Abstract
OBJECTIVE: To determine the predictive value of atrial natriuretic peptide (ANP), N-terminal pro-ANP (NT-proANP), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) for mortality and cardiovascular events in the general population in the absence of overt heart failure (HF).
PARTICIPANTS AND METHODS: We identified a community-based cohort of 2042 individuals. Those with stage C or D HF (n=45) and renal insufficiency (n=6) were excluded from the current study. Of the remaining individuals, 1769 (89%) underwent echocardiography and measurement of plasma ANP, NT-proANP, and NT-proBNP. Participants were followed up from January 1, 1997, to May 1, 2009, for mortality, HF, myocardial infarction (MI), and cerebrovascular accident; median follow-up was 9 years.
RESULTS: After adjustment for conventional clinical risk factors, NT-proANP had significant predictive value for mortality but not for HF, MI, or cerebrovascular accident, whereas ANP lacked any predictive value. The predictive value of NT-proANP for mortality was attenuated after adjustment for structural and functional cardiac abnormalities. In contrast, NT-proBNP had predictive value for mortality, HF, and MI after adjustment for conventional risk factors and retained significance for mortality and HF after adjustment for structural and functional cardiac abnormalities.
CONCLUSION: Our results suggest that NT-proBNP is a more robust cardiac biomarker compared with ANP or NT-proANP and is independently predictive of mortality and HF in the general population free of overt HF.
ANP = atrial natriuretic peptide; BMI = body mass index; BNP = B-type natriuretic peptide; CVA = cerebrovascular accident; HF = heart failure; HR = hazard ratio; MI = myocardial infarction; NT-proANP = N-terminal pro-ANP; NT-proBNP = N-terminal pro-BNP
The discovery of atrial natriuretic peptide (ANP) and B-type natriuretic peptide (BNP) and subsequent studies regarding their synthesis, release, and pleiotropic actions in the control of cardiorenal homeostasis have firmly established the heart as an endocrine organ. Both ANP and BNP are secreted from the heart primarily in response to myocardial stretch and as a result have diagnostic and prognostic utility among patients with heart failure (HF).1-5
In the absence of HF, we6 and others7-9 have reported that plasma BNP has independent prognostic value for mortality and cardiovascular morbidity beyond conventional cardiovascular risk factors. The predictive value of BNP beyond HF may reflect BNP release mechanisms beyond simple hemodynamics, including myocardial hypoxia and inflammation, which occur early in cardiovascular disease.10 Importantly, Wang et al7 from the Framingham Heart Study have also reported that N-terminal pro-ANP (NT-proANP) provides incremental predictive utility for mortality and cardiovascular events similar to that of BNP. Further, Lerman et al11 have previously reported the potential utility of NT-proANP as a biomarker to aid in the diagnosis of asymptomatic left ventricular dysfunction. Although these authors have asked for validation of these findings, to date, confirmation of the predictive utility of NT-proANP in an additional large, community-based study has not been reported. In addition, there are no published reports of the predictive utility of ANP in large, well-characterized community-based cohorts.
For editorial comment, see page 1143
The current study was designed to confirm and extend these previous reports.6,7,12 We sought to confirm the predictive utility of NT-proANP for mortality and cardiovascular events in the general population without HF. In addition, for the first time we evaluated the predictive utility of the biologically active ANP. Finally, we compared the diagnostic utility of ANP and NT-proANP with that of N-terminal proBNP (NT-proBNP), the most robust biomarker of the BNP assays for mortality and cardiovascular morbidity.6 We hypothesized that NT-proANP would have greater predictive utility than ANP because of its longer half-life and its greater stability in circulation. Further, we hypothesized that NT-proBNP would be a superior biomarker to NT-proANP because BNP, in contrast to ANP, is activated by hemodynamic and nonhemodynamic mechanisms in cardiovascular disease states.10,13-17
PARTICIPANTS AND METHODS
The Mayo Clinic and Olmsted Medical Center Institutional Review Boards approved this study.
The resources of the Rochester Epidemiology Project were used to identify a random sample of 2042 Olmsted County, Minnesota, residents aged 45 years or older. The design and selection criteria used in this community-based cohort study have been described previously, as have the characteristics of the Olmsted County population.18-21 Of the total sample, 45 individuals (2.2%) were excluded because of symptomatic HF (stage C and D HF by American College of Cardiology/American Heart Association guidelines22). An additional 6 individuals (0.3%) were excluded because of plasma creatinine levels greater than 2.0 mg/dL (to convert to umol/L, multiply by 88.4), which is consistent with previous reports.6,7,9 In the remaining 1991 individuals, baseline ANP, NT-proANP, and NT-proBNP levels were obtained in 1866 (94%), 1831 (92%), and 1932 (97%), respectively. Only the 1769 individuals (89%) who had assessment of all 3 assays (ANP, NT-proANP, and NT-proBNP) are included in our analysis.
Main Outcome Measures
The Rochester Epidemiology Project maintains a unified medical record including mortality data for Olmsted County. Participants were followed up from January 1, 1997, until May 1, 2009, when they were censored. This length of study provided a median (25th, 75th percentile) follow-up of 9.1 (8.5, 9.9) years for mortality. Participants were also followed up for the first incidence of HF, myocardial infarction (MI), stroke, or transient ischemic attack. Stroke and transient ischemic attack were grouped together under the term cerebrovascular accident (CVA). Analyses were restricted to participants who had never had the outcome being studied. Heart failure was defined as International Classification of Diseases, Ninth Revision (ICD-9) code 402 or 428. Cerebrovascular accident included ICD-9 codes 430-438. Myocardial infarction was defined as ICD-9 code 410 or 412. The median (25th, 75th percentile) follow-up for HF, MI, and CVA was 9.0 (7.5, 9.8), 9.0 (7.7, 9.8), and 8.9 (6.6, 9.8) years, respectively.
Doppler Echocardiography
A single echocardiologist (M.M.R.), who was blinded to natriuretic peptide values, interpreted all echocardiograms. In each participant, ejection fraction, left ventricular mass/hypertrophy, left atrial size/enlargement, and valvular stenosis/regurgitation were assessed, and diastolic function was categorized as previously described.19,23-26
Natriuretic Peptide Assays
Blood samples were collected in EDTA Vacutainers (Becton, Dickinson and Company), placed on ice, centrifuged within 2 hours, separated into multiple aliquots, and placed in a freezer at –80°C. Each assay was performed with a new aliquot that had never been thawed. Both NT-proANP and ANP were measured by radioimmunoassay as described previously.5,7 Plasma NT-proBNP was measured by electrochemiluminescence immunoassay as described previously.21,27 Interassay and intra-assay coefficients of variation for NT-proANP, ANP, and NT-proBNP were 9% and 6%, 24% and 7%, and 3.1% and 2.5%, respectively.
Statistical Analyses
Continuous variables were expressed as mean ± standard deviation and categorical variables by percentage. The Kaplan-Meier method was used to estimate survival and event-free rates. Cox proportional hazards regression was used to assess the association of outcomes with clinical and echocardiographic variables and natriuretic peptide levels. Only participants who had never had the outcome being studied were included in the analyses. Hazard ratios (HRs) were calculated from unadjusted analyses; after adjustment for age, sex, and body mass index (BMI); and from a multivariable model (multivariable model 1) with conventional cardiovascular risk factors (age, sex, BMI, diabetes mellitus, hypertension, smoking status, total cholesterol, coronary artery disease, and serum creatinine). Body mass index was analyzed as a categorical variable (<20, 20-25, 26-30, and >30). A final model (multivariable model 2) included both conventional cardiovascular risk factors and echocardiographic abnormalities (ejection fraction <50%, diastolic dysfunction, valvular dysfunction, left ventricular hypertrophy, left atrial enlargement, and wall motion abnormalities).
RESULTS
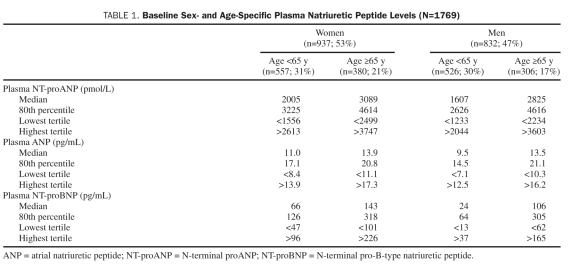
Of the 1769 participants, 937 (53%) were women. Mean age (SD) was 62 (10) years. Body mass index (SD) was 28 (5), and the incidence of hypertension and diabetes was 29% and 7%, respectively. Age- and sex-specific median, 80th percentile, and tertile values for NT-proANP, ANP, and NT-proBNP are represented in Table 1. Female sex and age greater than 65 years were associated with higher NT-proANP, ANP, and NT-proBNP values. Importantly, the NT-proBNP age- and sex-specific 80th percentile and highest tertile values are well below current HF diagnosis thresholds.21 There were 173 deaths and 162 HF, 107 MI, and 241 CVA events.
TABLE 1.
Baseline Sex- and Age-Specific Plasma Natriuretic Peptide Levels (N=1769)
N-Terminal Pro-ANP
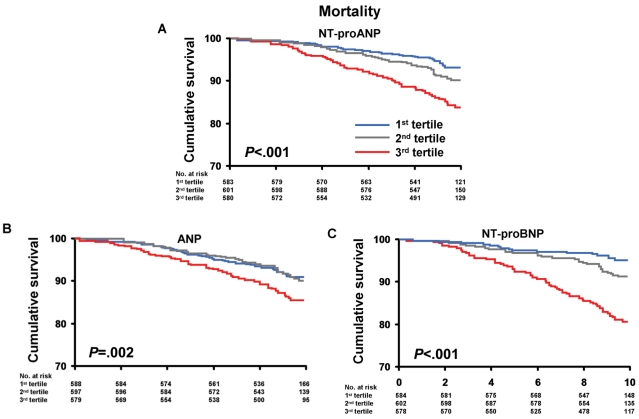
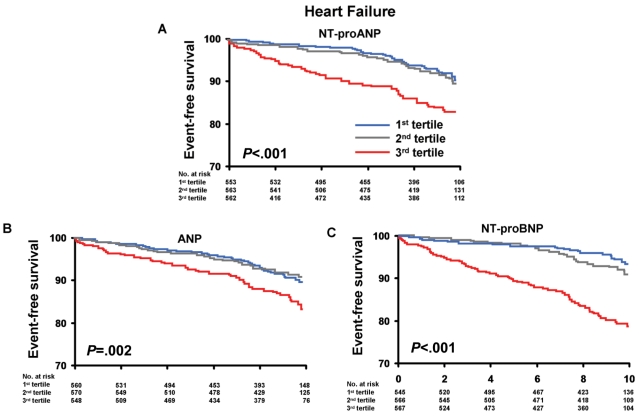
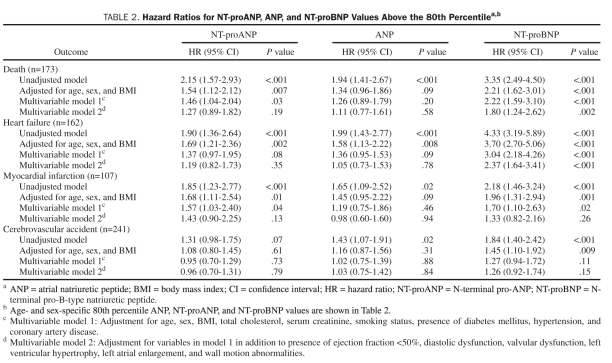
Mortality and HF according to age- and sex-specific tertiles of NT-proANP are shown in Figures 1A and 2A. The unadjusted incidence of mortality and HF events significantly increased with increasing tertiles of NT-proANP. Adjusted HRs for mortality, HF, MI, and CVA according to baseline NT-proANP (above the age- and sex-specific 80th percentile) are shown in Table 2. Models adjusted for age, sex, and BMI suggest that NT-proANP is associated with increased mortality, HF, and MI. After adjustment for conventional clinical risk factors (multivariable model 1), including hypertension, coronary artery disease, serum creatinine, and diabetes mellitus, NT-proANP retained significant predictive value for mortality. After adjustment for cardiac structural and functional abnormalities (multivariable model 2), NT-proANP lost all predictive value.
FIGURE 1.
Kaplan-Meier curves for mortality in the sample population according to age- and sex-specific tertiles of N-terminal pro-atrial natriuretic peptide (NT-proANP) (A), atrial natriuretic peptide (ANP) (B), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (C). Age- and sex-specific tertile values of NT-proANP, ANP, and NT-proBNP are shown in Table 2. P value is for trend across the tertiles.
FIGURE 2.
Kaplan-Meier curves for heart failure in the sample population according to age- and sex-specific tertiles of N-terminal pro-atrial natriuretic peptide (NT-proANP) (A), atrial natriuretic peptide (ANP) (B), and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (C). Age- and sex-specific tertile values of NT-proANP, ANP, and NT-proBNP are shown in Table 2. P value is for trend across the tertiles.
TABLE 2.
Hazard Ratios for NT-proANP, ANP, and NT-proBNP Values Above the 80th Percentilea,b
Atrial Natriuretic Peptide
Mortality and HF according to age- and sex-specific tertiles of ANP are shown in Figures 1B and 2B. As with NT-proANP, the unadjusted incidence of mortality and HF events increased with increasing tertiles of ANP. Adjusted HRs for mortality and cardiovascular morbidity according to baseline ANP are shown in Table 2. Models adjusted for age, sex, and BMI suggest that ANP is associated with increased risk of HF (but not mortality, MI, or CVA). Adjustment for conventional clinical risk factors (multivariable model 1) attenuated any significant increased risk associated with higher ANP levels for all outcomes.
N-Terminal Pro-BNP
Mortality and HF according to age- and sex-specific tertiles of NT-proBNP are shown in Figures 1C and 2C. As with NT-proANP, the unadjusted incidence of mortality and HF events significantly increased with increasing tertiles of NT-proBNP. Adjusted HRs for mortality and cardiovascular morbidity according to baseline NT-proBNP are shown in Table 2. Models adjusted for age, sex, and BMI suggest that higher NT-proBNP levels are associated with increased risk of mortality, HF, MI, and CVA. After adjustment for conventional clinical risk factors (multivariable model 1), elevated NT-proBNP remained significantly associated with increased mortality, HF, and MI (but not CVA). In multivariable model 2, which adjusts for structural and functional cardiac abnormalities in addition to conventional clinical risk factors, NT-proBNP retained predictive significance for mortality and HF (but not MI or CVA).
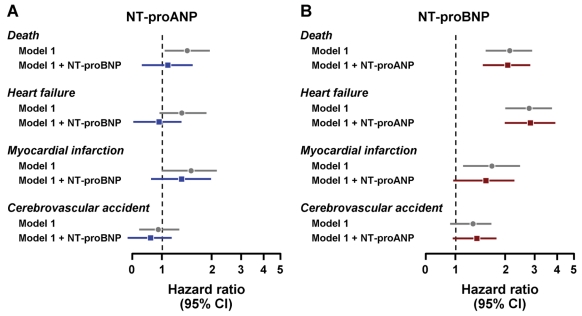
We next assessed whether the addition of NT-proBNP to multivariable models would attenuate the predictive value of an elevated NT-proANP (the more robust of the ANP-based biomarkers) for mortality and cardiovascular morbidity. When NT-proBNP was added to multivariable model 1, which adjusts for conventional clinical risk factors, all significant predictive value for NT-proANP was lost (Figure 3A). In contrast, NT-proBNP retained significant predictive value for mortality and HF (but not MI or CVA) after the addition of NT-proANP to multivariable model 1 (Figure 3B).
FIGURE 3.
Hazard ratios for N-terminal pro-atrial natriuretic peptide (NT-proANP) (A) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) (B) for mortality, heart failure, myocardial infarction, and cerebrovascular accident. Multivariable model 1 is adjusted for age, sex, body mass index, total cholesterol, serum creatinine, presence of diabetes mellitus, hypertension, and coronary artery disease. In panel A, NT-proBNP is added to multivariable model 1, and in panel B, NT-proANP is added to multivariable model 1. Data are presented as hazard ratios with 95% confidence intervals (CIs).
DISCUSSION
To our knowledge, the current study is the first to analyze and compare the predictive significance of NT-proANP, ANP, and NT-proBNP in a large, well-characterized community-based cohort without HF. Our results demonstrate that NT-proANP possesses significant predictive ability for mortality beyond conventional clinical risk factors. In contrast, ANP entirely lacked significant predictive value for mortality or cardiovascular morbidity. Importantly, minimally elevated NT-proBNP (well below levels observed in HF) was independently predictive for mortality, HF, and MI after adjustment for clinical risk factors and remained predictive for mortality and HF even after adjustment for echocardiographic structural and functional abnormalities. These findings suggest that NT-proBNP is superior to ANP and NT-proANP as a biomarker for mortality and cardiovascular morbidity in the general population without HF. Further, our results support the conclusion that mild NT-proBNP elevation may detect preclinical cardiovascular disease, which has important prognostic implications.
For the first time, in a large community-based cohort, we report the association of ANP with mortality and cardiovascular morbidity. Importantly, ANP did not provide incremental predictive value beyond age, sex, and BMI, which suggests that ANP levels are not independently associated with adverse cardiovascular events in the general population. In contrast to ANP, our results suggest that NT-proANP has significant predictive value for mortality, HF, and MI in the general population after adjustment for basic confounders. Further, adjustment for additional clinical cardiovascular risk factors (multivariable model 1) did not attenuate the predictive significance of NT-proANP for mortality. The mechanism of the more robust predictive utility of NT-proANP compared with ANP is likely due to differential processing. While NT-proANP is co-secreted with ANP into circulation in equimolar amounts, NT-proANP is not subject to the same enzymatic degradation and receptor binding as ANP.1 This results in higher and more stable levels of circulating NT-proANP compared with ANP and likely accounts for the greater predictive significance of NT-proANP compared with ANP.
When NT-proANP and NT-proBNP were compared as predictive biomarkers, NT-proBNP had independent predictive value for mortality, HF, and MI beyond conventional clinical risk factors, whereas NT-proANP was not independently predictive of HF or MI. Further, the addition of NT-proANP to multivariable model 1 (Figure 3B) did not attenuate the significant predictive value of NT-proBNP for mortality and HF. In contrast, the predictive value of NT-proANP for all outcomes was lost when NT-proBNP was added to multivariable model 1 (Figure 3A). Finally, NT-proBNP was independently predictive of mortality and HF even after adjustment for structural abnormalities of the heart (multivariable model 2), whereas the predictive significance of NT-proANP was markedly attenuated after adjustment for structural abnormalities. Taken together, these results support the conclusion that NT-proBNP is a more robust biomarker than NT-proANP in the absence of HF.
The mechanism for the greater predictive value of NT-proBNP compared with NT-proANP is likely multifactorial. One potential mechanism is related to the half-life of NT-proBNP, which is longer than that of NT-proANP, and this may result in more stable levels of NT-proBNP compared with NT-proANP. We also speculate that differential stimuli for release is an important mechanism for the greater predictive value of BNP compared with ANP. Studies suggest that ANP may be more a modulator of acute hemodynamics and blood pressure. In contrast, BNP release is stimulated by myocardial stretch but is also related to structural abnormalities including fibrosis as well as myocardial and peripheral ischemia.10, 13-17 Thus, elevation of NT-proBNP in the absence of HF may better correlate with a broader range of disease states associated with higher cardiovascular risk.
A major strength of this study is the 9 years of follow-up, in which the risk of mortality and cardiovascular morbidity grows progressively among those with elevated NT-proBNP values. This key observation strongly suggests that subtle alterations in myocardial structure and function identified by minimal NT-proBNP elevation may serve as a biomarker for preclinical cardiovascular disease and aid in identifing high-risk individuals. The implications for prevention suggest the need for studies to attempt to intervene to reduce circulating NT-proBNP in high-risk individuals to determine whether such a strategy represents an opportunity to reduce cardiovascular disease.
CONCLUSION
We report that NT-proANP is an independent biomarker for mortality but not for HF, MI, or CVA and that ANP entirely lacks independent predictive value in a large community-based cohort in the absence of HF. Furthermore, our results suggest that NT-proBNP is a more robust cardiac biomarker compared with NT-proANP and is independently predictive for mortality and cardiovascular morbidity beyond conventional clinical risk factors and structural abnormalities of the heart.
Footnotes
This work was supported by grants from the National Institutes of Health (R01 HL36634, P01 HL76611, R01 HL55502, R01 AG034676) and the Mayo Clinic. Dr Cataliotti was supported by the Doris Duke Charitable Foundation (CSDA 2006064).
REFERENCES
- 1. Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handb Exp Pharmacol. 2009(191):341–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McCullough PA, Nowak RM, McCord J, et al. B-type natriuretic peptide and clinical judgment in emergency diagnosis of heart failure: analysis from Breathing Not Properly (BNP) Multinational Study. Circulation. 2002;106(4):416–422 [DOI] [PubMed] [Google Scholar]
- 3. Maisel AS, Krishnaswamy P, Nowak RM, et al. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347(3):161–167 [DOI] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Gubens MA, Fonarow GC, Konstam MA, Stevenson LW, Shekelle PG. Cost-effectiveness of screening with B-type natriuretic peptide to identify patients with reduced left ventricular ejection fraction. J Am Coll Cardiol. 2004;43(6):1019–1026 [DOI] [PubMed] [Google Scholar]
- 5. Burnett JC, Jr, Kao PC, Hu DC, et al. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231(4742):1145–1147 [DOI] [PubMed] [Google Scholar]
- 6. McKie PM, Rodeheffer RJ, Cataliotti A, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide: biomarkers for mortality in a large community-based cohort free of heart failure. Hypertension. 2006;47(5):874–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang TJ, Larson MG, Levy D, et al. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350(7):655–663 [DOI] [PubMed] [Google Scholar]
- 8. Rosenberg J, Schou M, Gustafsson F, Badskjaer J, Hildebrandt P. Prognostic threshold levels of NT-proBNP testing in primary care. Eur Heart J. 2009;30(1):66–73 [DOI] [PubMed] [Google Scholar]
- 9. Kistorp C, Raymond I, Pedersen F, Gustafsson F, Faber J, Hildebrandt P. N-terminal pro-brain natriuretic peptide, C-reactive protein, and urinary albumin levels as predictors of mortality and cardiovascular events in older adults. JAMA. 2005;293(13):1609–1616 [DOI] [PubMed] [Google Scholar]
- 10. McKie PM, Burnett JC., Jr B-type natriuretic peptide as a biomarker beyond heart failure: speculations and opportunities. Mayo Clin Proc. 2005;80(8):1029–1036 [DOI] [PubMed] [Google Scholar]
- 11. Lerman A, Gibbons RJ, Rodeheffer RJ, et al. Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet. 1993;341(8853):1105–1109 [DOI] [PubMed] [Google Scholar]
- 12. McKie PM, Cataliotti A, Lahr BD, et al. The prognostic value of N-terminal pro-B-type natriuretic peptide for death and cardiovascular events in healthy normal and stage A/B heart failure subjects. J Am Coll Cardiol. 2010;55(19):2140–2147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kuhn M, Volker K, Schwarz K, et al. The natriuretic peptide/guanylyl cyclase: a system functions as a stress-responsive regulator of angiogenesis in mice. J Clin Invest. 2009;119(7):2019–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. de Bold AJ, Bruneau BG, Kuroski de Bold ML. Mechanical and neuroendocrine regulation of the endocrine heart. Cardiovasc Res. 1996;31(1):7–18 [PubMed] [Google Scholar]
- 15. Shimoike H, Iwai N, Kinoshita M. Differential regulation of natriuretic peptide genes in infarcted rat hearts. Clin Exp Pharmacol Physiol. 1997;24(1):23–30 [DOI] [PubMed] [Google Scholar]
- 16. Langenickel T, Pagel I, Hohnel K, Dietz R, Willenbrock R. Differential regulation of cardiac ANP and BNP mRNA in different stages of experimental heart failure. Am J Physiol Heart Circ Physiol. 2000;278(5):H1500–H1506 [DOI] [PubMed] [Google Scholar]
- 17. Hall C. NT-ProBNP: the mechanism behind the marker. J Card Fail. 2005;11(5, suppl):S81–S83 [DOI] [PubMed] [Google Scholar]
- 18. Senni M, Tribouilloy CM, Rodeheffer RJ, et al. Congestive heart failure in the community: a study of all incident cases in Olmsted County, Minnesota, in 1991. Circulation. 1998;98(21):2282–2289 [DOI] [PubMed] [Google Scholar]
- 19. Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202 [DOI] [PubMed] [Google Scholar]
- 20. Jacobsen SJ, Mahoney DW, Redfield MM, Bailey KR, Burnett JC, Jr, Rodeheffer RJ. Participation bias in a population-based echocardiography study. Ann Epidemiol. 2004;14(8):579–584 [DOI] [PubMed] [Google Scholar]
- 21. Costello-Boerrigter LC, Boerrigter G, Redfield MM, et al. Amino-terminal pro-B-type natriuretic peptide and B-type natriuretic peptide in the general community: determinants and detection of left ventricular dysfunction. J Am Coll Cardiol. 2006;47(2):345–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). Circulation. 2005;112(12):e154–e235 [DOI] [PubMed] [Google Scholar]
- 23. Nishimura RA, Tajik AJ. Evaluation of diastolic filling of left ventricle in health and disease: Doppler echocardiography is the clinician’s Rosetta Stone. J Am Coll Cardiol. 1997;30(1):8–18 [DOI] [PubMed] [Google Scholar]
- 24. Devereux RB, Alonso DR, Lutas EM, et al. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57(6):450–458 [DOI] [PubMed] [Google Scholar]
- 25. Pritchett AM, Jacobsen SJ, Mahoney DW, Rodeheffer RJ, Bailey KR, Redfield MM. Left atrial volume as an index of left atrial size: a population-based study. J Am Coll Cardiol. 2003;41(6):1036–1043 [DOI] [PubMed] [Google Scholar]
- 26. Levy D, Savage DD, Garrison RJ, Anderson KM, Kannel WB, Castelli WP. Echocardiographic criteria for left ventricular hypertrophy: the Framingham Heart Study. Am J Cardiol. 1987;59(9):956–960 [DOI] [PubMed] [Google Scholar]
- 27. Collinson PO, Barnes SC, Gaze DC, Galasko G, Lahiri A, Senior R. Analytical performance of the N terminal pro B type natriuretic peptide (NT-proBNP) assay on the Elecsys 1010 and 2010 analysers. Eur J Heart Fail. 2004;6(3):365–368 [DOI] [PubMed] [Google Scholar]