Abstract
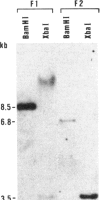
Two-cell and four-cell preimplantation mouse embryos were cocultured in vitro with fibroblasts producing the recombinant retrovirus M-MuLV-neo. No wildtype helper virus was detected in these cultures. Of the embryos that survived the in vitro cultivation and the reimplantation into foster mothers, 2 of 15 that were tested contained the proviral genome. The provirus integrated as a single copy at a unique site. We estimate that approximately equal to 20% of the cells in each of the two transgenic fetuses contained the provirus.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger S. A., Bernstein A. Characterization of a retrovirus shuttle vector capable of either proviral integration or extrachromosomal replication in mouse cells. Mol Cell Biol. 1985 Feb;5(2):305–312. doi: 10.1128/mcb.5.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Chen H. Y., Trumbauer M. E., Yagle M. K., Palmiter R. D. Factors affecting the efficiency of introducing foreign DNA into mice by microinjecting eggs. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4438–4442. doi: 10.1073/pnas.82.13.4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepko C. L., Roberts B. E., Mulligan R. C. Construction and applications of a highly transmissible murine retrovirus shuttle vector. Cell. 1984 Jul;37(3):1053–1062. doi: 10.1016/0092-8674(84)90440-9. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Fan H., Croker B. Infection of preimplantation mouse embryos and of newborn mice with leukemia virus: tissue distribution of viral DNA and RNA and leukemogenesis in the adult animal. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4008–4012. doi: 10.1073/pnas.72.10.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaenisch R., Jähner D., Nobis P., Simon I., Löhler J., Harbers K., Grotkopp D. Chromosomal position and activation of retroviral genomes inserted into the germ line of mice. Cell. 1981 May;24(2):519–529. doi: 10.1016/0092-8674(81)90343-3. [DOI] [PubMed] [Google Scholar]
- Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985 Jun 13;315(6020):594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- Karess R. E., Rubin G. M. Analysis of P transposable element functions in Drosophila. Cell. 1984 Aug;38(1):135–146. doi: 10.1016/0092-8674(84)90534-8. [DOI] [PubMed] [Google Scholar]
- Linney E., Davis B., Overhauser J., Chao E., Fan H. Non-function of a Moloney murine leukaemia virus regulatory sequence in F9 embryonal carcinoma cells. 1984 Mar 29-Apr 4Nature. 308(5958):470–472. doi: 10.1038/308470a0. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Ong E. S., Rosenfeld M. G., Verma I. M., Evans R. M. Infectious and selectable retrovirus containing an inducible rat growth hormone minigene. Science. 1984 Sep 7;225(4666):993–998. doi: 10.1126/science.6089340. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Yanagimachi R., Yanagimachi H. Ultrastructural localization of lectin-binding sites on the zonae pellucidae and plasma membranes of mammalian eggs. J Cell Biol. 1975 Aug;66(2):263–274. doi: 10.1083/jcb.66.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUNNER M. N., GATES A. Conception in prepuberal mice following artificially induced ovulation and mating. Nature. 1954 Jul 31;174(4422):222–223. doi: 10.1038/174222b0. [DOI] [PubMed] [Google Scholar]
- Rubenstein J. L., Nicolas J. F., Jacob F. Construction of a retrovirus capable of transducing and expressing genes in multipotential embryonic cells. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7137–7140. doi: 10.1073/pnas.81.22.7137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge J., Cutting A. E., Erdman V. D., Gautsch J. W. Integration-specific retrovirus expression in embryonal carcinoma cells. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6627–6631. doi: 10.1073/pnas.81.21.6627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Stuhlmann H., Cone R., Mulligan R. C., Jaenisch R. Introduction of a selectable gene into different animal tissue by a retrovirus recombinant vector. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7151–7155. doi: 10.1073/pnas.81.22.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo M., Gilboa E., Sherman M. I. Isolation of embryonal carcinoma cell lines that express integrated recombinant genes flanked by the Moloney murine leukemia virus long terminal repeat. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2422–2426. doi: 10.1073/pnas.82.8.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teich N. M., Weiss R. A., Martin G. R., Lowy D. R. Virus infection of murine teratocarcinoma stem cell lines. Cell. 1977 Dec;12(4):973–982. doi: 10.1016/0092-8674(77)90162-3. [DOI] [PubMed] [Google Scholar]
- Wagner E. F., Vanek M., Vennström B. Transfer of genes into embryonal carcinoma cells by retrovirus infection: efficient expression from an internal promoter. EMBO J. 1985 Mar;4(3):663–666. doi: 10.1002/j.1460-2075.1985.tb03680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]