Abstract
Background and Objectives
Breastfeeding, a public health priority, improves outcomes for infants. Methadone is dispensed as a racemic mixture; R-methadone is the active enantiomer. Pharmacologic data for R-methadone in breastmilk could improve risk–benefit decision-making for treatment of lactating women. This study estimated infant exposure to R- and S-methadone via breastmilk by theoretic infant dose (TID) and relative infant dose (RID) and reported the milk-to-maternal plasma (M/P) ratio.
Methods
Women treated with methadone doses of 40–200 mg/day (mean, 102 mg/day) provided concomitantly collected plasma and breastmilk samples 1–6 days after delivery. Most (16 of 20) samples were taken at the time of peak maternal plasma levels; thus infant exposure estimates are for maximum possible exposure. Concentrations of R- and S-methadone were measured in maternal plasma and breastmilk; M/P ratio, TID, and RID were calculated for each enantiomer and total methadone.
Results
The 20 participants were 18–38 years old and publicly insured; a quarter did not complete high school, and only one was not white. R-Methadone concentration was 1.3–3.0 times that of S-methadone in all breastmilk samples. The mean (SD) R-, S-, and total methadone M/P ratios were 0.52 (0.28), 0.28 (0.15), and 0.40 (0.21), respectively. Mean (range) R-, S-, and total methadone TID were 0.02 mg/kg/day (0.004–0.099), 0.013 mg/kg/day (0.002–0.071), and 0.033 mg/kg/day (0.006–0.170), respectively. Mean (range) RID of R-, S-, and total methadone were 2.7% (0.7–10.1%), 1.6% (0.3–7.2%), and 2.1% (0.52–8.8%), respectively.
Conclusions
R-Methadone is found in higher concentrations than S-methadone in breastmilk. Even at high methadone doses, breastmilk methadone concentrations were relatively low and support American Academy of Pediatrics recommendations that dose should not be a factor in determining whether women on methadone breastfeed.
Introduction
Breastmilk is the most complete form of nutrition for infants, with a range of benefits for health, growth, immunity, and development.1,2 Maternal illicit substance use is one of a short list of contraindications to breastfeeding due to the potential risks of passing drugs to the infant.3 The 1994 American Academy of Pediatrics (AAP) statement, “The Transfer of Drugs and Other Chemicals into Human Milk,”4 included methadone doses above 20 mg/day as a contraindication to breastfeeding. The updated 2001 statement removed the dose restriction and placed methadone in the table titled “Maternal medications usually compatible with breastfeeding.”3 Data to support this AAP policy change came from five case series published from 1975 to 2000, which together included fewer than 30 breastmilk samples.5–9 Publications after 2000 provided additional support for the AAP statement change.10–12
Recent epidemiologic studies demonstrated that breastfed infants of mothers on methadone have decreased severity of neonatal abstinence syndrome (NAS) and shorter hospital lengths of stay compared to formula-fed infants.13–15 The AAP and Academy of Breastfeeding Medicine statements indicate that breastfeeding is not contraindicated for women who are in a comprehensive methadone treatment program for their opiate addiction and demonstrate program adherence that includes no use of illicit or non-prescribed drugs.3,16
Despite medical society guidelines, women and their doctors worry about infant medication exposure during lactation.17–20 A wide variety of drug-, maternal-, and infant-specific factors impact infant exposure to medication via breastmilk, which makes conceptualization and actual measurement of infant exposure complex. Major factors include drug dissociation factor and fat solubility, maternal drug metabolism, plasma free (unbound) drug level, timing of medication dose relative to breastfeeding, drug bioavailability from the infant gut, and infant's drug metabolism. Previous studies suggest that on average the relative infant dose (RID) for methadone in breastmilk is 3%, but there is significant individual variability (range, 0.5–5%).5–8,11,12 Methadone metabolism rates differ greatly between individuals due to genetic variability in the activity of metabolizing cytochrome P-450 (CYP) isoforms.21–23
In the United States, methadone is given as a racemic mixture of equal parts of R- and S-methadone. The two enantiomers are metabolized at different rates and by different CYP isoforms. Older studies suggested that CYP3A4 was primarily responsible for methadone metabolism,23 but more recent studies suggest that CYP2B6 preferentially metabolizes S-methadone in humans and that CYP2C19 is partially responsible for R-methadone metabolism as well.24,25 There is significant heterogeneity in these cytochromes.26 CYP2B6 is interesting because only five drugs are known to be metabolized by it, and the known genotypes can be grouped into high and low activity. Thus, variation in CYP2B6 activity may explain some of the observed variability in effective methadone dosing and the variability of breastmilk levels of methadone. R-Methadone is 10 times more potent as a μ-opioid receptor antagonist than S-methadone.26,27 Therefore, the R-methadone concentration in milk is an important consideration. Enantiomer-specific methadone concentrations in breastmilk were reported in a single study of eight cases.10 Begg et al.10 found that R-methadone concentrations were nearly twice that of S-methadone in breastmilk. Characterization of infant exposure to methadone via breastmilk ideally includes assay of the more potent R-enantiomer. It is not known if the low concentrations of methadone in breastmilk are responsible for the improved NAS outcomes for breastfed infants. If it is, it is likely to be the R-methadone that has the effect. To date, this has only been studied in eight women at doses up to 100 mg/day. Given the wide individual variability in methadone metabolism, it is important to evaluate more women to determine the range of R-methadone specifically in breastmilk.
Most published data on breastmilk levels were generated when daily methadone doses were limited to 80 mg. However, federal regulations28 regarding methadone dosing have been liberalized based upon evidence that higher doses improved adherence to therapy and other outcomes29,30 and that individual variability in methadone metabolism is likely responsible for the large range of doses needed for therapeutic responses.31 This change in prescribing practices is reflected in current maternal dosing patterns. Studies to date have not found a consistent correlation between maternal methadone dose and methadone concentrations in breastmilk.7,11,12,32 Only one explored levels in women on high methadone doses (mean, 100 mg; range, 25–180 mg/day).12
In this study, we estimated infant exposure to R- and S-methadone via breastmilk when mothers receive higher, more clinically effective doses of racemic methadone. Infant exposure was estimated by theoretic infant dose (TID) and RID. In addition, enantiomer-specific milk-to-maternal plasma (M/P) ratios were determined. We then evaluated the 2001 AAP statement based upon our findings with higher maternal methadone doses and R-methadone-specific concentrations in breastmilk.
Subjects and Methods
Study population
Pregnant women in a comprehensive methadone treatment program were invited to participate in a longitudinal study of methadone pharmacokinetics. Women were eligible if they were 18–45 years old, had a singleton pregnancy, were human immunodeficiency virus negative, and spoke English. Twenty women provided concomitant plasma and breastmilk samples within 6 days after delivery. All were on daily methadone for at least a month prior to delivery, and most for longer. The methadone dose was not changed for at least 2 weeks prior to sample collection. Four women were taking another psychotropic medication (one each on sertraline, haloperidol, escitalopram, and clonazepam), and 85% were smoking cigarettes. Although other medications and smoking can impact drug metabolism, we were not able to control for this in the study.
Breastmilk
Breastmilk samples were collected from women regardless of their breastfeeding status. At sample collection, two women were fully breastfeeding, 13 were combining breastfeeding and formula feeding, and five were only formula feeding. Milk samples were collected when plasma methadone levels were in the range of peak levels (2–4 hours after dosing) in 16 of 20 samples and were obtained within 6 days of delivery based on the mother's availability. Women who were formula feeding or were only feeding their baby expressed breastmilk were asked to express milk using an electric breast pump from both breasts simultaneously for at least 10 minutes in order to obtain both foremilk and hindmilk. Samples from the two breasts were combined for analysis. Women who were breastfeeding were asked to express milk from only one breast for at least 10 minutes so that they could feed their infant from the nonexpressed side. Two women who preferred to use a manual pump expressed milk from a single breast for at least 10 minutes. A 10–15-mL aliquot of well-mixed breastmilk was retained for analysis; the remainder was fed to the infant. Milk samples were frozen to −80°C within 60 minutes of collection.
Maternal plasma methadone sample collection
Plasma and breastmilk samples were collected within a 15-minute time period. Maternal blood was collected by venipuncture into EDTA-containing tubes and was centrifuged in the cold within 60 minutes. Plasma was removed using polyethylene Pasteur pipettes into leak-proof polypropylene vials and frozen at −80°C.
Chiral methadone analysis
The plasma samples were analyzed for R- and S-methadone by the application of a modified chiral high-performance liquid chromatographic/ultraviolet detection method.33 The extraction procedure was done with 1-mL patient plasma samples or controls—the latter contain 0.4 μg of internal standard (racemic norfluoxetine) solution. After the pH of the samples was made alkaline, an extraction was done with 3:7 (vol/vol) methyl tert-butyl ether/n-hexane followed by re-extraction with hydrochloric acid and evaporation to dryness in a Savant Speed-Vac centrifuge evaporator (Thermo Fisher Scientific, Waltham, MA). The residues were reconstituted with 100 μL of mobile phase from which 80 μL was injected via an autoanalyzer. High-performance liquid chromatographic/ultraviolet detection conditions are as follows: column, Astec Cyclobond™ I 2000 RSP (Sigma-Aldrich, Atlanta, GA), film thickness 5 μm, 25 cm × 4.6 mm i.d.; guard column, Astec Cyclobond I, film thickness 5 μm, 5 cm × 4.6 mm i.d.; mobile phase, 9:11:80 (by volume) methanol:acetonitrile:pH 5.7 triethylamine phosphate buffer; detection, ultraviolet absorbance, 210 nm; calculation, weighted linear regression of peak height ratio versus concentration for each compound; and retention times (in minutes), R-methadone 10.1, S-methadone 11.8, S-norfluoxetine 41.3 (internal standard I), and R-norfluoxetine 45.5 (internal standard II). The limit of quantitative detectability is 0.005 mg/mL, and the mean percentage coefficients of variation range from 8.6 (high controls) to 12.7 (low controls).
For milk samples, the “Method of Standard Addition” was adapted in which milk samples are the actual matrices for the preparation of a standard curve. One-milliliter aliquots of milk were spiked with methadone standard to create seven to nine standards and controls of chiral methadone. After initial acidification followed by an n-heptane wash, with the latter being discarded, the samples were processed similarly as with plasma samples. A plot of added chiral methadone concentrations versus peak height ratios was constructed, and concentrations of chiral methadones were obtained from the x-axis intercepts.34,35
M/P ratio
The M/P ratio was calculated by dividing the breastmilk methadone concentration by maternal plasma methadone concentration for concomitantly collected samples. In general, the concentration of unbound drug in maternal plasma is an important determinant of the medication concentration in breastmilk. However, we did not measure plasma protein binding for these samples.
TID
TID estimates the quantity of drug delivered to an infant via breastmilk in a given day. The major assumption is that the methadone concentrations measured in our single samples of maternal plasma and breastmilk reflect the concentration across the entire day. Because most of the samples were obtained at peak maternal plasma concentration, and for methadone this correlates with peak breastmilk concentrations,11 our estimate is the maximum possible infant exposure to methadone via breastmilk. Also, the calculation assumes a standard volume of milk (0.15 L/kg/day):36 TID (mg/kg/day) = (maximum mg/L concentration of methadone) × (0.15 L/kg/day). No adjustment was made for partial or non-breastfeeding. For example, if the peak methadone concentration in the breastmilk is 0.5 mg/L, the TID is calculated as follows: (0.5 mg/L × 0.15 L/kg/day) = 0.075 mg/kg/day.
RID
RID is a method of relating the potential dose of the drug delivered to the infant to that of the mother. The calculation adjusts for both maternal and infant weight. The calculation is completed as follows: RID (%) = TID/(maternal daily mg/day methadone dose/maternal kg weight). For example, using the same example from above, if a mother's daily methadone dose is 100 mg/day and she weighs 70 kg, her weight-adjusted dose is 1.43 mg/kg/day. In general, the RID would be calculated as follows: (0.075 mg/kg/day/1.43 mg/kg/day) × 100 = 5.2%. An RID of < 10% is often considered acceptable with lactation.36,37 Women's measured third trimester weight was used in our calculations because a weight at delivery was not obtained. In order to see what the impact of a lower weight would be on RID, we recalculated RID using maternal third trimester weight minus 5 kg, the average weight loss at the time of delivery from delivery of the baby, placenta, and amniotic fluid.52
Analysis
The M/P ratio, TID, and RID were calculated for each participant for each enantiomer and total methadone, and means and ranges were determined. The ratio of R- to S-methadone was calculated for breastmilk and maternal plasma. We assessed the relationships between (1) maternal methadone dose and R-, S-, and total methadone concentrations in breastmilk and (2) concentrations of R-, S-, and total methadone concentrations in breastmilk and infant age in days when breastmilk samples were collected. We categorized maternal dose into four groups of nearly equal size (40–70, 75–90, 100–125, and 170–200 mg/day). The intervals postpartum when breastmilk samples were collected were also grouped into four categories of nearly equal size (≤1 day, 2 days, 3 days, and 4–6 days). The concentrations of methadone in breastmilk were compared across categories with analyses of variance. Associations between mothers' R-, S-, and total plasma concentrations of methadone and breastmilk concentrations of R-, S-, and total methadone were computed with Pearson correlations. In post hoc analyses, breastmilk concentrations of methadone were compared between samples collected at peak and trough.
This study was approved by the University of Pittsburgh Institutional Review Board. All women provided signed informed consent.
Results
Sample characteristics
The characteristics of the 20 women who provided concomitant breastmilk and plasma samples are summarized in Table 1. Most of these women were single, white, high school–educated, and multiparous. Women received a mean daily methadone dose of 102 mg (SD 46 mg) at delivery with a range from 40 to 200 mg/day. Most infants were born at term (85% at least 37 weeks' gestation; mean gestational age, 38.5 weeks [SD 1.7 weeks]) and with appropriate for gestational age anthropometric measurements: mean birth weight, 2,978.5 g (SD 542.7 g); mean length, 49.4 cm (SD 1.9 cm); and head circumference, 32.7 cm (SD 1.8 cm). Of the three (15%) infants who weighed less than 2,500 g at birth, only one was born preterm.
Table 1.
Demographic Characteristics of Study Participants (n = 20)
| Characteristic | n (%) |
|---|---|
| Maternal | |
| Age (<30 years) | 15 (75) |
| Race (white) | 19 (95) |
| Education | |
| Less than high school | 5 (25) |
| High school/GED | 9 (45) |
| More than high school | 6 (30) |
| Married or living as married | 7 (35) |
| Medicaid | 20 (100) |
| Primigravida | 5 (25) |
| Primiparous | 7 (35) |
| Hepatitis C | 10 (50) |
| Vaginal delivery | 17 (85) |
| Infant | |
| Male | 15 (75) |
| Preterm (<37 weeks of gestation) | 3 (15) |
| Birth weight <2,500 g | 3 (15) |
| Breastfed | |
| Ever | 15 (75) |
| At hospital discharge (n = 19) | 11 (58) |
M/P ratio, RID, and TID results
The concentrations of R- and S-methadone in breastmilk and maternal plasma are provided for each participant in Table 2 (ordered by maternal dose). The relative concentrations of R- and S-methadone in maternal plasma were variable. Some women had higher concentrations of R- than S-methadone, and vice versa. In contrast, the relative concentrations of R- and S-methadone in breastmilk were consistent; all had higher concentrations of R- than S-methadone regardless of the relative concentration of R- to S-methadone in maternal plasma.
Table 2.
Enantiomer-Specific Methadone Concentrations (mg/L) in Breastmilk and Maternal Plasma
| |
|
|
Breastmilk |
Maternal plasma |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Dose (mg/day) | Peak or trough | R-Methadone | S-Methadone | Total methadone | R/S-Methadone | R-Methadone | S-Methadone | Total methadone | R/S-Methadone |
| 1 | 40 | Peak | 0.0606 | 0.0413 | 0.1019 | 1.47 | 0.1220 | 0.150 | 0.272 | 0.81 |
| 2 | 50 | Peak | 0.0302 | 0.0174 | 0.0476 | 1.74 | 0.0876 | 0.0974 | 0.185 | 0.89 |
| 3 | 60 | Peak | 0.0866 | 0.0549 | 0.1415 | 1.58 | 0.1840 | 0.274 | 0.458 | 0.67 |
| 4 | 60 | Peak | 0.0720 | 0.0340 | 0.1060 | 2.12 | 0.0938 | 0.102 | 0.1958 | 0.92 |
| 5 | 70 | Peak | 0.0252 | 0.0146 | 0.0398 | 1.73 | 0.4720 | 0.644 | 1.116 | 0.73 |
| 6 | 75 | Trough | 0.0367 | 0.0119 | 0.0486 | 3.08 | 0.0787 | 0.0363 | 0.115 | 2.17 |
| 7 | 75 | Peak | 0.0714 | 0.0529 | 0.1243 | 1.35 | 0.1630 | 0.198 | 0.361 | 0.82 |
| 8 | 77 | Peak | 0.1430 | 0.0970 | 0.2400 | 1.47 | 0.2700 | 0.343 | 0.613 | 0.79 |
| 9 | 80 | Trough | 0.0617 | 0.0328 | 0.0945 | 1.88 | 0.1660 | 0.170 | 0.336 | 0.98 |
| 10 | 90 | Trough | 0.0342 | 0.0152 | 0.0494 | 2.25 | 0.1890 | 0.160 | 0.349 | 1.18 |
| 11 | 90 | Peak | 0.0661 | 0.0438 | 0.1099 | 1.51 | 0.256 | 0.284 | 0.540 | 0.90 |
| 12 | 100 | Peak | 0.2210 | 0.0998 | 0.3208 | 2.21 | 0.1740 | 0.1470 | 0.321 | 1.18 |
| 13 | 100 | Peak | 0.1080 | 0.0668 | 0.1748 | 1.61 | 0.2530 | 0.2740 | 0.527 | 0.92 |
| 14 | 110 | Peak | 0.1830 | 0.1160 | 0.2990 | 1.58 | 0.2790 | 0.2710 | 0.550 | 1.03 |
| 15 | 115 | Peak | 0.6590 | 0.4710 | 1.1300 | 1.40 | 0.7200 | 1.0450 | 1.765 | 0.69 |
| 16 | 123 | Trough | 0.0670 | 0.0310 | 0.0980 | 2.16 | 0.1740 | 0.1650 | 0.339 | 1.05 |
| 17 | 170 | Peak | 0.1700 | 0.0995 | 0.2695 | 1.71 | 0.1880 | 0.1980 | 0.386 | 0.95 |
| 18 | 177 | Peak | 0.0970 | 0.0381 | 0.1351 | 2.55 | 0.2480 | 0.1770 | 0.425 | 1.40 |
| 19 | 180 | Peak | 0.1670 | 0.0826 | 0.2496 | 2.02 | 0.4420 | 0.3590 | 0.801 | 1.23 |
| 20 | 200 | Peak | 0.3590 | 0.2530 | 0.6120 | 1.42 | 0.5480 | 0.6410 | 1.189 | 0.85 |
| Mean | 102.1 | — | 0.1359 | 0.0837 | 0.2196 | 1.84 | 0.2554 | 0.2868 | 0.5422 | 1.01 |
| SD | 46.2 | — | 0.1473 | 0.1063 | 0.2531 | 0.45 | 0.1672 | 0.2384 | 0.4017 | 0.33 |
| Range | 40–200 | — | 0.025–0.659 | 0.012–0.471 | 0.040–1.13 | 1.35–3.08 | 0.079–0.720 | 0.036–1.045 | 0.115–1.765 | 0.67–2.17 |
Peak indicates that the sample was obtained between 2 to 4 hours after the mother took her daily methadone dose. Trough indicates that the sample was obtained at least 20 hours after the mother took her last methadone dose.
The means of the M/P ratios, TIDs and RIDs for R-, S-, and total methadone are provided in Table 3. The M/P ratio for R-methadone is higher than for S-methadone with wide individual variability. In one sample obtained at peak (3.6 hours after the mother's dose), the M/P ratio for R-methadone exceeded 1. Like the M/P ratio, TID for both R- and S-methadone ranged widely. Among the 20 study participants, 19 had an R-methadone RID between 0.7 and 4.7%; one woman had an R-methadone RID of 10%. Her daily methadone dose was in the mid-range for the sample.
Table 3.
Measures of Infant Exposure to Enantiomer-Specific Methadone via Breastmilk and Milk-to-Maternal Plasma Ratio
| R-Methadone | S-Methadone | Total methadone | |
|---|---|---|---|
| Mean (SD) TID (mg/kg/day) | 0.020 (0.022) | 0.013 (0.016) | 0.033 (0.038) |
| Range | 0.004–0.099 | 0.002–0.071 | 0.006–0.170 |
| Mean (SD) RID (%) | 2.7 (2.1) | 1.6 (1.5) | 2.1 (1.8) |
| Range | 0.66–10.1 | 0.33–7.2 | 0.52–8.7 |
| Mean (SD) M/P ratio | 0.52 (0.28) | 0.28 (0.15) | 0.40 (0.21) |
| Range | 0.05–1.27 | 0.02–0.68 | 0.04–1.0 |
M/P, milk-to-maternal plasma ratio; RID, relative infant dose; TID, theoretic infant dose.
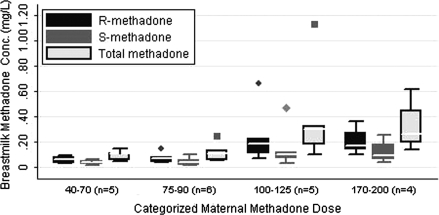
Associations between maternal methadone dose and breastmilk methadone concentrations (Fig. 1)
FIG. 1.
Maternal methadone dose and breastmilk methadone concentrations. Boxes represent interquartile range (IQR) of observed values with the median value represented with a contrasting line within the box. The whiskers represent adjacent values, defined as the value closest to (75% value + 3/2[IQR]) and whose absolute value is less than the cut-point for the adjacent value. Markers outside the whiskers represent any values outside of the adjacent values.38
As seen in Table 2, there is one breastmilk sample (ID #15) with methadone concentrations much higher than the other samples. Because this outlier significantly impacted the analysis of the correlation between maternal methadone dose and breastmilk concentration of methadone, the analyses were completed with and without this sample.
Without the outlier, analysis of variance demonstrated a significant trend of increased methadone concentrations in breastmilk across the dose categories (coefficient for dose category: R-methadone dose = 0.05 [95% confidence interval 0.02, 0.08], S-methadone dose = 0.03 [95% confidence interval 0.01, 0.05], total methadone dose = 0.08 [95% confidence interval 0.03, 0.13]). Coefficients obtained when the outlier was included remained significant for R-methadone dose and total methadone dose but not S-methadone dose.
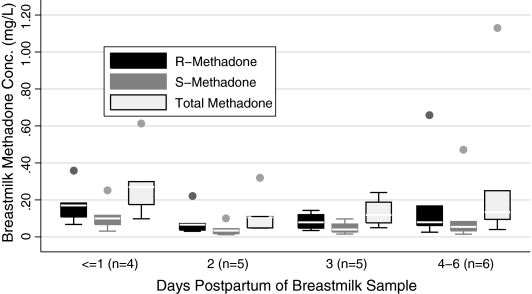
Associations between breastmilk methadone concentrations and breastmilk sampling day (Fig. 2)
FIG. 2.
Breastmilk methadone concentrations and age in days of sample collection. Boxes represent IQR of observed values with the median value represented with a contrasting line within the box. The whiskers represent adjacent values, defined as the value closest to (75% value + 3/2[IQR]) and whose absolute value is less than the cut-point for the adjacent value. Markers outside the whiskers represent any values outside of the adjacent values.38
The outlier did not impact the results of the analysis of breastmilk methadone concentration and postpartum sampling day. Analysis of variance demonstrated no significant association between breastmilk methadone concentration and days of breastmilk sampling.
Associations between breastmilk concentration and maternal plasma methadone concentrations
There were significant correlations between breastmilk concentrations and maternal plasma concentrations of R-, S-, and total methadone. Correlations ranged from 0.89 to 0.98 for maternal plasma and breastmilk concentrations (p < 0.001 for all) and from 0.51 to 0.59 for maternal plasma and breastmilk concentrations with the outlier sample removed (p ranges from 0.04 to 0.01).
Peak versus trough sampling
Four samples were obtained at trough rather than peak plasma levels. Therefore, we compared the R-, S-, and total methadone concentrations as well as M/P ratio, TID, and RID between peak and trough samples. As expected, samples taken at trough predicted less exposure to methadone via breastmilk, but the values for trough samples were all within the range for peak samples and not significantly different between groups (p = 0.11 for all without the outlier, p = 0.2 with the outlier included). For example, the M/P ratio range for peak samples was 0.05–1.27, and the trough M/P ratio range was 0.18–0.47, which falls within the range for peak samples.
Discussion
We found that breastmilk concentrations of total methadone were highly variable and ranged from 0.04 to 1.10 mg/L. R-Methadone, the active enantiomer, was found in higher concentrations in breastmilk than the less active S-methadone enantiomer, regardless of M/P ratio of R- to S-methadone. Drug transfer from maternal plasma to breastmilk depends significantly on the concentration of unbound drug in maternal plasma. R-Methadone is less protein bound than S-methadone, thus explaining the paradox of R-/S-methadone ratios in plasma and breastmilk.39 In all but one woman, the maximum RID of R-methadone was less than 4.7%; one woman had an R-methadone RID of 10.1%. Total methadone RID ranged from 0.5% to 8.6%. These values should be interpreted as the maximum possible daily infant exposure from breastmilk. Most of the single samples were taken at peak methadone levels; this level was assumed to be present in all feedings throughout the day. In reality, breastmilk methadone concentrations vary across the day as methadone is metabolized and excreted. Jansson et al.11 demonstrated a doubling of the methadone concentration in breastmilk from trough to peak.
Lower S-methadone levels in breastmilk are interesting in light of recent study findings that suggest that S-methadone may be responsible for the QT prolongation, especially in CYP2B6 slow metabolizers.40,41 There have not been any reported cases of prolonged QT among infants exposed to methadone via breastmilk. The large variation in methadone concentrations in maternal plasma and breastmilk may be explained in part by difference in cytochrome activity. For example, it is plausible that the outlier sample came from a woman who is a slow metabolizer of methadone, emphasizing the point made by Begg et al.10 for individualizing management as much as possible.
Women in our study were taking much higher methadone doses than reported in most of the previous studies. The mean methadone dose in our study was over 100 mg/day. Unlike others, we did find significant correlations between the mother's dose and methadone concentrations in breastmilk or maternal plasma.7,11,12
Breastfeeding is associated with lower treatment rates, lower medication doses required for pharmacological treatment of NAS, and shorter hospital lengths of stay for infants with NAS.13–15 Breastfeeding could reduce NAS severity through a variety of mechanisms. For example, breastmilk constituents might improve digestibility and reduce gastrointestinal symptoms associated with NAS. Breastmilk is emptied more quickly from the stomach and small intestines than formula, which results in smaller volume and more frequent feedings than for formula-fed infants. Breastfeeding and the associated skin-to-skin contact between mother and infant modify the maternal and infant hormonal milieu. Women who breastfeed have higher oxytocin and prolactin levels than women who formula feed.42,43 Skin-to-skin maternal infant contact improves infant temperature regulation and maternal–infant attachment for preterm infants44,45 and decreases maternal cortisol levels and stress.46 Breastfeeding may modify the detrimental effects of smoking on infants.47,48 Finally, small quantities of methadone in breastmilk could slow the rate of decline of methadone in infant plasma and reduce symptoms of NAS.
This hypothesis that small quantities of methadone in breastmilk could decrease NAS severity deserves further evaluation. We observed one woman with nearly double the RID of R-methadone than all other women in the study. This subject was treated within the mid-range dose of methadone for the group (115 mg). It is possible that this subject is a slow metabolizer of methadone and thus developed unusually high plasma methadone concentrations. She breastfed her infant briefly, and her infant did not receive pharmacological treatment for NAS. If these study findings and the two published case reports of acute onset of NAS after abrupt cessation of breastfeeding49 are combined, the notion that small amounts of R-methadone in breastmilk reduce NAS appears plausible in some mother–baby dyads.
The breastfeeding rate among women on methadone in the United States was reported in two studies. McCarthy and co-workers reported that among women in their California-based methadone program with integrated obstetric, pediatric, and substance abuse services, in 2005, 46% breastfed compared with 32% in 2000.12,50 Wachman et al.51 reported that in their inner-city Boston hospital, among women eligible to breastfeed, only 24% initiated breastfeeding. In our small sample of women, 75% initiated breastfeeding, and 55% were partially breastfeeding at the infant's hospital discharge. The high initiation rate may be due in part to study selection criteria, which excluded human immunodeficiency virus–infected women and women actively using illicit drugs. Women in the study did receive breastfeeding encouragement and support from the study staff, but that was not the aim of this study. This high rate of any breastfeeding demonstrates that women on methadone can be supported to breastfeed. However, the low rate of exclusive breastfeeding is consistent with the other published reports and indicates additional need for support for this population.
There are several study limitations. First, women provided a single breastmilk sample. Four of the samples were taken at trough, rather than peak, which could result in underestimation of the TID and RID. We repeated these calculations for just the 16 peak samples, and the mean total methadone TID increased minimally (from 0.0329 mg/kg/day to 0.0384 mg/kg/day), and the mean total methadone RID increased from 2.1% to 2.4%. Samples were taken from women regardless of breastfeeding status. Milk specimens from women who are fully, partially, and not at all breastfeeding are highly variable with respect to fat content, and methadone is a lipophilic drug. Samples were collected across the first 6 days of life when the fat content of breastmilk is highly variable. The inconsistency in when postpartum samples were obtained and how much women were breastfeeding could explain the wide range of levels seen in the breastmilk samples. Furthermore, women were instructed to express milk for at least 10 minutes, which may not completely achieve hindmilk expression. Hindmilk has relatively greater fat content; therefore, an incomplete collection of breastmilk underestimates the methadone concentration. However, the methadone levels we report are consistent with previously reported study findings, including our finding of higher concentrations of R- than S-methadone in breastmilk.10 Finally, for the RID calculation, maternal third trimester weight was used, which likely overestimates maternal weight after birth. To determine the potential impact of lower maternal weight on RID, the RID calculations were rerun assuming a 5 kg weight loss, an estimate of the average weight loss at delivery.52 The mean R-, S-, and total methadone RIDs increased by 0.1–0.2% using the lower weight. These results do not change the study findings or conclusions.
Conclusions
In conclusion, even at high maternal methadone doses the R-methadone TID is very low; all infants are estimated to receive less than 0.1 mg/kg/day methadone via breastmilk. Our methodology overestimates the true amount of methadone exposure via breastmilk, and therefore our data support the 2001 AAP statement that mothers on high methadone doses should be supported to breastfeed if they remain in and adherent to their comprehensive methadone treatment program. Additional research is needed to evaluate potential contribution of low concentrations of methadone enantiomers in breastmilk to prevention or improved outcomes of NAS.
Acknowledgments
This study was funded by The Children's Hospital of Pittsburgh Research Advisory Committee and The Gerber Foundation. Study visits were conducted in the Magee-Womens Hospital Clinical and Translational Research Center, which is funded by the National Institutes of Health (grant M01RR00056). Medela, Inc. supported this study by providing the pump supplies to collect the breastmilk samples and educational materials. D.L.B.'s contribution to this work was supported by grant K12 HD043441 (BIRCWH Award). Support for the time of K.L.W., J.C.H., and J.M.P. was funded by grant R01 MH 075921 (K.L.W., Principal Investigator). We would like to thank Yael Klionsky for her help with data cleaning and preparation and Dr. Catherine Friedman for her careful review of the manuscript.
Disclosure Statement
D.L.B. received breastfeeding supplies for this study from Medela, Inc. K.L.W. served on an advisory board for Eli Lilly Corporation and received a donation of matching estradiol and placebo transdermal patches for an National Institute of Mental Health–funded randomized clinical trial from Novogyne (Novartis). J.M.P., B.H.H., J.C.H., and M.T. declare no competing interests exist.
References
- 1.Office on Women's Health. Health and Human Services Blueprint for Action on Breastfeeding. Washington, DC: 2000. U.S. Department of Health and Human Services. [Google Scholar]
- 2.Gartner LM. Morton J. Lawrence RA, et al. Breastfeeding and the use of human milk. Pediatrics. 2005;115:496–506. doi: 10.1542/peds.2004-2491. [DOI] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics Committee on Drugs. Transfer of drugs and other chemicals into human milk. Pediatrics. 2001;108:776–789. [PubMed] [Google Scholar]
- 4.American Academy of Pediatrics Committee on Drugs. The transfer of drugs and other chemicals into human milk. Pediatrics. 1994;93:137–150. [PubMed] [Google Scholar]
- 5.Geraghty B. Graham EA. Logan B, et al. Methadone levels in breast milk. J Hum Lact. 1997;13:227–230. doi: 10.1177/089033449701300312. [DOI] [PubMed] [Google Scholar]
- 6.Wojnar-Horton RE. Kristensen JH. Yapp P, et al. Methadone distribution and excretion into breast milk of clients in a methadone maintenance programme. Br J Clin Pharmacol. 1997;44:543–547. doi: 10.1046/j.1365-2125.1997.t01-1-00624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blinick G. Inturrisi CE. Jerez E, et al. Methadone assays in pregnant women and progeny. Am J Obstet Gynecol. 1975;121:617–621. doi: 10.1016/0002-9378(75)90461-5. [DOI] [PubMed] [Google Scholar]
- 8.Kreek MJ. Methadone disposition during the perinatal period in humans. Pharmacol Biochem Behav. 1979;11(Suppl):7–13. [PubMed] [Google Scholar]
- 9.Pond SM. Kreek MJ. Tong TG, et al. Altered methadone pharmacokinetics in methadone-maintained pregnant women. J Pharmacol Exp Ther. 1985;233:1–6. [PubMed] [Google Scholar]
- 10.Begg EJ. Malpas TJ. Hackett LP, et al. Distribution of R- and S-methadone into human milk during multiple, medium to high oral dosing. Br J Clin Pharmacol. 2001;52:681–685. doi: 10.1046/j.1365-2125.2001.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansson LM. Choo RE. Harrow C, et al. Concentrations of methadone in breast milk and plasma in the immediate perinatal period. J Hum Lact. 2007;23:184–190. doi: 10.1177/0890334407300336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCarthy JJ. Posey BL. Methadone levels in human milk. J Hum Lact. 2000;16:115–120. doi: 10.1177/089033440001600206. [DOI] [PubMed] [Google Scholar]
- 13.Abdel-Latif ME. Pinner J. Clews S, et al. Effects of breast milk on the severity and outcome of neonatal abstinence syndrome among infants of drug-dependent mothers. Pediatrics. 2006;117:e1163–e1169. doi: 10.1542/peds.2005-1561. [DOI] [PubMed] [Google Scholar]
- 14.Arlettaz R. Kashiwagi M. Das-Kundu S, et al. Methadone maintenance program in pregnancy in a Swiss perinatal center (II): Neonatal outcome and social resources. Acta Obstet Gynecol Scand. 2005;84:145–150. doi: 10.1111/j.0001-6349.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 15.Ballard JL. Treatment of neonatal abstinence syndrome with breast milk containing methadone. J Perinat Neonatal Nurs. 2002;15:76–85. doi: 10.1097/00005237-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 16.Academy of Breastfeeding Medicine Protocol Committee. ABM clinical protocol #21: Guidelines for breastfeeding and the drug-dependent woman. Breastfeed Med. 2009;4:225–228. doi: 10.1089/bfm.2009.9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gendron MP. Martin B. Oraichi D, et al. Health care providers' requests to Teratogen Information Services on medication use during pregnancy and lactation. Eur J Clin Pharmacol. 2009;65:523–531. doi: 10.1007/s00228-008-0611-6. [DOI] [PubMed] [Google Scholar]
- 18.Ito S. Koren G. Einarson TR. Maternal noncompliance with antibiotics during breastfeeding. Ann Pharmacother. 1993;27:40–42. doi: 10.1177/106002809302700110. [DOI] [PubMed] [Google Scholar]
- 19.Ito S. Lieu M. Chan W, et al. Continuing drug therapy while breastfeeding. Part 1. Common misconceptions of patients. Can Fam Physician. 1999;45:897–899. [PMC free article] [PubMed] [Google Scholar]
- 20.Ito S. Moretti M. Liau M, et al. Initiation and duration of breast-feeding in women receiving antiepileptics. Am J Obstet Gynecol. 1995;172:881–886. doi: 10.1016/0002-9378(95)90015-2. [DOI] [PubMed] [Google Scholar]
- 21.Eap CB. Bertschy G. Baumann P, et al. High interindividual variability of methadone enantiomer blood levels to dose ratios. Arch Gen Psychiatry. 1998;55:89–90. doi: 10.1001/archpsyc.55.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Foster DJ. Somogyi AA. Bochner F. Methadone N-demethylation in human liver microsomes: Lack of stereoselectivity and involvement of CYP3A4. Br J Clin Pharmacol. 1999;47:403–412. doi: 10.1046/j.1365-2125.1999.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gerber JG. Rhodes RJ. Gal J. Stereoselective metabolism of methadone N-demethylation by cytochrome P4502B6 and 2C19. Chirality. 2004;16:36–44. doi: 10.1002/chir.10303. [DOI] [PubMed] [Google Scholar]
- 24.Totah RA. Sheffels P. Roberts T, et al. Role of CYP2B6 in stereoselective human methadone metabolism. Anesthesiology. 2008;108:363–374. doi: 10.1097/ALN.0b013e3181642938. [DOI] [PubMed] [Google Scholar]
- 25.Kharasch ED. Hoffer C. Whittington D, et al. Methadone pharmacokinetics are independent of cytochrome P4503A (CYP3A) activity and gastrointestinal drug transport. Anesthesiology. 2009;110:660–672. doi: 10.1097/ALN.0b013e3181986a9a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crettol S. Déglon J-J. Besson J, et al. Methadone enantiomer plasma levels, CYP2B6, CYP2C19, and CYP2C9 genotypes, and response to treatment. Clin Pharmacol Ther. 2005;78:593–604. doi: 10.1016/j.clpt.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 27.Kristensen K. Christensen CB. Christrup LL. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci. 1995;56:PL45–PL50. doi: 10.1016/0024-3205(94)00426-s. [DOI] [PubMed] [Google Scholar]
- 28.Institute of Medicine Committee on Federal Regulation of Methadone Treatment. Federal Regulation of Methadone Treatment. National Academy Press; Washington, DC: 1995. Division of Biobehavioral Sciences and Mental Disorders. [Google Scholar]
- 29.Leavitt SB. Shinderman M. Maxwell S, et al. When “enough” is not enough: New perspectives on optimal methadone maintenance dose. Mt Sinai J Med. 2000;67:404–411. [PubMed] [Google Scholar]
- 30.Eap CB. Bourquin M. Martin J, et al. Plasma concentrations of the enantiomers of methadone and therapeutic response in methadone maintenance treatment. Drug Alcohol Depend. 2000;61:47–54. doi: 10.1016/s0376-8716(00)00121-6. [DOI] [PubMed] [Google Scholar]
- 31.Lehotay DC. George S. Etter ML, et al. Free and bound enantiomers of methadone and its metabolite, EDDP in methadone maintenance treatment: Relationship to dosage? Clin Biochem. 2005;38:1088–1094. doi: 10.1016/j.clinbiochem.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Jansson LM. Choo R. Velez ML, et al. Methadone maintenance and breastfeeding in the neonatal period. Pediatrics. 2008;121:106–114. doi: 10.1542/peds.2007-1182. [DOI] [PubMed] [Google Scholar]
- 33.Foster DJ. Somogyi AA. White JM, et al. Population pharmacokinetics of (R)-, (S)- and rac-methadone in methadone maintenance patients. Br J Clin Pharmacol. 2004;57:742–755. doi: 10.1111/j.1365-2125.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kristensen JH. Ilett KF. Dusci LJ, et al. Distribution and excretion of sertraline and N-desmethylsertraline in human milk. Br J Clin Pharmacol. 1998;45:453–457. doi: 10.1046/j.1365-2125.1998.00705.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moses-Kolko EL. Meltzer CC. Helsel JC, et al. No interruption of lactation is needed after 11C-WAY 100635 or 11C-raclopride PET. J Nucl Med. 2005;46:1765. [PubMed] [Google Scholar]
- 36.Bennett P. Use of the monographs on drugs. In: Bennett P, editor. Drugs and Human Lactation. Elsevier; Amsterdam: 1996. pp. 67–74. [Google Scholar]
- 37.Hale TW. Medications and Mother's Milk. 13th. Hale Publishing; Amarillo, TX: 2008. [Google Scholar]
- 38.Tukey JW. Exploratory Data Analysis. Addison-Wesley; Reading, MA: 1977. [Google Scholar]
- 39.Eap CB. Cuendet C. Baumann P. Binding of d-methadone, l-methadone, and dl-methadone to proteins in plasma of healthy volunteers: Role of the variants of alpha 1-acid glycoprotein. Clin Pharmacol Ther. 1990;47:338–346. doi: 10.1038/clpt.1990.37. [DOI] [PubMed] [Google Scholar]
- 40.Eap CB. Crettol S. Rougier JS, et al. Stereoselective block of hERG channel by (S)-methadone and QT interval prolongation in CYP2B6 slow metabolizers. Clin Pharmacol Ther. 2007;81:719–728. doi: 10.1038/sj.clpt.6100120. [DOI] [PubMed] [Google Scholar]
- 41.Lin C. Somberg T. Molnar J, et al. The effects of chiral isolates of methadone on the cardiac potassium channel IKr. Cardiology. 2009;113:59–65. doi: 10.1159/000167043. [DOI] [PubMed] [Google Scholar]
- 42.Adamopoulos DA. Kapolla N. Prolactin concentration in milk and plasma of puerperal women and patients with galactorrhea. J Endocrinol Invest. 1984;7:273–276. doi: 10.1007/BF03351001. [DOI] [PubMed] [Google Scholar]
- 43.Nissen E. Uvnas-Moberg K. Svensson K, et al. Different patterns of oxytocin, prolactin but not cortisol release during breastfeeding in women delivered by caesarean section or by the vaginal route. Early Hum Dev. 1996;45:103–118. doi: 10.1016/0378-3782(96)01725-2. [DOI] [PubMed] [Google Scholar]
- 44.Ibe OE. Austin T. Sullivan K, et al. A comparison of kangaroo mother care and conventional incubator care for thermal regulation of infants < 2000 g in Nigeria using continuous ambulatory temperature monitoring. Ann Trop Paediatr. 2004;24:245–251. doi: 10.1179/027249304225019082. [DOI] [PubMed] [Google Scholar]
- 45.Cattaneo A. Davanzo R. Worku B, et al. Kangaroo mother care for low birthweight infants: A randomized controlled trial in different settings. Acta Paediatr. 1998;87:976–985. doi: 10.1080/080352598750031653. [DOI] [PubMed] [Google Scholar]
- 46.Morelius E. Theodorsson E. Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116:1105–1113. doi: 10.1542/peds.2004-2440. [DOI] [PubMed] [Google Scholar]
- 47.Chantry CJ. Howard CR. Auinger P. Full breastfeeding duration and associated decrease in respiratory tract infection in US children. Pediatrics. 2006;117:425–432. doi: 10.1542/peds.2004-2283. [DOI] [PubMed] [Google Scholar]
- 48.Woodward A. Douglas RM. Graham NM, et al. Acute respiratory illness in Adelaide children: Breast feeding modifies the effect of passive smoking. J Epidemiol Community Health. 1990;44:224–230. doi: 10.1136/jech.44.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malpas TJ. Darlow BA. Neonatal abstinence syndrome following abrupt cessation of breastfeeding. N Z Med J. 1999;112:12–13. [PubMed] [Google Scholar]
- 50.McCarthy JJ. Leamon MH. Parr MS, et al. High-dose methadone maintenance in pregnancy: Maternal and neonatal outcomes. Am J Obstet Gynecol. 2005;193:606–610. doi: 10.1016/j.ajog.2005.03.072. [DOI] [PubMed] [Google Scholar]
- 51.Wachman E. Byun J. Philipp B. Breastfeeding rates among mothers of infants with neonatal abstinence syndrome. Breastfeed Med. 2010;5:159–164. doi: 10.1089/bfm.2009.0079. [DOI] [PubMed] [Google Scholar]
- 52.Crowell DT. Weight change in the postpartum period. A review of the literature. J Nurse Midwifery. 1995;40:418–423. doi: 10.1016/0091-2182(95)00049-p. [DOI] [PubMed] [Google Scholar]