Abstract
Investigators have typically relied on a single or few discrete time points as measures of polychlorinated biphenyl (PCB) body burden, however health effects are more likely to be the result of integrative exposure in time, optionally expressed as an area under the time curve (AUC) of PCB serum concentration. Using data from a subgroup of 93 infants from a birth cohort in eastern Slovakia—a region highly polluted by PCBs—we fit a system type model, customized to our longitudinal measures of serum PCB concentrations in cord, 6, 16, and, 45 month blood specimens. The most abundant congener, PCB 153, was chosen for modeling purposes. In addition to currently used methods of exposure assessment, our approach estimates a concentration time profile for each subject, taking into account mean residence time of PCB 153 molecules in the body, duration of breast feeding, hypothetical PCB 153 concentration in steady-state without breast feeding and alternately without normal food intake. Hypothetical PCB 153 concentration in steady-state without normal food intake correlates with AUC (r=0.84, p<0.001) as well as with duration of breast feeding (r=0.64, p<0.001). It makes possible to determine each subject’s exposure profile expressed as AUC of PCBs serum concentration with a minimum model parameters. PCB body burden in most infants was strongly associated with duration of breast feeding in most, but not all children, was apparent from model output.
Keywords: Polychlorinated biphenyls, Breast feeding, Toxicokinetics, Modeling, Mean residence time, Children
1. Introduction
Polychlorinated biphenyls (PCBs) are a member of the organochlorine family, and though they are no longer produced, they are still found in the environment and in numerous wildlife species, as well as human tissues. Human exposure to PCBs first occurs in utero through transplacental transfer of PCBs from mother to infant. After birth, infants are exposed to PCBs via breast feeding, and later through intake of contaminated foods. To assess the association between PCB exposure and health outcomes, investigators have typically relied on discrete time points as a measure of PCB body burden. Examples include PCB concentrations measured in maternal blood taken during pregnancy (Chevrier et al., 2008; Darnerud et al., 2010; Glynn et al., 2008; Hertz-Picciotto et al., 2008; Lopez-Espinoza et al., 2009; McGlynn et al., 2009; Roze et al., 2009; Terrell et al., 2009; Wilhelm et al., 2008), cord blood (Brucker-Davis et al., 2008; Dallaire et al., 2006; Otake et al., 2007; Sagiv et al., 2010; Tan et al., 2009), placental tissue (Laisi et al., 2008; Reichrtova et al., 1999; Wang et al., 2005), breast milk (Darnerud et al., 2010; Glynn et al., 2008; Heilman et al., 2006), or PCB concentrations measured postnatally in the blood of infants and children (Barr et al., 2006; Darnerud et al., 2010; Grimalt et al., 2010; Sunyer et al., 2008). While these exposures are correlated across time, they may not accurately reflect PCB body burden for several reasons. First, the likelihood of PCB toxicity from a particular exposure scenario may be most strongly related to the maximum concentration (Cmax) of PCBs in the target tissue (i.e. a “peak” exposure), or a cumulative measure of PCBs over time. Second, estimating a maximum concentration or “peak” exposure is difficult in most longitudinal studies since few PCB concentrations are determined, and often, these determinations are spaced widely in time. Finally, calculating a cumulative measure of PCB exposure (a widely used metric for such an exposure scenario is the “area under the concentration curve” (AUC)) also requires serial measures of PCB concentration in the developing infant and child. Complicating the issue of assessing exposure is that for developmental effects, the chemical time course may also have to coincide with the window of susceptibility for a particular gestational or postnatal event (Young et al., 1996), leading to situations where either AUC or Cmax or the levels within a narrow time window may be the more appropriate dose metric (Verner et al., 2010)..
With the aim of examining several developmental health outcomes in relation to PCB exposures occurring during pregnancy and into postnatal life, we launched a birth cohort study of mother-infant pairs (Hertz-Picciotto et al., 2003) living in an area of eastern Slovakia with significant environmental contamination (Kočan et al., 1994). The objective of this work was to use longitudinally obtained measurements to develop more useful exposure metrics which could be applied to evaluate exposure-outcome associations.
2. Materials and methods
2.1 Study subjects
A cohort of mothers with newborns was recruited from 2 regions of eastern Slovakia as described elsewhere (Hertz-Picciotto et al., 2003; Sonneborn et al., 2008b). Information on breastfeeding was collected using questionnaires administered during follow up visits at 6, 16, and 45 months of age.
2.2 Analyses
Blood samples from children were collected from umbilical cord and at 6, 16 and 45 months of age by a trained nurse using venipuncture. Samples were stored in a refrigerator and within 2 hours transported to the Biochemical Department, where they were centrifuged and serum aliquots were divided into test tubes. Blood sera were stored frozen at −18°C until transport to the Slovak Medical University for PCB analyses. Written informed consent was obtained from parents. The study was approved by the Ethics Committee of the Slovak Medical University. The concentration of 15 PCB congeners (PCB IUPAC #28, #52, #101, #105, #114, #118, #123, #138, #153, #156, #157, #167, #170, #180, and #189) were determined in the sera samples by high-resolution gas chromatography with electron capture detection (Čonka et al., 2005; Kočan et al., 1994). Total serum lipids were estimated using enzymatic summation method (Akins et al., 1989). Funding permitted to complete all chemical analyses except 93 serum samples taken at the age of 6 months.
2.3 Model description
The lipid adjusted serum concentration of the most abundant PCB congener, #153, was used for modeling the time-course of PCB concentration. The measured PCB concentration after birth C(t), can be described by the relationship:
| (1), |
where t is time, C(0)=C0 is PCB concentration at birth and ΔC(t) is an increase of PCB concentration given by environment, mainly by breast feeding with a transfer rate Ibf and normal food intake with a transfer rate If (index bf means breast feeding and f food).
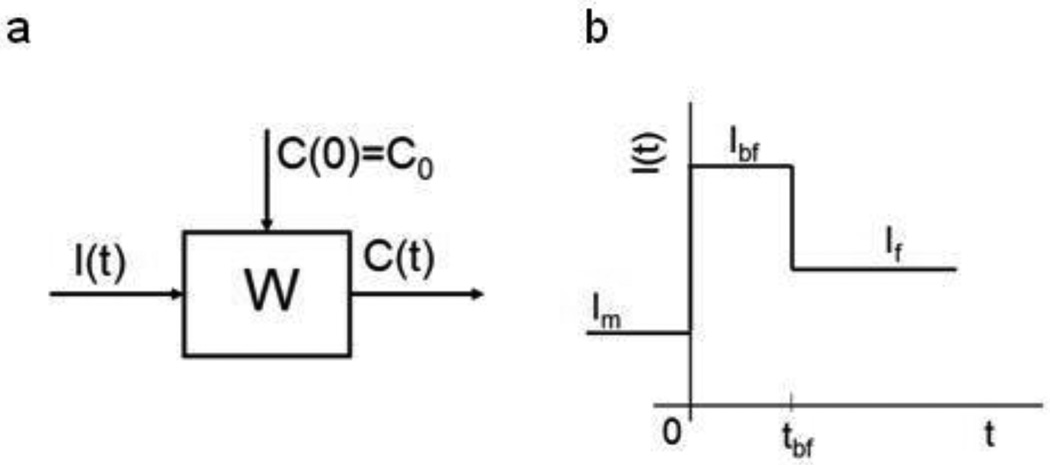
Suppose that the principle of superposition holds for system W studied (Figure 1a), we can describe the function ΔC(t) by convolution of the weighting function of the system of the child W(t) and function of effect of PCB I(t):
| (2) |
It holds for function I(t) (Figure 1b):
I(t) = Ibf for t<tbf and I(t) = If for t>tbf, where tbf is duration of breast feeding.
By means of Laplace transform (Debnath, 1995) we can write for ΔC the equation
| (3), |
where s is Laplace operator and H(s) is transfer function of the system PCB concentration increase of the child ΔC depending on rate of PCB I(t).
Figure 1.
a: Definition of system W. I, C is the input respectively the output function of the system. C(0) - Initial condition of system, t- time. b: Time course of input function I(t), where t is time, Im, Ibf and If are hypothetical transfer rates of PCB from mother to fetus, from mother milk to infant and from normal food to infant, respectively. tbf is duration of breast feeding.
Due to small number of measured PCB serum concentrations after birth, we apply to system studied the simplest model in form of the transfer function
where G, gain of the system, is a parameter that characterizes the static properties of the system in steady state and MT (mean time) is a parameter that characterizes the dynamic properties of the system (Dedík and Ďurišová, 1999). The value 1/G is clearance of the system. Knowing values of tbf, Ibf , If and the measured PCB concentrations, the values of G and MT of the model studied could be assessed in a similar way as previously (Dedík et al., 1997).
As we do not know values of Ibf and If , we cannot estimate the gain of the system G from the measured PCB concentration-time profile and from data on duration of breast feeding tbf. We have to consider the value of parameter G equal one and the transfer function of model as
| (4) |
With respect to this we cannot estimatee clearance of the system and instead of values Ibf and If, we can assess only the limit PCB serum concentration values for the limiting case of permanent breast feeding, tbf →∞ as
and without breast feeding tbf =0
Instead of function I(t) we shall consider for entering function of the system the function L(t) for which it holds:
For analytical solution of the model ΔC(t) then holds
| (5) |
| (6) |
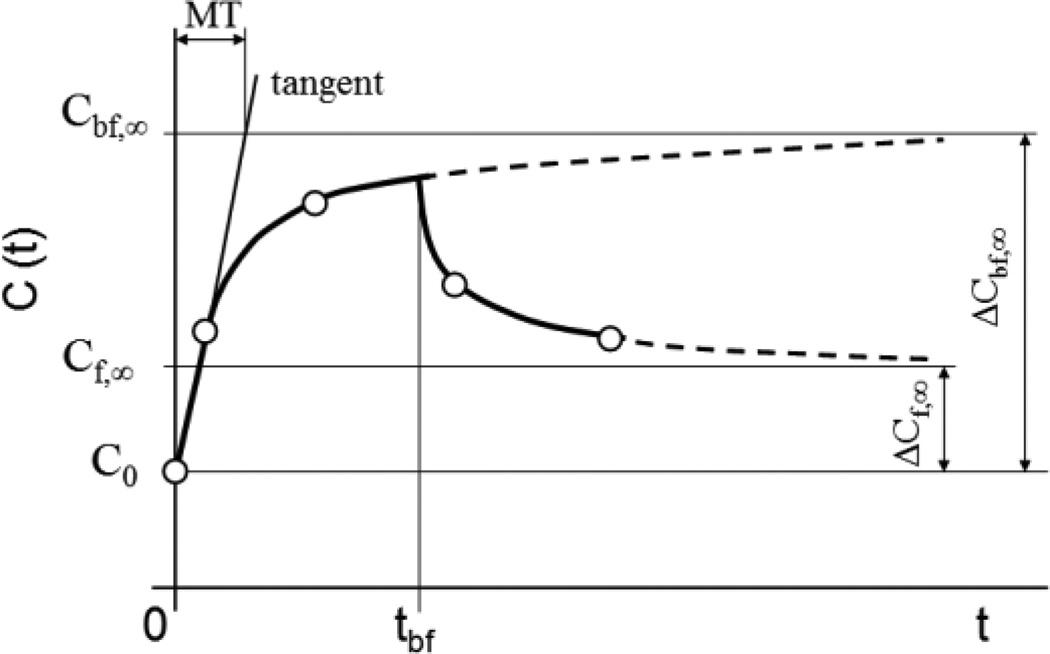
Figure 2 illustrates equations (1), (5) and (6).
Figure 2.
Illustration of system output function C(t), where t is time, and of the model parameters used for approximation of the concentration of PCB in serum of infants. tbf is duration of breast feeding, Cbf,∞ is a hypothetical limit value of PCB concentration increase for t and tbf converging to infinity and Cf,∞ is a hypothetical limit value of PCB concentration increase for t converging to infinity and tbf converging to zero and MT is mean time of PCB in the body of infants.
Vector λ of estimated model parameters will then be:
The parameters of vector λ were assessed by Monte Carlo simulations.
3. Results
Statistics on concentration of PCB 153 in cord blood serum C0 and at 6, 16 and 45 months of age (C6, C16 and C45, respectively) and duration of breast feeding tbf are shown in upper part of Table 1. The concentration data for all time intervals were positively skewed and lognormally distributed (one-sample Kolmogorov-Smirnov Test). The following weight increase in g in children of our cohort has been observed (means±SE): birth weight 3277.87±486.93, 6 months 7791.78±1098.81, 16 months 11802.13±1508.45 and 45 months 17241.94±2986.03.
Table 1.
Descriptive statistics on duration of breast feeding tbf (months) and PCB 153 serum concentration (ng/g lipids) measured in cord blood serum and venous serum at 6, 16 and 45 months of age (C0, C6, C16 and C45, respectively) (upper part) and on computed parameters MT, AUC, Cbf, ∞, Cf, ∞, Cbf, ∞ /C0, Cf, ∞/ C0 and Cf, ∞ / Cbf, ∞ describing kinetics of PCB 153 serum concentration in 93 infants from birth to month 45 of age (lower part).
| Mean±SD | Minimum | 5th percentile |
25th percentile |
Median | 75th percentile |
95th percentile |
Maximum | |
|---|---|---|---|---|---|---|---|---|
| tbf | 8.8±8.6 | 0 | 0.4 | 2.8 | 5 | 14 | 26.9 | 39 |
| C0 | 288.8±215.1 | 57.7 | 89.8 | 155 | 209.8 | 359.8 | 808.1 | 1108.4 |
| C6 | 335.7±398.5 | 1.7 | 27.7 | 107.3 | 256.6 | 384.9 | 1253.9 | 2444.9 |
| C16 | 401.8±598.9 | 6.1 | 22.5 | 81.2 | 203.1 | 467.1 | 1602.8 | 3503.7 |
| C45 | 276.5±349.6 | 16.7 | 28.5 | 74.2 | 156.9 | 326.4 | 1092.8 | 1918.8 |
| MT | 9.3±14.2 | 0.2 | 0.4 | 1.2 | 2.9 | 12 | 45.9 | 75 |
| AUC | 15621.5±21503.8 | 920 | 1515 | 4420 | 9180 | 16850 | 62480 | 124000 |
| Cbf. ∞ | 669±814 | 70 | 139.1 | 254.2 | 420.2 | 726.6 | 3485 | 4080 |
| Cf. ∞ | 313.9±358.3 | 14.3 | 25.5 | 75.1 | 168.6 | 440.8 | 1084 | 1820 |
| Cbf. ∞/C0 | 2.5±2.2 | 1 | 1 | 1.1 | 1.5 | 3.1 | 8.3 | 10.8 |
| Cf. ∞/C0 | 1.4±1.5 | 0.1 | 0.1 | 0.3 | 0.9 | 1.9 | 4.9 | 6.5 |
| Cf. ∞/Cbf. ∞ | 0.5±0.3 | 0.3 | 0.1 | 0.2 | 0.4 | 0.7 | 1.1 | 1.8 |
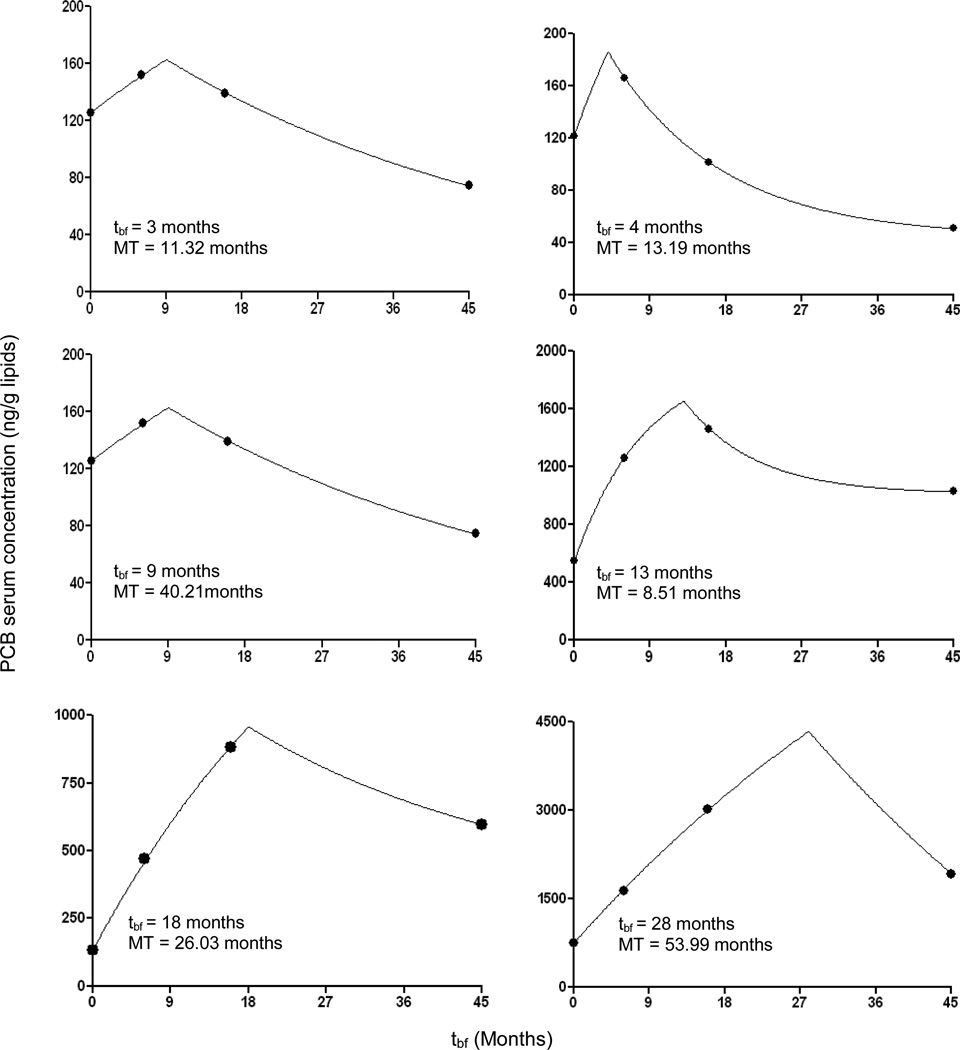
The capability of the suggested model to describe the PCB 153 serum concentration in infants with various duration of breast feeding can be seen from Figure 3. The model describes well the PCB 153 serum concentration and at the time of weaning the massive uptake of PCB 153 ceased which was reflected by the onset of serum PCB 153 decrease.
Figure 3.
The measured PCB 153 serum concentrations and the calculated curve for six children with markedly different duration of breast feeding tbf. Note the markedly different vertical scales.
Figure 4 is showing PCB 153 measured serum concentrations and the calculated approximations for 12 infants with equal duration of breast feeding of 16 months and rank ordered according to the estimated MT. It can be seen that in the 2 children in which the concentration increased after weaning, the longest MTs, 108 and 134 months, were observed. The kinetics of PCB 153 serum concentration of each child was described by parameters MT, Cbf,∞ Cf,∞ and AUC (Table 1 lower part). The individual values of Cbf,∞ and Cf,∞ were normalized with regard to C0 in order to illustrate better the kinetics of PCB 153 in the body.
Figure 4.
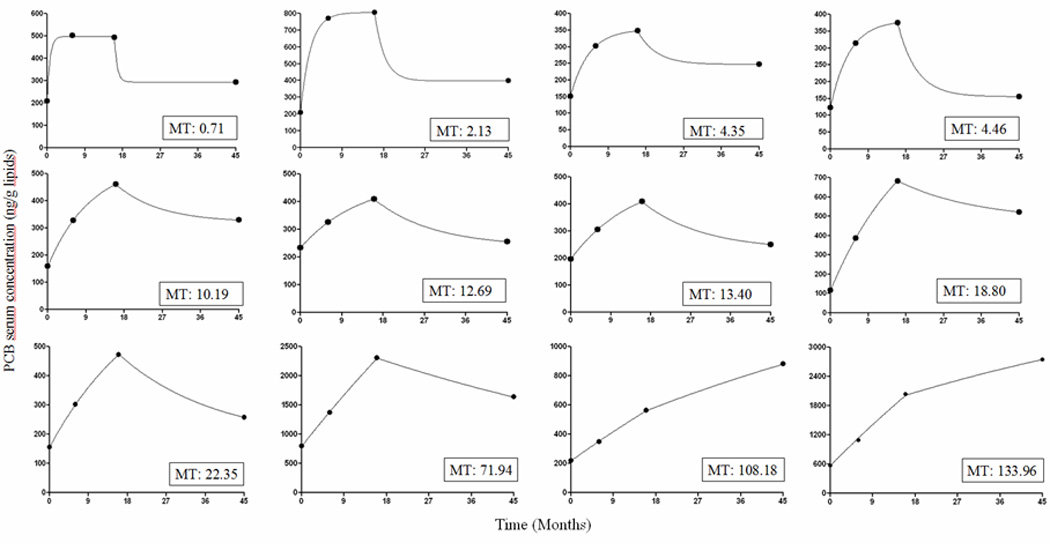
Model approximation of PCB 153 serum concentration values in 12 infants all breast fed 16 months and rank ordered according to increasing mean time (MT) in months.
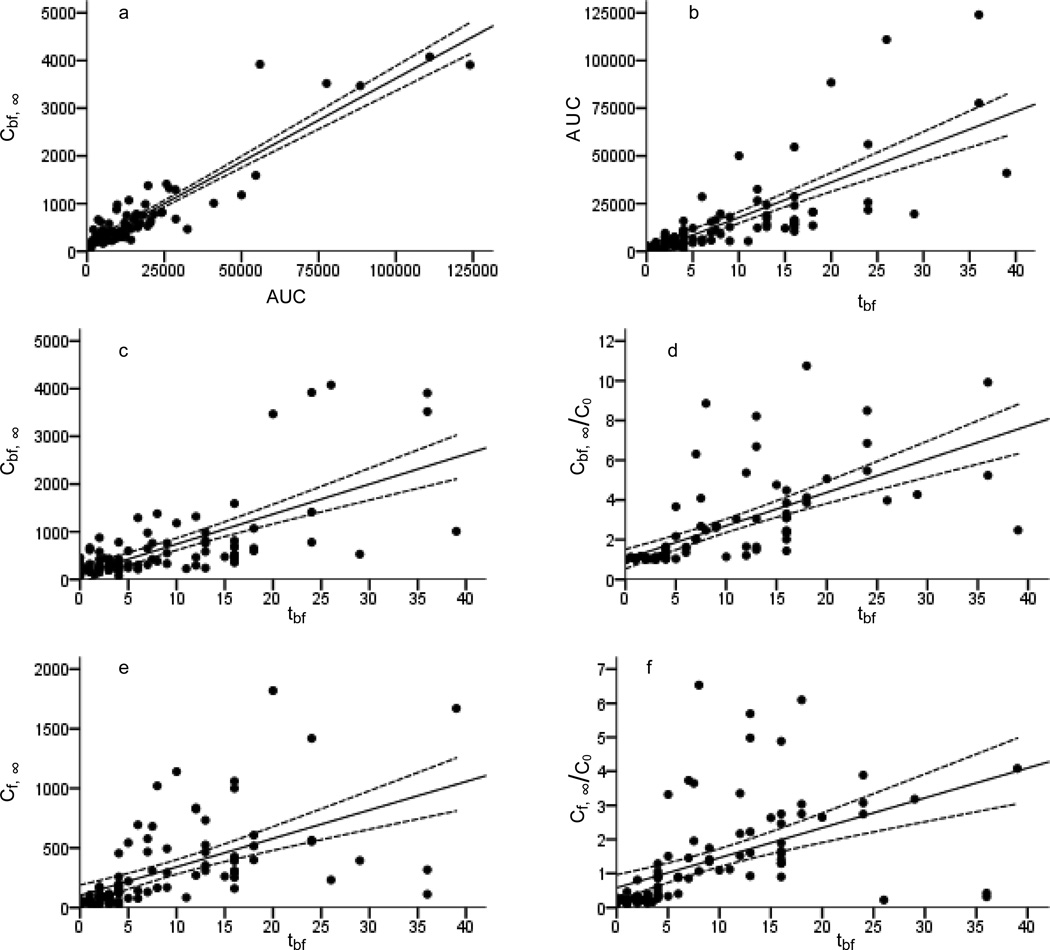
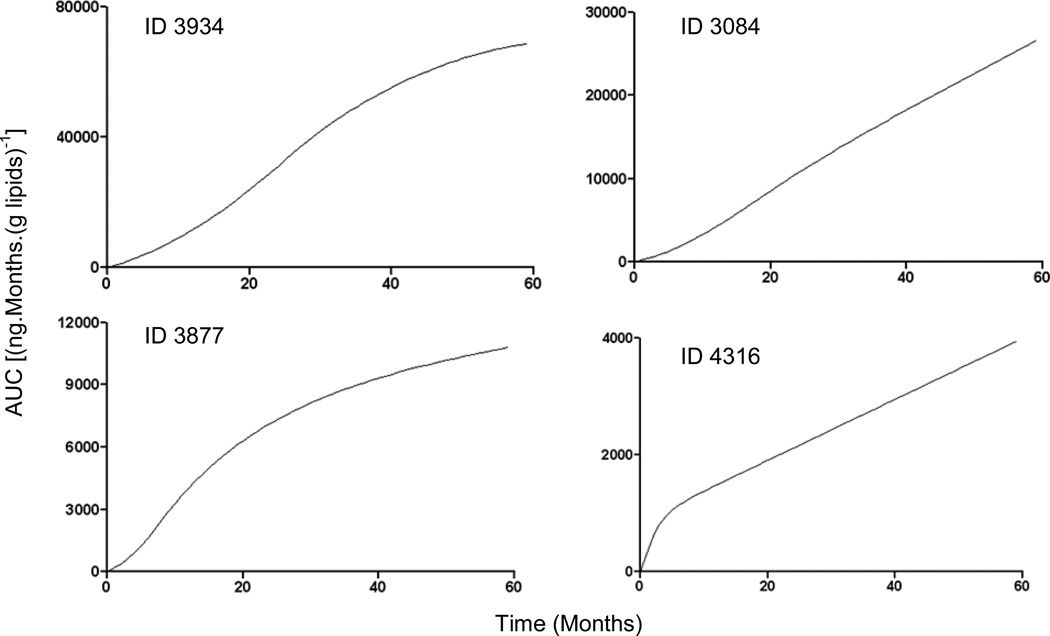
Spearman's correlations in Figure 5 show that Cbf,∞ is related to AUC (Figure 5a: r=0.84, p<0.001) and that tbf predicts AUC (Figure 5b: r=0.86, p<0.001), Cbf, ∞ (Figure 5c: r=0.64, p<0.001), Cbf, ∞/C0 (Figure 5d: r=0.85, p<0.001), Cf, ∞ ( Figure 5e: r=0.78, p<0.001) and Cf, ∞/C0 (Figure 5f: r=0.78, p<0.001). MT was related to AUC (r=0.6, p<0.001), Cbf, ∞ (r=0.55, p<0.001), Cbf, ∞/C0 (r=0.71, p<0.001), Cf, ∞ (r=0.60, p<0.001) and Cf, ∞/C0 (r=0.59, p<0.001). The ratio Cbf, ∞/Cf, ∞ for 89 infants was >1, only for 4 infants the build up of PCB 153 body burden via normal food intake was greater than via milk. The model enables estimation of an AUC for each individual child. To demonstrate this, AUC values were calculated for increasing time for 4 children with largely varying duration of breast feeding (Figure 6).
Figure 5.
Spearman's correlations between the parameter values AUC vs. Cbf, ∞ and tbf vs. AUC, Cbf, ∞, Cbf, ∞/C0, Cf, ∞ and Cf, ∞ /C0 describing kinetics of PCB 153 serum concentration in 93 infants from birth to month 45 of age.
Figure 6.
Time dependence of AUC in four children with different duration of breast feeding, tbf. For child ID=3934, 3084, 3877 and 4316 tbf was 24, 18, 7, 1 months and Cbf, inf/C0 was 11.92, 3.97, 14.4, 1.95, respectively. Note the markedly different vertical scales.
4. Discussion
The children of our cohort have been born with widely varying concentrations of PCB 153 in serum, which is reflected by the nearly 9-fold ratio of the 95th to the 5th percentiles of PCB 153 concentration. This compares well to a report summarizing 10 earlier studies which showed a range of ratios from 3.8 to 12.3 with a median of 7.5 (Longnecker et al., 2003).
The developed model approximating PCB 153 in the body of infants belongs to the category of “system models” in distinction to the “deterministic pharmacokinetic models” (Ďurišová and Dedík, 2005) and helps the analyst to understand the functionality of the system. The good fit of the model to our data, confirmed by Akaike's criterion value (Yamaoka et al., 1978), indicates that PCB very probably behaves in the body of our children in a single phase and with regard to its lipophilicity presumably in the body fat and its elimination kinetics is of first order. Our model assumes instant mixing of PCB 153 in the body and distribution into fat, linear response to changes in dose and constant intake rate during breast feeding, recently confirmed (LaKind et al., 2009). From model equations and model parameters of each subject we can calculate AUC for each child for selected time limits. Recently this approach was applied, using a physiologically based pharmacokinetic model, in a study of neurobehavioral performance in children (Verner et al., 2010). Thus the model developed here gives an opportunity to relate the health outcomes of each participant to their own individual PCB 153 exposure profile.
Our results differentiating between PCB exposures due to breast feeding and normal food intake in individual infants based purely on serial PCB serum concentration measurements, complement data of others in this direction. Direct comparison is uneasy as some authors base their estimates besides concentration measurements on inherent model assumptions or do not treat PCBs in their prediction but related polychlorinated dibenzodioxins, polychlorinated dibenzofurans or mixtures of congeners. First to be mentioned are the single time point predictions of PCB serum concentrations: Ayotte et al., (2003) explained 72% of PCB-153 plasma concentration variance at 6 months postpartum using a multivariate model that included maternal PCB 153 plasma lipid concentration, breast feeding duration, and the sum of two skin-fold thicknesses. Jacobson et al., (1989) studied determinants to PCB plasma concentration in 4-year old children and found that maternal PCB milk level and breast feeding duration jointly explained 60% of the variance. The PCB levels in cord blood and human milk and the duration of breast feeding explained the 75% variance of the plasma PCB level at 42 months (Lanting et al., 1998). In another study (Patandin et al., 1997) the duration of breast feeding, breast milk PCB concentration, and the child’s weight showed statistically significant associations with the child’s plasma lipid concentration at 3.5 years of age. Next are several studies where the PCBs body burden was modeled as a continuous variable in contrast to single time point prediction. In an earlier paper body burden of dioxin-like compounds in Inuits from birth to age 75 years was calculated using a physiologically based pharmacokinetic (PBPK) model (Ayotte et al., 1996). A similar approach and maternal blood levels made possible to predict PCB concentrations in infant blood, breast milk and cord blood (Verner et al., 2009). Our data can less easily be compared with those derived to assess exposure to polychlorinated dibenzodioxins, polychlorinated dibenzofurans or dioxin-like compounds (Kerger et al., 2007; Kreuzer et al., 1997; LaKind et al., 2000; Lorber and Phillips, 2002; Patandin et al., 1999).
A few limitations of our approach should be mentioned. First, the current model does not take into account the role of specific food sources. In an earlier work (Sonneborn et al., 2008a), we have shown that consumption of locally produced pork and other fatty foods resulted in higher concentrations of PCB levels in the mothers, as compared with those who purchased such foods from retail stores. It is possible, therefore, that these same foodstuffs might affect the child’s PCB levels as well. This might, in fact, explain the curves of those children where PCBs continued to rise after weaning. Secondly, possibly as a consequence of variability in food types consumed (specifically, amount of fat) and in the sources of those foods, the extreme variability in estimated model parameters might be overestimated. In other words, the implicit assumption of our model is that normal food consumption is uniform across the study population. Elaborating our model might narrow the range of parameter estimates.
MT is a basic model parameter in biological and technical disciplines for description of first order linear systems by first order differential equations with constant coefficients and their solution in time. It is the average time a substance spends within a specified region of space and its potential for concentration-effect assessments is obvious. It is visualized in Figure 2 as a tangent to response intersecting the baseline at t=0 (Buffham and Kropholler, 1973). In specified settings it is a measure of the rate of elimination of a xenobiotic (Karol, 1990; Veng-Pedersen, 1989a; Veng-Pedersen, 1989b). In our scenario the parameter MT reflects the rate at which the body of the child handles PCB 153 reflecting a large scale of mechanisms, including increasing fat content. Thus the greater the rate of PCB 153 serum decrease is, the smaller is the MT value. MT besides other factors may be related to activity of enzymes metabolizing PCB. We reported about high variability of PCB metabolism processes in humans (Hovander et al., 2006; Linderholm et al., 2007; Park et al., 2007; Park et al., 2008). The hypothesis whether MT is related to expression of enzymes metabolizing PCB in children, for which considerably shorter apparent half lives compared to adults were reported (Milbrath MD et al., 2009), has to be examined.
5. Conclusions
The need to compare health outcomes observed in children of our cohort, characterized by relatively high and variable PCB body burdens, with time integrated individual PCB exposures, prompted us to develop an original approach 1. customized to our time series concentration data, 2. differentiating between breast feeding and normal food PCB intake and 3. characterizing each subject’s toxicokinetic profile with a minimum model parameters enabling calculation of an area under the time curve of PCB serum concentration.
Serum PCB 153 was determined serially in infants from birth to 45 months
So far published PCB exposure assessments did not suite our longitudinal study design
Original model fitted to data describes kinetics of PCB in each subject individually
Mean residence time of PCB molecules in the body and AUCs were evaluated
It is differentiated between PCB intake by breast feeding and normal food intake
Acknowledgements
This research received support from National Institutes of Health grant R01-CA096525, from the Slovak Research and Development Agency under the contract No. LPP-0164-07 and EU projects INTARESE (no. 018385), HEIMTSA (no. 036913-2), ENVIRISK (no. 044232) and OBELIX (no. 227391) and in part by the Intramural Research Program of the National Institutes of Health, National Institute of Environmental Health Sciences.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akins JR, Waldrep K, Bernert JT., Jr The estimation of total serum lipids by a completely enzymatic ‘summation’ method. Clin. Chim. Acta. 1989;184:219–226. doi: 10.1016/0009-8981(89)90054-5. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Carrier G, Dewailly E. Health risk assessment for Inuit newborns exposed to dioxin-like compounds through breast feeding. Chemosphere. 1996;32:531–542. doi: 10.1016/0045-6535(96)00003-3. [DOI] [PubMed] [Google Scholar]
- Ayotte P, Muckle G, Jacobson JL, Jacobson SW, Dewailly E. Assessment of Pre- and Postnatal Exposure to Polychlorinated Biphenyls: Lessons from the Inuit Cohort Study. Environ. Health Perspect. 2003;111:1253–1258. doi: 10.1289/ehp.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr DB, Weihe P, Davis MD, Needham LL, Grandjean P. Serum polychlorinated biphenyl and organochlorine insecticide concentrations in a Faroese birth cohort. Chemosphere. 2006;62:1167–1182. doi: 10.1016/j.chemosphere.2005.06.063. [DOI] [PubMed] [Google Scholar]
- Brucker-Davis F, Wagner-Mahler K, Delattre I, Ducot B, Ferrari P, Bongain A, Kurzenne JY, Mas JC, Fénichel P. Cryptorchidism Study Group from Nice Area. Cryptorchidism at birth in Nice area (France) is associated with higher prenatal exposure to PCBs and DDE, as assessed by colostrum concentrations. Hum. Reprod. 2008;23:708–1718. doi: 10.1093/humrep/den186. [DOI] [PubMed] [Google Scholar]
- Buffham BA, Kropholler HW. Tracer kinetics: some general properties, the mean residence time and applications to phase and chemical equilibria. Chemical Engineering Science. 1973;28:1081–1089. [Google Scholar]
- Chevrier J, Eskenazi B, Holland N, Bradman A, Barr DB. Effects of exposure to polychlorinated biphenyls and organochlorine pesticides on thyroid function during pregnancy. Am. J. Epidemiol. 2008;168:298–310. doi: 10.1093/aje/kwn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Čonka K, Drobná B, Kočan A, Petrík J. Simple solid-phase extraction method for determination of polychlorinated biphenyls and selected organochlorine pesticides in human serum. J. Chromatogr. A. 2005;1084:33–38. doi: 10.1016/j.chroma.2004.11.029. [DOI] [PubMed] [Google Scholar]
- Dallaire F, Dewailly E, Vézina C, Muckle G, Weber JP, Bruneau S, Ayotte P. Effect of prenatal exposure to polychlorinated biphenyls on incidence of acute respiratory infections in preschool Inuit children. Environ. Health Perspect. 2006;114:1301–1305. doi: 10.1289/ehp.8683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnerud PO, Lignell S, Glynn A, Aune M, Törnkvist A, Stridsberg M. POP levels in breast milk and maternal serum and thyroid hormone levels in mother-child pairs from Uppsala. Sweden. Environ. Int. 2010;36:180–187. doi: 10.1016/j.envint.2009.11.001. [DOI] [PubMed] [Google Scholar]
- Debnath L. Integral Transforms and Their Applications. Berlin: Springer Verlag; 1995. [Google Scholar]
- Dedík L, Ďurišová M. System approach in technical, environmental, and bio-medical studies. Bratislava: Publishing House of Slovak University of Technology; 1999. 168 pp. ISBN 80-227-1176- 4, http://www.uef.sav.sk/advanced.htm. [Google Scholar]
- Dedík L, Ďurišová M, Ághová Ľ. The frequency response method used in modelling environmental systems: a working example. Ecol. Model. 1997;101:175–184. [Google Scholar]
- Ďurišová M, Dedík L. New mathematical methods in pharmacokinetic modeling. Basic Clin. Pharmacol. Toxicol. 2005;96:335–342. doi: 10.1111/j.1742-7843.2005.pto_01.x. [DOI] [PubMed] [Google Scholar]
- Glynn A, Thuvander A, Aune M, Johannisson A, Darnerud PO, Ronquist G, Cnattingius S. Immune cell counts and risks of respiratory infections among infants exposed pre- and postnatally to organochlorine compounds: a prospective study. Environ. Health. 2008;7:62. doi: 10.1186/1476-069X-7-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimalt JO, Carrizo D, Garí M, Font-Ribera L, Ribas-Fito N, Torrent M, Sunyer J. An evaluation of the sexual differences in the accumulation of organochlorine compounds in children at birth and at the age of 4 years. Environ. Res. 2010;110:244–250. doi: 10.1016/j.envres.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Grandjean P, Weihe P, Nielsen F, Budtz-Jørgensen E. Reduced antibody responses to vaccinations in children exposed to polychlorinated biphenyls. PLoS. Med. 2006;3:e311. doi: 10.1371/journal.pmed.0030311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Trnovec T, Kočan A, Charles MJ, Čižnár P, Langer P, Šovčíková E, James R. PCBs and early childhood development in Slovakia: Study design and background. Fresen. Environ. Bull. 2003;12:208–214. [Google Scholar]
- Hertz-Picciotto I, Jusko TA, Willman EJ, Baker RJ, Keller JA, Teplin SW, Charles MJ. A cohort study of in utero polychlorinated biphenyl (PCB) exposures in relation to secondary sex ratio. Environ. Health. 2008;15:37. doi: 10.1186/1476-069X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hovander L, Linderholm L, Athanasiadou M, Athanassiadis I, Bignert A, Fängström B, Kocan A, Petrik J, Trnovec T, Bergman A. Levels of PCBs and their metabolites in the serum of residents of a highly contaminated area in eastern Slovakia. Environ. Sci. Technol. 2006;40:3696–3703. doi: 10.1021/es0525657. [DOI] [PubMed] [Google Scholar]
- Jacobson JL, Humphrey HE, Jacobson SW, Schantz SL, Mullin MD, Welch R. Determinants of polychlorinated biphenyls (PCBs), polybrominated biphenyls (PBBs), and dichlorodiphenyl trichloroethane (DDT) levels in the sera of young children. Am. J. Public Health. 1989;79:1401–1404. doi: 10.2105/ajph.79.10.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karol MD. Mean residence time and the meaning of AUMC/AUC. Biopharm Drug Dispos. 1990;11:179–181. doi: 10.1002/bdd.2510110210. [DOI] [PubMed] [Google Scholar]
- Kerger BD, Leung HW, Scott PK, Paustenbach DJ. An adaptable internal dose model for risk assessment of dietary and soil dioxin exposures in young children. Toxicol. Sci. 2007;100:224–237. doi: 10.1093/toxsci/kfm199. [DOI] [PubMed] [Google Scholar]
- Kočan A, Petrík J, Drobná B, Chovancová J. Levels of PCBs and some organochlorine pesticides in the human population of selected areas of the Slovak Republic. Chemosphere. 1994;29:2315–2325. doi: 10.1016/0045-6535(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Kreuzer PE, Csanády GyA, Baur C, Kessler W, Papke O, Greim H, Filser JG. 2,3,7,8 Tetrachlorodibenzo-p-dioxin (TCDD) and congeners in infants. A toxicokinetic model of human lifetime body burden by TCDD with special emphasis on its uptake by nutrition. Arch. Toxicol. 1997;71:383–400. doi: 10.1007/s002040050402. [DOI] [PubMed] [Google Scholar]
- Laisi S, Kiviranta H, Lukinmaa PL, Vartiainen T, Alaluusua S. Molar-incisor-hypomineralisation and dioxins: new findings. Eur. Arch. Paediatr. Dent. 2008;9(4):224–227. doi: 10.1007/BF03262639. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Park CN, Naiman DQ, Gudka NJ. Methodology for characterizing distributions of incremental body burdens of 2,3,7,8-TCDD and DDE from breast milk in North American nursing infants. J. Toxicol. Environ. Health Part A. 2000;59:605–639. doi: 10.1080/009841000156628. [DOI] [PubMed] [Google Scholar]
- LaKind JS, Berlin CM, Sjödin A, Turner W, Wang RY, Needham LL, Paul IM, Stokes JL, Naiman DQ, Patterson DG., Jr Do human milk concentrations of persistent organic chemicals really decline during lactation? Chemical concentrations during lactation and milk/serum partitioning. Environ Health Perspect. 2009;117:1625–1631. doi: 10.1289/ehp.0900876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanting CI, Fidler V, Huisman M, Boersma ER. Determinants of Polychlorinated Biphenyl Levels in Plasma from 42-Month-Old Children. Arch. Environ. Contam. Toxicol. 1998;35:135–139. doi: 10.1007/s002449900360. [DOI] [PubMed] [Google Scholar]
- Linderholm L, Park JS, Kocan A, Trnovec T, Athanasiadou M, Bergman K, Hertz-Picciotto I. Maternal and cord serum exposure to PCB and DDE methyl sulfone metabolites in eastern Slovakia. Chemosphere. 2007;69:403–410. doi: 10.1016/j.chemosphere.2007.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker MP, Wolff MS, Gladen BC, Brock JW, Grandjean P, Jacobson JL, Korrick SA, Rogan WJ, Weisglas-Kuperus N, Hertz-Picciotto I, Ayotte P, Stewart P, Winneke G, Charles MJ, Jacobson SW, Dewailly E, Boersma ER, Altshul LM, Heinzow B, Pagano JJ, Jensen AA. Comparison of polychlorinated biphenyl levels across studies of human neurodevelopment. Environ. Health. Perspect. 2003;111:65–70. doi: 10.1289/ehp.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Vizcaino E, Murcia M, Llop S, Espada M, Seco V, Marco A, Rebagliato M, Grimalt JO, Ballester F. Association between thyroid hormone levels and 4,4'-DDE concentrations in pregnant women (Valencia, Spain) Environ. Res. 2009;109:479–485. doi: 10.1016/j.envres.2009.02.003. [DOI] [PubMed] [Google Scholar]
- Lorber M, Phillips L. Infant Exposure to Dioxin-like Compounds in Breast Milk. Environ. Health Perspect. 2002;110:A 325–A 332. doi: 10.1289/ehp.021100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Guo X, Graubard BI, Brock JW, Klebanoff MA, Longnecker MP. Maternal pregnancy levels of polychlorinated biphenyls and risk of hypospadias and cryptorchidism in male offspring. Environ. Health Perspect. 2009;117:1472–1476. doi: 10.1289/ehp.0800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrath MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent half-lives of dioxins, furans, and polychlorinated biphenyls as a function of age, body fat, smoking status, and breast-feeding. Environ. Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake T, Yoshinaga J, Enomoto T, Matsuda M, Wakimoto T, Ikegami M, Suzuki E, Naruse H, Yamanaka T, Shibuya N, Yasumizu T, Kato N. Thyroid hormone status of newborns in relation to in utero exposure to PCBs and hydroxylated PCB metabolites. Environ. Res. 2007;105:240–246. doi: 10.1016/j.envres.2007.03.010. [DOI] [PubMed] [Google Scholar]
- Park JS, Bergman A, Linderholm L, Athanasiadou M, Kocan A, Petrik J, Drobna B, Trnovec T, Charles MJ, Hertz-Picciotto I. Placental transfer of polychlorinated biphenyls, their hydroxylated metabolites and pentachlorophenol in pregnant women from eastern Slovakia. Chemosphere. 2008;70:1676–1684. doi: 10.1016/j.chemosphere.2007.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Linderholm L, Charles MJ, Athanasiadou M, Petrik J, Kocan A, Drobna B, Trnovec T, Bergman A, Hertz-Picciotto I. Polychlorinated biphenyls and their hydroxylated metabolites (OH-PCBS) in pregnant women from eastern Slovakia. Environ. Health Perspect. 2007;115:20–27. doi: 10.1289/ehp.8913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, Dagnelie PC, Mulder PG, Op de Coul E, van der Veen JE, Weisglas-Kuperus N, Sauer PJ. Dietary Exposure to Polychlorinated Biphenyls and Dioxins from Infancy until Adulthood: A Comparison between Breast-feeding, Toddler, and Longterm Exposure. Environ. Health Perspect. 1999;107:45–51. doi: 10.1289/ehp.9910745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patandin S, Weisglas-Kuperus N, de Ridder MA, Koopman-Esseboom C, van Staveren WA, van der Paauw CG, Sauer PJ. Plasma Polychlorinated Biphenyl Levels in Dutch Preschool Children Either Breast-Fed or Formula-Fed during Infancy. Am. J. Public Health. 1997;87:1711–1714. doi: 10.2105/ajph.87.10.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichrtová E, Ciznár P, Prachar V, Palkovicová L, Veningerová M. Cord serum immunoglobulin E related to the environmental contamination of human placentas with organochlorine compounds. Environ. Health Perspect. 1999;107:895–899. doi: 10.1289/ehp.107-1566702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roze E, Meijer L, Bakker A, Van Braeckel KN, Sauer PJ, Bos AF. Prenatal exposure to organohalogens, including brominated flame retardants, influences motor, cognitive, and behavioral performance at school age. Environ. Health Perspect. 2009;117:1953–1958. doi: 10.1289/ehp.0901015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagiv SK, Thurston SW, Bellinger DC, Tolbert PE, Altshul LM, Korrick SA. Prenatal organochlorine exposure and behaviors associated with attention deficit hyperactivity disorder in school-aged children. Am. J. Epidemiol. 2010;171:593–601. doi: 10.1093/aje/kwp427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Babinska K, Nguyen DV, Palkovicova L, Trnovec T, Kocan A, Hertz-Picciotto I. Association of PCB Exposure to Food-specific Consumption and Sources in Eastern Slovakia. J. Expo. Anal. Environ. Epidemiol. 2008a;18:86–87. doi: 10.1038/jes.2008.1. [DOI] [PubMed] [Google Scholar]
- Sonneborn D, Park HY, Petrik J, Kocan A, Palkovicova L, Trnovec T, Nguyen D, Hertz-Picciotto I. Prenatal polychlorinated biphenyl exposures in eastern Slovakia modify effects of social factors on birthweight. Paediatr. Perinat. Epidemiol. 2008b;22:202–213. doi: 10.1111/j.1365-3016.2008.00929.x. [DOI] [PubMed] [Google Scholar]
- Sunyer J, Alvarez-Pedrerol M, To-Figueras J, Ribas-Fitó N, Grimalt JO, Herrero C. Urinary porphyrin excretion in children is associated with exposure to organochlorine compounds. Environ. Health Perspect. 2008;116:1407–1410. doi: 10.1289/ehp.11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Loganath A, Chong YS, Obbard JP. Exposure to persistent organic pollutants in utero and related maternal characteristics on birth outcomes: a multivariate data analysis approach. Chemosphere. 2009;74:428–433. doi: 10.1016/j.chemosphere.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Terrell ML, Berzen AK, Small CM, Cameron LL, Wirth JJ, Marcus M. A cohort study of the association between secondary sex ratio and parental exposure to polybrominated biphenyl (PBB) and polychlorinated biphenyl (PCB) Environ. Health. 2009;8:35. doi: 10.1186/1476-069X-8-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veng-Pedersen P. Mean time parameters in pharmacokinetics. Definition, computation and clinical implications (Part I) Clin Pharmacokinet. 1989a;17:345–366. doi: 10.2165/00003088-198917050-00004. [DOI] [PubMed] [Google Scholar]
- Veng-Pedersen P. Mean time parameters in pharmacokinetics. Definition, computation and clinical implications (Part II) Clin Pharmacokinet. 1989b;17:424–440. doi: 10.2165/00003088-198917060-00005. [DOI] [PubMed] [Google Scholar]
- Verner MA, Ayotte P, Muckle G, Charbonneau M, Haddad S. A physiologically based pharmacokinetic model for the assessment of infant exposure to persistent organic pollutants in epidemiologic studies. Environ. Health Perspect. 2009;117:481–487. doi: 10.1289/ehp.0800047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verner MA, Plusquellec P, Muckle G, Ayotte P, Dewailly E, Jacobson SW, Jacobson JL, Charbonneau M, Haddad S. Alteration of infant attention and activity by polychlorinated biphenyls: unravelling critical windows of susceptibility using physiologically based pharmacokinetic modeling. Neurotoxicology. 2010;31:424–431. doi: 10.1016/j.neuro.2010.05.011. [DOI] [PubMed] [Google Scholar]
- Wang SL, Su PH, Jong SB, Guo YL, Chou WL, Päpke O. In utero exposure to dioxins and polychlorinated biphenyls and its relations to thyroid function and growth hormone in newborns. Environ. Health Perspect. 2005;113:1645–1650. doi: 10.1289/ehp.7994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Wittsiepe J, Lemm F, Ranft U, Krämer U, Fürst P, Röseler SC, Greshake M, Imöhl M, Eberwein G, Rauchfuss K, Kraft M, Winneke G. The Duisburg birth cohort study: influence of the prenatal exposure to PCDD/Fs and dioxin-like PCBs on thyroid hormone status in newborns and neurodevelopment of infants until the age of 24 months. Mutat. Res. 2008;659:83–92. doi: 10.1016/j.mrrev.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Yamaoka K, Nakagawa T, Uno T. Application of Akaike's information criterion (AIC) in the evaluation of linear pharmacokinetics equations. J. Pharmacokinet. Biopharm. 1978;6:165–175. doi: 10.1007/BF01117450. [DOI] [PubMed] [Google Scholar]
- Young J, Schwetz B, Willhite C, Kacew S. Symposium on pharmacokinetics/pharmacodynamics in the developing system and impact on risk assessment: Executive summary. J. Toxicol. Environ. Health. 1996;49:339–355. [Google Scholar]