieland LS, Manheimer E, Berman BM. Development and classification of an operational definition of complementary and alternative medicine for the Cochrane collaboration. Altern Ther Health Med. 2011;17:50–59. [PMC free article] [PubMed] [Google Scholar]
- 19.Ruggie M. Mainstreaming complementary therapies: new directions in health care. Health Aff (Millwood) 2005;24:980–990. doi: 10.1377/hlthaff.24.4.980. [DOI] [PubMed] [Google Scholar]
- 20.Herman PM, Craig BM, Caspi O. Is complementary and alternative medicine (CAM) cost-effective? A systematic review. BMC Complement Altern Med. 2005;5:11. doi: 10.1186/1472-6882-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan AH, Wilson DH, Taylor AW. The escalating cost and prevalence of alternative medicine. Prev Med. 2002;35:166–173. doi: 10.1006/pmed.2002.1057. [DOI] [PubMed] [Google Scholar]
- 22.Lu AP, Jia HW, Xiao C, Lu QP. Theory of traditional Chinese medicine and therapeutic method of diseases. World J Gastroenterol. 2004;10:1854–1856. doi: 10.3748/wjg.v10.i13.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu F, Takahashi T, Moriya J, Kawaura K, Yamakawa J, Kusaka K, Itoh T, Morimoto S, Yamaguchi N, Kanda T. Traditional Chinese medicine and Kampo: a review from the distant past for the future. J Int Med Res. 2006;34:231–239. doi: 10.1177/147323000603400301. [DOI] [PubMed] [Google Scholar]
- 24.Li WF, Jiang JG, Chen J. Chinese medicine and its modernization demands. Arch Med Res. 2008;39:246–251. doi: 10.1016/j.arcmed.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 25.Debas HT, Laxminarayan R, Straus SE. Complementary and Alternative Medicine. 2006 [PubMed] [Google Scholar]
- 26.Khanna D, Sethi G, Ahn KS, Pandey MK, Kunnumakkara AB, Sung B, Aggarwal A, Aggarwal BB. Natural products as a gold mine for arthritis treatment. Curr Opin Pharmacol. 2007;7:344–351. doi: 10.1016/j.coph.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 27.Carroll RJ. Complementry and Alternative Medicine- History, Definitions, and What Is It Today? 2007:7–43. [Google Scholar]
- 28.Power M, Hopayian K. Exposing the evidence gap for complementary and alternative medicine to be integrated into science-based medicine. J R Soc Med. 2011;104:155–161. doi: 10.1258/jrsm.2011.100271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long AF. Outcome measurement in complementary and alternative medicine: unpicking the effects. J Altern Complement Med. 2002;8:777–786. doi: 10.1089/10755530260511793. [DOI] [PubMed] [Google Scholar]
- 30.Joos S. Review on efficacy and health services research studies of complementary and alternative medicine in inflammatory bowel disease. Chin J Integr Med. 2011;17:403–409. doi: 10.1007/s11655-011-0758-3. [DOI] [PubMed] [Google Scholar]
- 31. Robinson NG. Yunnan Paiyao. Http://CsuvetsColostateEdu/Pain/Articlespdf/YunnanPaiyao111206Pdf2007; 1-4.
- 32.Bergner P. Panax notoginseng Yunnan bai yao: A must for the first aid kit. Medical Herbalism. 1994;6:12. [Google Scholar]
- 33.Fan C, Song J, White CM. A comparision of the hemostatic effects of notoginseng and yun nan bai yao to placebo control. J Herb Pharmacother. 2005;5:1–5. [PubMed] [Google Scholar]
- 34.Tang ZL, Wang X, Yi B, Li ZL, Liang C, Wang XX. Effects of the preoperative administration of Yunnan Baiyao capsules on intraoperative blood loss in bimaxillary orthognathic surgery: a prospective, randomized, double-blind, placebo -controlled study. Int J Oral Maxillofac Surg. 2009;38:261–266. doi: 10.1016/j.ijom.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 35.Sarker R, Valkhoff VE, Zachos NC, Lin R, Cha B, Chen TE, Guggino S, Zizak M, de JH, Hogema B, Donowitz M. NHERF1 and NHERF2 are necessary for multiple but usually separate aspects of basal and acute regulation of NHE3 activity. Am J Physiol Cell Physiol. 2011;300:C771–C782. doi: 10.1152/ajpcell.00119.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alex P, Zachos NC, Nguyen T, Gonzales L, Chen TE, Conklin LS, Centola M, Li X. Distinct cytokine patterns identified from multiplex profiles of murine DSS and TNBS-induced colitis. Inflamm Bowel Dis. 2009;15:341–352. doi: 10.1002/ibd.20753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alex P, Ye M, Zachos NZ, Sipes J, Nguyen T, Suhodrev M, Gonzales L, Arora Z, Zhang T, Centola M, Guggino SE, Li X. Clc-5 Knockout Mice Exhibit Novel Immunomodulatory Effects And Are More Susceptible to Dextran Sulphate Sodium Induced Colitis. J Immunol. 2010;184:3988–3996. doi: 10.4049/jimmunol.0901657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seltana A, Basora N, Beaulieu JF. Intestinal epithelial wound healing assay in an epithelialmesenchymal co-culture system. Wound Repair Regen. 2010;18:114–122. doi: 10.1111/j.1524-475X.2009.00554.x. [DOI] [PubMed] [Google Scholar]
- 39.Xavier R, Podolsky DK. Commensal flora: wolf in sheep's clothing. Gastroenterology. 2005;128:1122–1126. doi: 10.1053/j.gastro.2005.02.053. [DOI] [PubMed] [Google Scholar]
- 40.Chichlowski M, Hale LP. Bacterial-mucosal interactions in inflammatory bowel disease: an alliance gone bad. Am J Physiol Gastrointest Liver Physiol. 2008;295:G1139–G1149. doi: 10.1152/ajpgi.90516.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li X, Conklin L, Alex P. New serological biomarkers of inflammatory bowel disease. World J Gastroenterol. 2008;14:5115–5124. doi: 10.3748/wjg.14.5115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sanchez Munoz F, Dominguez Lopez A, Yamamoto-Furusho JK. Role of cytokines in inflammatory bowel disease. World J Gastroenterol. 2008;14:4280–4288. doi: 10.3748/wjg.14.4280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Flynn A, Kane S. Mucosal healing in Crohn's disease and ulcerative colitis: what does it tell us? Curr Opin Gastroenterol. 2011;27:342–345. doi: 10.1097/MOG.0b013e3283455c8f. [DOI] [PubMed] [Google Scholar]
- 44.Baert F, Moortgat L, Van AG, Caenepeel P, Vergauwe P, de VM, Stokkers P, Hommes D, Rutgeerts P, Vermeire S, D'Haens G. Mucosal healing predicts sustained clinical remission in patients with early-stage Crohn's disease. Gastroenterology. 2010;138:463–468. doi: 10.1053/j.gastro.2009.09.056. [DOI] [PubMed] [Google Scholar]
- 45.Pineton de CG, Peyrin Biroulet L, Lemann M, Colombel JF. Clinical implications of mucosal healing for the management of IBD. Nat Rev Gastroenterol Hepatol. 2010;7:15–29. doi: 10.1038/nrgastro.2009.203. [DOI] [PubMed] [Google Scholar]
- 46.Lenaghan SC, Xia L, Zhang M. Identification of nanofibers in the Chinese herbal medicine: Yunnan Baiyao. J Biomed Nanotechnol. 2009;5:472–476. doi: 10.1166/jbn.2009.1056. [DOI] [PubMed] [Google Scholar]
- 47.Strober W, Fuss IJ. Proinflammatory cytokines in the pathogenesis of inflammatory bowel diseases. Gastroenterology. 2011;140:1756–1767. doi: 10.1053/j.gastro.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Melmed GY, Targan SR. Future biologic targets for IBD: potentials and pitfalls. Nat Rev Gastroenterol Hepatol. 2010;7:110–117. doi: 10.1038/nrgastro.2009.218. [DOI] [PubMed] [Google Scholar]
- 49.De ND, Sarra M, Cupi ML, Pallone F, Monteleone G. Targeting IL-23 and Th17-cytokines in inflammatory bowel diseases. Curr Pharm Des. 2010;16:3656–3660. doi: 10.2174/138161210794079164. [DOI] [PubMed] [Google Scholar]
- 50.Sandborn WJ. Current directions in IBD therapy: what goals are feasible with biological modifiers? Gastroenterology. 2008;135:1442–1447. doi: 10.1053/j.gastro.2008.09.053. [DOI] [PubMed] [Google Scholar]
- 51.Benson JM, Sachs CW, Treacy G, Zhou H, Pendley CE, Brodmerkel CM, Shankar G, Mascelli MA. Therapeutic targeting of the IL-12/23 pathways: generation and characterization of ustekinumab. Nat Biotechnol. 2011;29:615–624. doi: 10.1038/nbt.1903. [DOI] [PubMed] [Google Scholar]