Abstract
Every year more than 500,000 patients present to the emergency department with cocaine-associated complications, most commonly chest pain. Many of these patients undergo extensive work-up and treatment. Much of the evidence regarding cocaine’s cardiovascular effects, as well as the current management of cocaine-associated chest pain and acute coronary syndromes, is anecdotally derived and based on studies written more than 2 decades ago that involved only a few patients. Newer studies have brought into question many of the commonly held theories and practices regarding the etiology, diagnosis, and treatment of this common clinical scenario. However, there continues to be a paucity of prospective, randomized trials addressing this topic as it relates to clinical outcomes. We searched PubMed for English-language articles from 1960 to 2011 using the keywords cocaine, chest pain, coronary arteries, myocardial infarction, emergency department, cardiac biomarkers, electrocardiogram, coronary computed tomography, observation unit, β-blockers, benzodiazepines, nitroglycerin, calcium channel blockers, phentolamine, and cardiomyopathy; including various combinations of these terms. We reviewed the abstracts to confirm relevance, and then full articles were extracted. References from extracted articles were also reviewed for relevant articles. In this review, we critically evaluate the limited historical evidence underlying the current teachings on cocaine’s cardiovascular effects and management of cocaine-associated chest pain. We aim to update the reader on more recent, albeit small, studies on the emergency department evaluation and clinical and pharmacologic management of cocaine-associated chest pain. Finally, we summarize recent guidelines and review an algorithm based on the current best evidence.
ACS = acute coronary syndrome; AHA = American Heart Association; CCB = calcium channel blockers; CCP = cocaine-associated chest pain; DBP = diastolic blood pressure; ECG = electrocardiography; ED = emergency department; HR = heart rate; MAP = mean arterial pressure; MI = myocardial infarction; PCI = percutaneous coronary intervention; SBP = systolic blood pressure; STEMI = ST-segment elevation myocardial infarction; TIMI = Thrombolysis in Myocardial Infarction
Every year millions of Americans abuse cocaine, many of whom present to the emergency department (ED) with cocaine-related complications, most commonly chest pain. Unfortunately, much of the current thinking on cocaine and its cardiovascular effects is either anecdotally derived or based on small studies conducted more than 2 decades ago. Recently, newer studies have brought into question many of the commonly held theories and practices regarding the etiology, diagnosis, and management of cocaine-associated chest pain (CCP) and suspected myocardial ischemia.
We searched PubMed for English-language articles from 1960 to 2011 using the keywords cocaine, chest pain, coronary arteries, myocardial infarction, emergency department, cardiac biomarkers, electrocardiogram, coronary computed tomography, observation unit, β-blockers, benzodiazepines, nitroglycerin, calcium channel blockers, phentolamine, and cardiomyopathy; including various combinations of these terms. We reviewed the abstracts to confirm relevance, and then full articles were extracted. References from extracted articles were also reviewed for relevant articles. In this review, we aim to summarize the evidence on cocaine’s cardiovascular pathophysiology, as well as the evaluation and management of CCP, with a focus on critically evaluating the older data and updating the reader on more recent, albeit still limited, developments.
A BRIEF HISTORY
Cocaine is a naturally occurring substance found in South America’s Erythroxylum coca plant that has been in use for more than 5000 years.1 It was not until the 1800s that cocaine became widely used throughout the developed world. In 1859 Albert Niemann purified cocaine in Germany, and by the early 1900s physicians were using cocaine as the first local anesthetic and prescribing it as treatment for a variety of maladies. Cocaine was also introduced into food and beverages, including the original Coca-Cola recipe.1,2 As its popularity increased, the addictive and harmful properties of cocaine became apparent, forcing the US government to pass the Harrison Narcotic Act of 1914, allowing for regulation and taxation of cocaine and limiting its distribution to prescription only.2 Its use decreased in the 1920s and 1930s but, spurred on by Hollywood glorification and the counterculture movement, regained popularity in the 1960s.1 Widespread use continued into the 1980s, when new modes of consumption were popularized in the form of freebasing and crack.3 Cocaine has remained among the most popular illicit substances used in the United States for the past 40 years.
WIDESPREAD ABUSE IN AN AGING POPULATION
Cocaine is the second most widely used illegal drug and the most common to precipitate an ED visit. According to
Article Highlights
Cocaine is the second most widely used illegal drug and the most common to precipitate a visit to the ED
Cocaine use has become increasingly common in the population older than 50 years
Cocaine affects the cardiovascular system in a variety of ways, including hemodynamic alterations, coronary vasoconstriction, hypercoagulability, endothelial dysfunction, and direct myocardial and vascular toxicity
Clinical outcomes data regarding safe and appropriate pharmacologic therapy for cocaine-induced acute coronary syndromes, including the use of β-blockers, have yet to be satisfactorily studied
First-line pharmacologic therapy for CCP includes calcium channel blockers and nitroglycerin
The use of labetalol, a combined α- and β-blocker, is controversial and remains poorly studied but may be considered in CCP patients with refractory hypertension and tachycardia
The evaluation of CCP patients remains a clinical challenge, which may be aided by the addition of observation units, myocardial perfusion imaging, and coronary computed tomography
the 2008 National Survey on Drug Use and Health,4 approximately 36.8 million Americans older than 12 years had tried cocaine at least once in their lifetime, representing 14.7% of the population 12 years and older. Approximately 5.3 million (2.1%) had used cocaine in the past year, and 1.9 million (0.7%) had used cocaine within the past month. Although there was a slight decrease in new users in 2008, there were still 722,000 persons aged 12 years or older who used cocaine for the first time.
With such a large population of users, EDs and hospitals have become inundated with patients experiencing cocaine-related complications. In 2007 illicit drug use was responsible for more than 970,000 ED visits, with cocaine responsible for more than 550,000 (57%) of these.5 This number is likely an underestimate because of patient underreporting, denial,6 and potentially undertesting. Cocaine-precipitated ED visits were widely distributed among age groups, with patient ages ranging from younger than 5 years to older than 65 years, with the highest incidence occurring in the 35- to 44-year age group, followed by the 45- to 54-year age group.5
One population in which drug abuse is often underestimated is those older than 50 years. Use of illicit drugs among older Americans has steadily increased. Since 2002 the use of illicit drugs in the 50- to 59-year-old population has almost doubled, increasing from 2.7% to 4.6% in 2008. Cocaine has emerged as the illicit drug of choice in this population, now accounting for 63.8% of all illicit drug–related ED visits in patients older than 50 years.7
PATHOPHYSIOLOGY
Systemic Cardiovascular Hemodynamics
In humans, cocaine causes an acute increase in heart rate (HR), systolic blood pressure (SBP), diastolic blood pressure (DBP), and mean arterial pressure (MAP), although in some studies this effect has been modest.8,9 The increase is initially dose dependent,8-11 followed by an acute tachyphylaxis in which HR and blood pressure plateau, even with increasingly larger quantities of cocaine and increasing serum cocaine concentrations.9,11 Animal models have demonstrated that cocaine decreases the reuptake of norepinephrine and dopamine, thereby increasing its plasma concentration and causing increased sympathetic stimulation,12,13 but one human study questions this.
In 2002, Tuncel et al14 compared intrabrachial cocaine infusion, which eliminates the systemic baroreceptor response, with intranasal cocaine. Intrabrachial cocaine caused the expected increase in norepinephrine concentration, increased forearm vascular resistance, and decreased forearm blood flow, with no effect on MAP. However, with intranasal administration the opposite occurred, with subsequent decreases in forearm vascular resistance and increased forearm blood flow and MAP. In these patients, sympathetic nerve activity showed a mean ± SD decrease of 72%±7% (P<.01), likely a response to elevated MAP, whereas norepinephrine concentrations remained unchanged when compared with precocaine levels. That study demonstrated that intranasal administration of cocaine, while causing an increase in MAP, actually produces no change in norepinephrine concentration and a decrease in vascular resistance due to the baroreceptor response.
Coronary Artery Effects
Cocaine has been shown to have a direct effect on coronary vasoconstriction. Several studies have looked at coronary arteries after cocaine administration and have demonstrated a decrease in diameter, ranging from 11% to 45%,15-17 with greater constriction occurring in arteries with preexisting atherosclerotic disease15 and when combined with cigarette smoking.16 However, in the absence of plaque rupture and/or acute thrombosis, cocaine-induced vasoconstriction does not appear to cause complete coronary occlusion. Cocaine abuse has also been associated with accelerated coronary atherosclerosis18 and an increased prevalence of coronary artery aneurysms when compared with controls.19
Prothrombotic Effects
In addition to systemic and coronary vascular changes, cocaine has been found to cause alterations in platelet function and coagulation, with multiple cases reported of acute coronary artery thrombosis after cocaine use.20-22
Cocaine activates circulating platelets by increasing expression of platelet factor 4, thromboglobulin B, and P-selectin.23,24 Elevated levels of C-reactive protein, von Willebrand factor, and fibrinogen also affect coagulation, with no compensatory increase in fibrinolytic activity.25 Cocaine has also been shown to induce endothelial dysfunction by increasing the production and expression of endothelin 1, with concurrent decrease in nitric oxide production that leads to enhanced endothelial activation, platelet activation, and leukocyte adhesion.26,27 Moreover, cigarette smoking causes endothelial and platelet dysfunction28 and when used concurrently with cocaine may potentiate its harmful effects.
Myocardial Toxicity
Cocaine has also been shown to induce direct myocyte damage and cell death in the absence of myocardial ischemia. Endomyocardial biopsy of humans after cocaine abuse has revealed mononuclear inflammatory cell infiltration, contraction band, coagulative necrosis, and in some cases focal myocarditis.29,30 The mechanism of cellular injury is still not completely understood, but multiple theories of injury have been suggested from animal studies. Recently, Fan et al31 demonstrated that cocaine increases reactive oxygen species production, leading to severe oxidative damage and myocyte cell death. Chen et al32 found that cocaine leads to an upregulation of cellular adhesion molecules, which could explain the inflammatory changes that have been demonstrated in human myocardial biopsy specimens. These cumulative effects in long-term cocaine abusers may be responsible for the development of cardiomyopathy in some.33
Acute Aortic Syndromes
The aorta’s intima is susceptible to sheer force injury from profound hypertension and tachycardia that may accompany cocaine abuse. Cocaine has also been shown to cause decreased aortic elasticity34 and increased aortic vascular smooth muscle cell apoptosis.35 Furthermore, the accelerated atherosclerosis that occurs in long-term cocaine abusers, as well as those with concomitant smoking, may predispose cocaine-abusing patients to acute aortic syndromes.36
Hsue et al37 reported their 20-year experience in an inner-city hospital: 14 (37%) of 38 patients reported cocaine abuse preceding their aortic dissection. Preexisting hypertension and being a younger black male in an inner-city population appeared to correlate with their seemingly high incidence. Daniel et al38 reported their 15-year experience: 16 (9.8%) of 164 individuals had used cocaine within 24 hours. The International Registry for Aortic Dissection reported that only 5 (0.5%) of 921 cases of aortic dissection were associated with cocaine use, and 4 of these were descending aortic dissections.36
CCP and MI
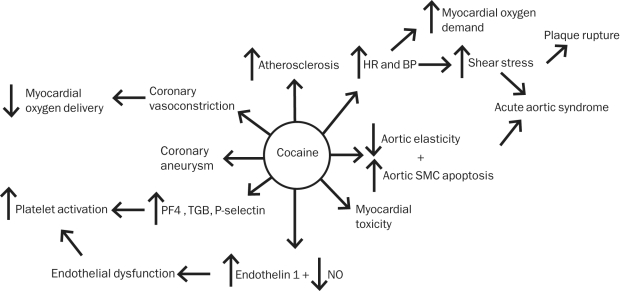
Cocaine’s myriad effects on the cardiovascular system all serve to increase the risk of myocardial ischemia (Figure 1). Alterations in cardiovascular hemodynamics, including increased HR and blood pressure, lead to an increase in myocardial oxygen demand and shear stress, which may increase the risk of plaque rupture. Concurrently, coronary vasoconstriction causes a decrease in oxygen delivery. Both platelet and endothelial dysfunction lead to a transient hypercoagulable state, increasing the risk of coronary artery thrombosis and platelet aggregation, further decreasing cardiac perfusion, and increasing the risk of acute coronary syndrome (ACS).
FIGURE 1.
Cocaine’s pathologic cardiovascular effects that lead to chest pain. BP = blood pressure; HR = heart rate; NO = nitric oxide; PF4 = platelet factor 4; SMC = smooth muscle cell; TGB = thromboglobulin B.
The reported frequency of patients presenting with CCP with actual myocardial infarction (MI) varied considerably among studies, ranging from 0.7% to 31%; however, most investigators have found a prevalence between 0.7% and 6%.39-41
The association between MI and cocaine use within 1 to 2 hours of presentation appears to be unequivocal and parallels the physiologic effects and serum drug levels. In one study, cocaine was shown to elevate the relative risk of MI 23.7 times during the first hour after consumption.42 Most investigators have shown that most cardiovascular complications arising from cocaine use occur within 12 hours of presentation,43 but it is likely that ischemic events days after intoxication can also be attributed to active cocaine metabolites.
Cocaine reaches peak blood levels at various times, depending on the method of administration. With intranasal cocaine, peak blood levels are reached in 40 to 90 minutes, with an average half-life of 75 minutes. When cocaine is smoked, peak blood levels are reached in only 3 minutes, with a half-life of 88 minutes.42 Cocaine’s clearance is almost entirely due to metabolic breakdown to benzoylecgonine and ecgonine methyl ester.32 These metabolites are detectable for more than 48 hours after cocaine administration and have been shown to be metabolically active on the cardiovascular system, causing an increase in MAP, coronary artery vasoconstriction, and widening of the QRS complex even as serum levels of cocaine are decreasing or become undetectable.44,45 In the setting of concomitant alcohol use, cocaine’s effects are prolonged and enhanced due to slowed clearance and the creation of an additional active metabolite, cocaethylene.46-49
In a retrospective European study of 479 ACS patients younger than 50 years admitted to a critical care unit from 2001 to 2008,50 a total of 24 patients (5%) had admitted to recent cocaine abuse or tested positive on urine drug screening. These patients had a higher troponin level (52.9 vs 23.4 ng/mL; P<.001), lower left ventricular ejection fraction (44.5% vs 52.2%; P=.049), and increased mortality (8.3 vs 0.8%; P=.03) compared with ACS patients who did not use cocaine.
In addition to myocardial ischemia and MI, the differential diagnosis of CCP includes musculoskeletal pain, pneumonia, pneumothorax, myocarditis, endocarditis, pulmonary embolus, and aortic dissection.51
EVALUATION OF CCP
With more than 500,000 patients presenting annually to the ED with cocaine-associated complications, most commonly CCP, an accurate and efficient evaluation and diagnostic strategy remain a clinical challenge.
Patient history
The diagnosis of myocardial ischemia in patients with recent cocaine use by patient history alone is difficult, with many of the common clinical signs and symptoms of MI being mimicked by cocaine use itself. Chest pain is the most common symptom described by cocaine users, but diaphoresis and shortness of breath are also frequent.41 However, in CCP patients, these symptoms have not been shown to have any predictive value in diagnosing MI.40
Electrocardiogram
Electrocardiographic (ECG) changes have been examined in patients with CCP to determine their diagnostic value. The Cocaine Associated Chest Pain Study Group40 looked at the ECGs of 242 patients with CCP and found the sensitivity for diagnosing MI to be 35.7%, with a specificity of 89.9% and a positive predictive value of 17.9%. Another study, by Amin et al,52 found a similarly low positive predictive value in diagnosing acute MI. These studies demonstrate that, although the ECG has utility in this setting, its poor sensitivity and positive predictive value make it unreliable in accurately distinguishing benign CCP from actual ischemia. Further complicating the picture is the frequency of normal variant ECG patterns, such as early repolarization in younger patients,53 that can lead to misinterpretation.54
Cardiac Biomarkers
The sensitivity and specificity of cardiac biomarkers in diagnosing MI in the setting of recent cocaine use have also been examined. Studies have shown that the specificity of creatine kinase MB and myoglobin in diagnosing MI may be decreased in the setting of cocaine use, but the specificity of troponin is not affected.55 Patients with CCP who have an elevated troponin concentration have a high incidence of underlying coronary disease.56,57 Although troponin elevation is highly specific for myocyte injury in any clinical scenario, it does not indicate the mechanism of injury, which may include plaque rupture, coronary vasoconstriction that leads to decreased oxygen delivery, demand ischemia on preexisting atherosclerosis, acute inflammation, direct myocardial toxicity, or all of these.58
TIMI Risk Stratification
Chase et al59 examined the utility of applying the validated Thrombolysis in Myocardial Infarction (TIMI) risk score as a way to predict which patients with CCP are at high risk of adverse cardiovascular events. More than half of the composite cardiovascular outcomes occurred in patients with a TIMI score of 1 or less, and the conclusion was that the TIMI risk score had no clinically useful predictive value in this particular patient population.
Observation Unit
Because the incidence of acute MI in CCP appears to be relatively low (0.7%-6%), the onset of complications is typically within 12 hours, the history and ECG are often unreliable, and the TIMI risk stratification is ineffective, the use of an observation unit has been suggested as a cost-effective and safe management option. Two groups of authors have looked at outcomes of low- to intermediate-risk patients discharged from an observation unit. Weber et al60 found that of 302 patients, 1.6% experienced an MI during the 30-day follow-up. Cunningham et al61 found that of the 219 patients followed up for 1 year, no MIs were reported.
Future Diagnostic Options
Rest myocardial perfusion imaging has been recently studied as a way to predict significant underlying coronary disease in patients with CCP. Kontos et al62 performed early rest perfusion imaging on 216 low- to moderate-risk patients presenting with acute CCP. Only 5 patients had positive results, 2 of whom experienced subsequent elevation of cardiac troponin levels. Of the 211 patients with negative scans, none were subsequently diagnosed as having MI, and at 30-day follow-up, there were no adverse cardiovascular events in patients with negative imaging results.
Coronary computed tomography may have a role in treating patients who present with CCP. With its high negative predictive value, coronary computed tomography has been shown to be highly correlative with cardiac catheterization results63 and could be used to help identify patients at low risk of ACSs. In a recent prospective study, Walsh et al64 examined 59 patients presenting with CCP. All patients had a nonischemic ECG, TIMI risk score of 2 or less, and computed tomography results showing less than 50% coronary artery stenosis. At 30-day follow-up there were no reports of fatal MI or death in any of the patients.
PHARMACOLOGIC MANAGEMENT OF CCP AND MYOCARDIAL ISCHEMIA
Antiplatelet Therapy
Aspirin is recommended in all patients without contraindications in whom an ACS is suspected.58,65 Aspirin has never been examined specifically in patients presenting with CCP, but given the data demonstrating cocaine’s prothrombotic effects on platelet and endothelial function, it is reasonable to use aspirin. Further research of antiplatelet therapy with thienopyridines, glycoprotein IIB/IIIA inhibitors, and direct endothelin antagonists is warranted; however, decisions on their clinical use in CCP should follow similar guidelines extrapolated from a general ACS population.
Benzodiazepines and Nitroglycerin
Benzodiazepines with their anxiolytic properties66 may help decrease adrenergic stimulation in CCP. A 2008 scientific statement from the American Heart Association (AHA) Acute Cardiac Care Committee of the Council on Clinical Cardiology recommended early consideration of benzodiazepine use in patients with CCP.67 In a small, prospective study, nitroglycerin reversed coronary artery vasoconstriction in 9 patients.15 In addition to data from animal studies and case reports,66 2 randomized, prospective studies have compared benzodiazepine and nitroglycerin therapy in CCP patients.
Baumann et al68 found that both benzodiazepines and nitroglycerin when given to CCP patients improved chest pain and decreased cardiac output and cardiac index, but no significant difference was found between the 2 classes and no benefit was found for combination therapy. Of the 40 patients included in the study, only 5 were eventually diagnosed as having an ACS.
Honderick et al69 compared combination therapy of benzodiazepines and nitroglycerin vs nitroglycerin alone in the treatment of CCP. They concluded that combination therapy was more efficacious than nitroglycerin alone in relieving chest pain. Although the patients experienced symptomatic relief, none of the 27 patients were diagnosed as having ACS; thus, it is difficult to extrapolate the findings to a higher-risk CCP patient population.
These studies show significant symptomatic relief and favorable hemodynamic changes from both benzodiazepines and nitroglycerin. However, both studies were underpowered and significantly limited by the fact that only 5 of 67 patients were eventually diagnosed as having ACS, and neither study analyzed major adverse cardiovascular outcomes.
Calcium Channel Blockers
Two studies have evaluated calcium channel blockers (CCBs) in humans in the setting of recent cocaine administration. One study showed improvement of coronary vasoconstriction with diltiazem, and the other showed improved hemodynamics with no adverse outcomes with verapamil.70,71 The remainder of the data regarding this class of drugs are based on animal models, which have produced contradictory results.72,73 Currently, CCBs combined with nitroglycerin are recommended for the treatment of CCP patients with ST-segment elevation or depression accompanying ischemic chest discomfort. Caution is advised in patients with left ventricular dysfunction, and CCBs should be avoided in those with congestive heart failure and/or low output states.58
β-Blockers
In most patients with ACSs, β-blockers have been shown in multiple randomized trials to decrease morbidity and mortality and are recommended orally within the first 24 hours in the absence of contraindications.58,65,74,75 Recent cocaine abuse is considered one such contraindication because of concern for precipitating coronary vasoconstriction due to unopposed α-receptor stimulation.67 Only 2 human studies have prospectively evaluated the direct coronary effects of β-blockers after cocaine use.17,76 In the first study published in 1990, Lange et al76 administered intracoronary propranolol after intranasal cocaine to 10 patients and observed a significant increase in coronary vascular resistance and a decrease in coronary sinus blood flow. In the second study published in 1993, Boehrer et al17 compared intravenous labetalol, a combined α- and β-blocker, vs saline after cocaine administration and found that the 9 patients who received labetalol showed no decrease in the cross-sectional area of the coronary artery. Animal studies, including canine and porcine models, also suggested a negative effect with propranolol and a neutral effect with labetalol.77-79 These limited data from only 2 human studies involving a total of 19 patients provide the basis for avoidance of β-blockers in patients with CCP listed in current guidelines and scientific statements.58,67
Regarding systemic cardiovascular hemodynamics, most of the data with β-blockers in the setting of recent cocaine use are derived from case reports, animal studies, and small retrospective case studies and include both selective and nonselective β-blockers. The results are inconsistent with studies that show both improvement80-83 and worsening84-86 of hemodynamics.
Because of significant patient underreporting or denial6 and often delayed results from urine drug screening, CCP patients may receive β-blockers before the treating physician is aware of the cocaine abuse. In a retrospective study by Dattilo et al,83 17% of 363 cocaine-positive patients received β-blockers (66% received metoprolol, atenolol, or propranolol; 21% received combined α- and β-blockers [labetalol or carvedilol]; and 13% received both types). Any type of β-blocker administration was associated with a significantly lower incidence of MI. Rangel et al82 retrospectively studied 331 patients with cocaine-positive urine drug screen results, of which 151 (46%) received β-blockers (metoprolol in 85%). β-Blocker therapy was associated with a significantly greater decrease in SBP, with no difference in the rate of adverse outcomes, peak troponin levels, or ECG changes. There was a trend toward decreased posthospitalization mortality.
In a subsequent randomized trial of CCP patients given the combined α- and β-blocker labetolol or diltiazem, Hoskins et al70 found that both medications were associated with a similar and statistically significant decrease in blood pressure and HR, with no adverse outcomes.
Carvedilol, another combined α- and β-blocker, has been shown to be beneficial in the setting of acute MI when given intravenously followed by oral dosing.54 In stable patients without chest pain, carvedilol given orally before crack cocaine administration appeared to reduce HR, SBP, and DBP.87 Carvedilol has never been studied in a CCP population, but because of its higher ratio of β:α blockade compared with labetalol (10:1 vs 4:1),53 it theoretically may not be as safe.
With regard to the safe timing of delayed β-blocker therapy in the days after exposure to cocaine, no data are available. Given the extensive active metabolites that are detectable for up to 48 hours after cocaine use,44,45 caution with β-blockade during this time is reasonable.
Phentolamine
Phentolamine, a direct α-receptor antagonist, has been shown in case reports to relieve chest pain in patients presenting with CCP.88,89 In one prospective study it was shown to reverse cocaine-induced coronary artery vasoconstriction in 6 patients when given as an intracoronary infusion.90 More research is required before phentolamine can be recommended as a first-line agent.
Novel Medical Therapy
Dexmedetomidine is a centrally acting α2-agonist that may be a novel therapeutic agent to treat cocaine-induced hypertension and tachycardia and possibly reduce the incidence of cocaine-induced MI. A report published by Menon et al91 examined dexmedetomidine compared with placebo in patients after cocaine administration. They found that dexmedetomidine reversed increases in sympathetic nerve activity, HR, and SBP.
THROMBOLYSIS AND PERCUTANEOUS CORONARY INTERVENTION IN COCAINE-INDUCED ST-SEGMENT ELEVATION MYOCARDIAL INFARCTION
The decision to use fibrinolytics or percutaneous coronary intervention (PCI) in cocaine-associated ST-segment elevation myocardial infarction (STEMI) should generally follow traditional STEMI therapy with one caveat. Medical therapy with nitroglycerin or CCBs should be administered first, and urgent revascularization procedures should be performed only if there is no immediate resolution of ST-segment elevation.58,65 Although PCI is generally superior to fibrinolytics in STEMI,65 a comparison has never been performed in cocaine abusers. Thrombolysis has been given successfully in this patient population,92 but life-threatening complications have also been reported.93 For those receiving stents, increased rates of in-stent thrombosis have been reported, which has been attributed to antiplatelet therapy nonadherence and/or continued drug use.94,95 Therefore, if stenting is performed, preference should be given to baremetal stents over drug-eluting stents to limit the duration of mandated dual-antiplatelet therapy.96
SUMMARY OF GUIDELINES AND SCIENTIFIC STATEMENTS
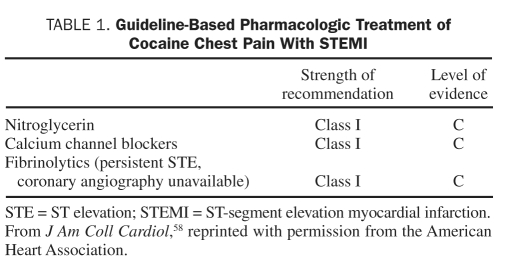
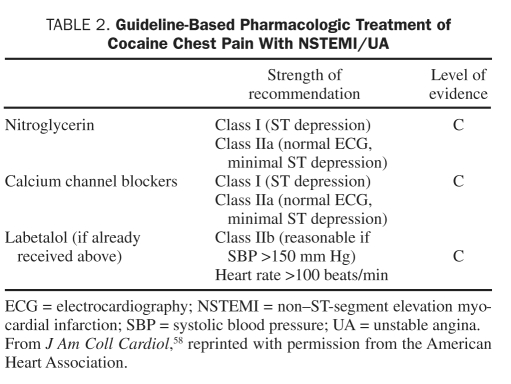
In the 2011 American College of Cardiology Foundation/AHA Focused Update Incorporated Into the American College of Cardiology/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non-STEMI,58 no changes were made to the treatment of CCP patients with suspected MI. These guidelines recommend that patients who present with signs and symptoms suggestive of ischemia first be assessed for the likelihood that their symptoms represent an ACS. A history of cocaine abuse in the absence of other higher-risk features suggests a lower risk. The guidelines classify treatment recommendations based on the relationship between benefit and risk, as well as the level of evidence from the literature. Class I recommendations imply that the benefit far outweighs the risk, class IIa suggests a significant benefit, class IIb implies efficacy is less well established, and class III indicates that the therapy is harmful. The level of evidence estimates the certainty of treatment effect with level A based on multiple randomized trials or meta-analyses, level B based on a single randomized trial or nonrandomized data, and level C based on expert opinion. The recommendations regarding management of CCP are all of evidence level C, reflecting the overall lack of data on this topic (Tables 1 and 2).
TABLE 1.
Guideline-Based Pharmacologic Treatment of Cocaine Chest Pain With STEMI
TABLE 2.
Guideline-Based Pharmacologic Treatment of Cocaine Chest Pain With NSTEMI/UA
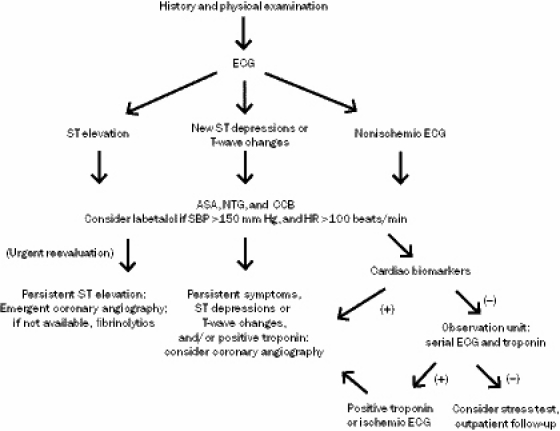
Patients with ST-segment elevation or depressions on ECG initially should receive nitroglycerin and CCBs (class I, evidence level C). Patients with persistent chest discomfort and ST-segment elevation should receive fibrinolytic therapy or, if a catheterization laboratory is available, undergo coronary angiography, with PCI if a significant flow-limiting lesion is detected (class I, evidence level C). Patients with CCP and a normal ECG or minimal ST-segment deviation suggestive of ischemia should also be given nitroglycerin and CCBs (class IIa, evidence level C). Coronary angiography is recommended in patients with new ST depressions or isolated T-wave changes that are refractory to nitroglycerin and CCB therapy (class IIa, evidence level C). Combined α- and β-blockers are considered reasonable for CCP with hypertension (SBP >150 mm Hg) and tachycardia (HR >100 beats/min) only if nitroglycerin or a CCB has been recently given (class IIb, evidence level C) (Figure 2).
FIGURE 2.
Evaluation and management of cocaine-associated chest pain. ASA = aspirin; CCB = calcium channel blocker; ECG = electrocardiography; HR = heart rate; NTG = nitroglycerin; SBP = systolic blood pressure.
These guidelines differ slightly from the 2008 scientific statement from the AHA Acute Cardiac Care Committee of the Council on Clinical Cardiology regarding management of CCP.67 The 2008 scientific statement gives benzodiazepines a class I, evidence level C rating and phentolamine a class IIb, evidence level C rating; neither medication is mentioned in the updated 2011 guidelines.58 Also in the 2008 scientific statement, CCBs are listed as class IIb, evidence level C, whereas nitroglycerin is class I, evidence level B, and labetolol and β-blockers in general are listed as class III, evidence level C.
CONCLUSION
Cocaine has been used for more than 5000 years. Its abuse remains prevalent and increasingly so among older patients, with large numbers of patients presenting to the ED each year with CCP. Cocaine increases SBP and HR and induces coronary vasoconstriction. In addition, it leads to a prothrombotic state, direct myocardial toxicity, and accelerated atherosclerosis; however, our understanding of the underlying mechanisms remains limited. Despite these pathologic alterations, the incidence of MI is relatively low. Standard monitoring with ECG and serial troponins in an observation unit appears to be safe, and although few data exist, treatment with aspirin, CCBs, and nitroglycerin is recommended. Whether β-blockers are clinically harmful or beneficial requires investigation, and these agents should be considered only when combined with α-blockade. Because of cocaine’s social stigma, an overall assumed minority of patients with cocaine-induced cardiovascular complications, and the lack of financial incentives, large prospective, randomized trials will likely never be performed.
REFERENCES
- 1. Gold M. Cocaine. New York, NY: Plenum Publishing Corporation; 1993:11–24 [Google Scholar]
- 2. Bock G. Cocaine: Scientific and Social Dimensions. New York, NY: John Wiley and Sons Inc; 1992:7–19 [Google Scholar]
- 3. History of Cocaine. http://www.historyofcocaine.net/ Accessed August 16, 2011
- 4. US Department of Health and Human Services (DHS); Office of Applied Studies Results from the 2008 National Survey on Drug Use and Health: National Findings. http://oas.samhsa.gov/nsduh/2k8nsduh/2k8Results.cfm Accessed August 16, 2011
- 5. US Department of Health and Human Services (DHS), Substance Abuse and Mental Health Service Administration, Office of Applied Studies Drug Abuse Warning Network, 2007: National Estimates of Drug-Related Emergency Department Visits. Rockville, MD: 2010. https://dawninfo.samhsa.gov/files/ED2007/DAWN2k7ED.pdf Accessed August 16, 2011 [Google Scholar]
- 6. Harrison L. The validity of self-reported drug use in survey research: an overview and critique of research methods. NIDA Res Monogr. 1997;167:17–36 [PubMed] [Google Scholar]
- 7. US Department of Health and Human Services (DHS), Substance Abuse and Mental Health Service Administration, Office of Applied Studies The DAWN Report: Emergency Department Visits Involving Illicit Drug Use by Older Adults: 2008. Rockville, MD: Published September 9, 2010. http://oas.samhsa.gov/2k10/DAWN015/IllicitAbuseHTML.pdf Accessed August 16, 2011 [Google Scholar]
- 8. Boehrer JD, Moliterno DJ, Willard JE, et al. Hemodynamic effects of intranasal cocaine in humans. J Am Coll Cardiol. 1992;20:90–93 [DOI] [PubMed] [Google Scholar]
- 9. Foltin RW, Ward AS, Haney M, et al. The effects of escalating doses of smoked cocaine in humans. Drug Alcohol Depend. 2003;70:149–157 [DOI] [PubMed] [Google Scholar]
- 10. Resnick RB, Kestenbaum RS, Schwartz ELK. Acute systemic effects of cocaine in man: a controlled study by intranasal and intravenous routes. Science. 1977;195(4279):696–698 [DOI] [PubMed] [Google Scholar]
- 11. Fischman MW, Schuster CR, Javaid J, et al. Acute tolerance development to the cardiovascular and subjective effects of cocaine. J Pharmacol Exp Ther. 1985;235(3):677–682 [PubMed] [Google Scholar]
- 12. Whitby LG, Hertting G, Axelrod J. Effect of Cocaine on the disposition of noradrenaline labeled with tritium. Nature. 1960;187:604–605 [DOI] [PubMed] [Google Scholar]
- 13. Muscholl E. Effect of cocaine and related drugs on the uptake of noradrenaline by heart and spleen. Br J Pharmacol Chemother. 1961;16:352–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tuncel M, Wang Z, Arbique D, et al. Mechanism of the blood pressure–raising effect of cocaine in humans. Circulation. 2002;105(9):1054–1059 [DOI] [PubMed] [Google Scholar]
- 15. Brogan WC, III, Lange RA, Kim AS, Moliterno DJ, Hillis LD. Alleviation of cocaine-induced coronary vasoconstriction by nitroglycerin. J Am Coll Cardiol. 1991;18(2):581–586 [DOI] [PubMed] [Google Scholar]
- 16. Moliterno DJ, Willard JE, Lange RA, et al. Coronary-artery vasoconstriction induced by cocaine, cigarette smoking, or both. N Engl J Med. 1994;330(7):454–459 [DOI] [PubMed] [Google Scholar]
- 17. Boehrer JD, Moliterno DJ, Willard JE, Hillis LD, Lange RA. Influence of labetalol on cocaine-induced coronary vasoconstriction in humans. Am J Med. 1993;94(6):608–610 [DOI] [PubMed] [Google Scholar]
- 18. Dressler FA, Malekzadeh S, Roberts WC. Quantitative analysis of amounts of coronary arterial narrowing in cocaine addicts. Am J Cardiol. 1990;65(5):303–308 [DOI] [PubMed] [Google Scholar]
- 19. Satran A, Bart BA, Henry CR, et al. Increased prevalence of coronary artery aneurysms among cocaine users. Circulation. 2005;111(19):2424–2429 [DOI] [PubMed] [Google Scholar]
- 20. Apostolakis E, Tsigkas G, Baikoussis NG, Koniari I, Alexopoulos D. Acute left main coronary artery thrombosis due to cocaine use. J Cardiothorac Surg. 2010;5:65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Robaei D, Grieve SM, Nelson GC, Bhindi R, Figtree GA. Cocaine-induced epicardial coronary artery thrombosis resulting in extensive myocardial injury assessed by cardiac magnetic resonance imaging. Eur Heart J. 2010;31(20):2446. [DOI] [PubMed] [Google Scholar]
- 22. Mongeon FP, Rinfret S. Left main coronary artery occlusion with myocardial infarction in a cocaine user. Successful angioplasty with a drug-eluting stent. Can J Cardiol. 2008;24(5):e30–e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Heesch CM, Wilhelm CR, Ristich J, Adnane J, Bontempo FA, Wagner WRY. Cocaine activates platelets and increases the formation of circulating platelet containing microaggregates in humans. Heart. 2000;83(6):688–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kugelmass AD, Shannon RP, Yeo EL, Ware JA. Intravenous cocaine induces platelet activation in the conscious dog. Circulation. 1995;91(5):1336–1340 [DOI] [PubMed] [Google Scholar]
- 25. Siegel AJ, Mendelson JH, Sholar MB, et al. Effect of cocaine usage on C-reactive protein, von Willebrand factor, and fibrinogen. Am J Cardiol. 2002;89(9):1133–1135 [DOI] [PubMed] [Google Scholar]
- 26. Pradhan L, Mondal D, Chandra S, Ali M, Agrawal KC. Molecular analysis of cocaine-induced endothelial dysfunction: role of endothelin-1 and nitric oxide. Cardiovasc Toxicol. 2008;8(4):161–171 [DOI] [PubMed] [Google Scholar]
- 27. Böhm F, Pernow J. The importance of endothelin-1 for vascular dysfunction in cardiovascular disease. Cardiovasc Res. 2007;76(1):8–18 [DOI] [PubMed] [Google Scholar]
- 28. Jonas MA, Oates JA, Ockene JK, Hennekens CH; American Heart Association Statement on smoking and cardiovascular disease for healthcare professionals. Circulation. 1992;86:1664–1669 [DOI] [PubMed] [Google Scholar]
- 29. Iacobellis G, Kemp W. Cardiomyocyte apoptosis in cocaine-induced myocarditis with involvement of bundle of His and left bundle branch. Int J Cardiol. 2006;112(1):116–118 [DOI] [PubMed] [Google Scholar]
- 30. Peng SK, French WJ, Pelikan PC. Direct cocaine cardiotoxicity demonstrated by endomyocardial biopsy. Arch Pathol Lab Med. 1989;113(8):842–845 [PubMed] [Google Scholar]
- 31. Fan L, Sawbridge D, George V, et al. Chronic cocaine-induced cardiac oxidative stress and mitogen-activated protein kinase activation: the role of Nox2 oxidase. J Pharmacol Exp Ther. 2009;328(1):99–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen Y, Ke Q, Xiao YF, et al. Cocaine and catecholamines enhance inflammatory cell retention in the coronary circulation of mice by upregulation of adhesion molecules. Am J Physiol Heart Circ Physiol. 2005;288(5):H2323–H2331 [DOI] [PubMed] [Google Scholar]
- 33. Bertolet BD, Freund G, Martin CA, Perchalski DL, Williams CM, Pepine CJ. Unrecognized left ventricular dysfunction in an apparently healthy cocaine abuse population. Clin Cardiol. 1990;13(5):323–328 [DOI] [PubMed] [Google Scholar]
- 34. Eisenberg MJ, Yakel DL, Mendelson J, Redberg RF, Jones RT, Foster E. Immediate effects of intravenous cocaine on the thoracic aorta and coronary arteries: a transesophageal echocardiographic study. Chest. 1996;110(1):147–154 [DOI] [PubMed] [Google Scholar]
- 35. Su J, Li J, Li W, Altura B, Altura B. Cocaine induces apoptosis in primary cultured rat aortic vascular smooth muscle cells: possible relationship to aortic dissection, atherosclerosis, and hypertension. Int J Toxicol. 2004;23(4):233–237 [DOI] [PubMed] [Google Scholar]
- 36. Eagle KA, Isselbacher EM, DeSanctis RW. Cocaine-related aortic dissection in perspective. Circulation. 2002;105:1529–1530 [DOI] [PubMed] [Google Scholar]
- 37. Hsue PY, Salinas C, Bolger AF, et al. Acute aortic dissection induced by crack cocaine. Circulation. 2002;105:1592–1595 [DOI] [PubMed] [Google Scholar]
- 38. Daniel JC, Huynh TT, Zhou W, et al. Acute aortic dissection associated with use of cocaine. J Vasc Surg. 2007;46:427–433 [DOI] [PubMed] [Google Scholar]
- 39. Feldman JA, Fish SS, Beshansky JR, Griffith JL, Woolard RH, Selker HP. Acute cardiac ischemia in patients with cocaine-associated complaints: results of a multicenter trial. Ann Emerg Med. 2000;36(5):469–476 [DOI] [PubMed] [Google Scholar]
- 40. Hollander JE, Hoffman RS, Gennis P, et al. ; Cocaine Associated Chest Pain (COCHPA) Study Group Prospective multicenter evaluation of cocaine-associated chest pain. Acad Emerg Med. 1994;1(4):330–339 [DOI] [PubMed] [Google Scholar]
- 41. Brody SL, Slovis CM, Wrenn KD. Cocaine-related medical problems: consecutive series of 233 patients. Am J Med. 1990;88(4):325–331 [DOI] [PubMed] [Google Scholar]
- 42. Lakowski JM, Galloway MP, White FJ. Cocaine Pharmacology, Physiology and Clinical Strategy. Boca Raton, FL: CRC Press; 1992:35–51 [Google Scholar]
- 43. Hollander JE, Hoffman RS, Burstein JL, Shih RD, Thode HC, Jr; Cocaine-Associated Myocardial Infarction Study Group Cocaine-associated myocardial infarction: mortality and complications. Arch Intern Med. 1995;155(10):1081–1086 [PubMed] [Google Scholar]
- 44. Erzouki HK, Baum I, Goldberg SR, Schindler CW. Comparison of the effects of cocaine and its metabolites on cardiovascular function in anesthetized rats. J Cardiovasc Pharmacol. 1993;22(4):557–563 [DOI] [PubMed] [Google Scholar]
- 45. Brogan WC, III, Lange RA, Glamann DB, Hillis LD. Recurrent coronary vasoconstriction caused by intranasal cocaine: possible role for metabolites. Ann Intern Med. 1992;116(7):556–561 [DOI] [PubMed] [Google Scholar]
- 46. Jatlow P, Elsworth JD, Bradberry CW, et al. Cocaethylene: a neuropharmacologically active metabolite associated with concurrent cocaine-ethanol ingestion. Life Sci. 1991;48(18):1787–1794 [DOI] [PubMed] [Google Scholar]
- 47. Hearn WL, Rose S, Wagner J, Ciarleglio A, Mash DC. Cocaethylene is more potent than cocaine in mediating lethality. Pharmacol Biochem Behav. 1991;39(2):531–533 [DOI] [PubMed] [Google Scholar]
- 48. Wilson LD, French S. Cocaethylene’s effects on coronary artery blood flow and cardiac function in a canine model. J Toxicol Clin Toxicol. 2002;40(5):535–546 [DOI] [PubMed] [Google Scholar]
- 49. Wilson LD, Malik M, Willson H. Cocaine and ethanol: combined effects on coronary artery blood flow and myocardial function in dogs. Acad Emerg Med. 2009;16(7):646–655 [DOI] [PubMed] [Google Scholar]
- 50. Carrillo X, Curós A, Muga R, et al. Acute coronary syndrome and cocaine use: 8-year prevalence and inhospital outcomes. Eur Heart J. 2011;32:1244–1250 [DOI] [PubMed] [Google Scholar]
- 51. Jones JH, Weir W. Cocaine-induced chest pain. Clin Lab Med. 2006;26:127–146 [DOI] [PubMed] [Google Scholar]
- 52. Amin M, Gabelman G, Karpel J, Buttrick P. Acute myocardial infarction and chest pain syndromes after cocaine use. Am J Cardiol. 1990;66(20):1434–1437 [DOI] [PubMed] [Google Scholar]
- 53. Frishman WH. Carvedilol. N Engl J Med. 1998;339(24):1759–1765 [DOI] [PubMed] [Google Scholar]
- 54. Basu S, Senior R, Raval U, van der Does R, Bruckner T, Lahiri A. Beneficial effects of intravenous and oral carvedilol treatment in acute myocardial infarction: a placebo-controlled, randomized trial. Circulation. 1997;96(1):183–191 [DOI] [PubMed] [Google Scholar]
- 55. Hollander JE, Levitt MA, Young GP, Briglia E, Wetli CV, Gawad Y. Effect of recent cocaine use on the specificity of cardiac markers for diagnosis of acute myocardial infarction. Am Heart J. 1998;135(2 Pt 1):245–252 [DOI] [PubMed] [Google Scholar]
- 56. Kontos MC, Anderson FP, Ornato JP, Tatum JL, Jesse RL. Utility of troponin I in patients with cocaine-associated chest pain. Acad Emerg Med. 2002;9(10):1007–1013 [DOI] [PubMed] [Google Scholar]
- 57. Kontos MC, Jesse RL, Tatum JL, Ornato JP. Coronary angiographic findings in patients with cocaine-associated chest pain. J Emerg Med. 2003;24(1):9–13 [DOI] [PubMed] [Google Scholar]
- 58. Wright RS, Anderson JL, Adams CD, et al. 2011 ACCF/AHA Focused Update Incorporated Into the ACC/AHA 2007 Guidelines for the Management of Patients with Unstable Angina/Non ST-Elevation Myocardial Infarction: a Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2011;57:e15–e367 [DOI] [PubMed] [Google Scholar]
- 59. Chase M, Brown AM, Robey JL, et al. Application of the TIMI risk score in ED patients with cocaine-associated chest pain. Am J Emerg Med. 2007;25(9):1015–1018 [DOI] [PubMed] [Google Scholar]
- 60. Weber JE, Shofer FS, Larkin GL, Kalaria AS, Hollander JE. Validation of a brief observation period for patients with cocaine-associated chest pain. N Engl J Med. 2003;348(6):510–517 [DOI] [PubMed] [Google Scholar]
- 61. Cunningham R, Walton MA, Weber JE, et al. One-year medical outcomes and emergency department recidivism after emergency department observation for cocaine-associated chest pain. Ann Emerg Med. 2009;53(3):310–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kontos MC, Schmidt KL, Nicholson CS, Ornato JP, Jesse RL, Tatum JL. Myocardial perfusion imaging with technetium-99m sestamibi in patients with cocaine-associated chest pain. Ann Emerg Med. 1999;33(6):639–645 [PubMed] [Google Scholar]
- 63. Hollander JE, Chang AM, Shofer FS, et al. One-year outcomes following coronary computerized tomographic angiography for evaluation of emergency department patients with potential acute coronary syndrome. Acad Emerg Med. 2009;16(8):693–698 [DOI] [PubMed] [Google Scholar]
- 64. Walsh K, Chang AM, Perrone J, et al. Coronary computerized tomography angiography for rapid discharge of low-risk patients with cocaine-associated chest pain. J Med Toxicol. 2009;5(3):111–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Antman EM, Anbe DT, Armstrong PW, et al. ACC/AHA Guidelines for the Management of Patients With ST-Elevation Myocardial Infarction—Executive Summary: a Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation. 2004;110:588–636 [DOI] [PubMed] [Google Scholar]
- 66. Hollander JE. The management of cocaine-associated myocardial ischemia. N Engl J Med. 1995;333(19):1267–1272 [DOI] [PubMed] [Google Scholar]
- 67. McCord J, Jneid H, Hollander J, et al. Management of cocaine-associated chest pain and myocardial infarction: a scientific statement from the American Heart Association Acute Cardiac Care Committee of the Council on Clinical Cardiology. Circulation. 2008;117:1897–1907 [DOI] [PubMed] [Google Scholar]
- 68. Baumann BM, Perrone J, Hornig SE, Shofer FS, Hollander JE. Randomized, double-blind, placebo-controlled trial of diazepam, nitroglycerin, or both for treatment of patients with potential cocaine-associated acute coronary syndromes. Acad Emerg Med. 2000;7(8):878–885 [DOI] [PubMed] [Google Scholar]
- 69. Honderick T, Williams D, Seaberg D, Wears R. A prospective, randomized, controlled trial of benzodiazepines and nitroglycerin or nitroglycerin alone in the treatment of cocaine-associated acute coronary syndromes. Am J Emerg Med. 2003;21(1):39–42 [DOI] [PubMed] [Google Scholar]
- 70. Hoskins MH, Leleiko RM, Ramos JJ, Sola S, Caneer PM, Khan BV. Effects of labetalol on hemodynamic parameters and soluble biomarkers of inflammation in acute coronary syndrome in patients with active cocaine use. J Cardiovasc Pharmacol Ther. 2010;15(1):47–52 [DOI] [PubMed] [Google Scholar]
- 71. Negus BH, Willard JE, Hillis LD, et al. Alleviation of cocaine-induced coronary vasoconstriction with intravenous verapamil. Am J Cardiol. 1994;73(7):510–513 [DOI] [PubMed] [Google Scholar]
- 72. Derlet RW, Albertson TE. Potentiation of cocaine toxicity with calcium channel blockers. Am J Emerg Med. 1989;7(5):464–468 [DOI] [PubMed] [Google Scholar]
- 73. Nahas G, Trouvé R, Demus JR, von Sitbon M. A calcium-channel blocker as antidote to the cardiac effects of cocaine intoxication. N Engl J Med. 1985;313(8):519–520 [DOI] [PubMed] [Google Scholar]
- 74. First International Study of Infarct Survival Collaborative Group Randomised trial of intravenous atenolol among 16 027 cases of suspected acute myocardial infarction: ISIS-1. Lancet. 1986;2:57–66 [PubMed] [Google Scholar]
- 75. The MIAMI Trial Research Group Metoprolol in acute myocardial infarction: patient population. Am J Cardiol. 1985;56:10G–14G [PubMed] [Google Scholar]
- 76. Lange RA, Cigarroa RG, Flores ED, et al. Potentiation of cocaine, induced coronary vasoconstriction by beta adrenergic blockade. Ann Intern Med. 1990;112:897–903 [DOI] [PubMed] [Google Scholar]
- 77. Shannon RP, Stambler BS, Komamura K, Ihara T, Vatner SF. Cholinergic modulation of the coronary vasoconstriction induced by cocaine in conscious dogs. Circulation. 1993;87(3):939–949 [DOI] [PubMed] [Google Scholar]
- 78. Kenny D, Pagel PS, Warltier DC. Attenuation of the systemic and coronary hemodynamic effects of cocaine in conscious dogs: propranolol versus labetolol. Basic Res Cardiol. 1992;87:465–477 [DOI] [PubMed] [Google Scholar]
- 79. Vargas R, Gillis RA, Ramwell PW. Propranolol promotes cocaine-induced spasm of porcine coronary artery. J Pharmacol Exp Ther. 1991;257:644–646 [PubMed] [Google Scholar]
- 80. Rappolt RT, Gay G, Inaba DS, Rappolt NR. Propranolol in cocaine toxicity [letter]. Lancet. 1976;2(7986):640–641 [DOI] [PubMed] [Google Scholar]
- 81. Catravas JD, Waters IW. Acute cocaine intoxication in the conscious dog: studies on the mechanism of lethality. J Pharmacol Exp Ther. 1981;217(2):350–356 [PubMed] [Google Scholar]
- 82. Rangel C, Shu RG, Lazar LD, Vittinghoff E, Hsue PY, Marcus GM. Beta-blockers for chest pain associated with recent cocaine use. Arch Intern Med. 2010;170(10):874–879 [DOI] [PubMed] [Google Scholar]
- 83. Dattilo PB, Hailpern SM, Fearon K, Sohal D, Nordin C. Beta-blockers are associated with reduced risk of myocardial infarction after cocaine use. Ann Emerg Med. 2008;51(2):117–125 [DOI] [PubMed] [Google Scholar]
- 84. Ramoska E, Sacchetti AD. Propranolol-induced hypertension in treatment of cocaine intoxication. Ann Emerg Med. 1985;14(11):1112–1113 [DOI] [PubMed] [Google Scholar]
- 85. Smith M, Garner D, Niemann JT. Pharmacologic interventions after an LD50 cocaine insult in a chronically instrumented rat model: are beta-blockers contraindicated? Ann Emerg Med. 1991;20(7):768–771 [DOI] [PubMed] [Google Scholar]
- 86. Sand IC, Brody SL, Wrenn KD, Slovis CM. Experience with esmolol for the treatment of cocaine-associated cardiovascular complications. Am J Emerg Med. 1991;9(2):161–163 [DOI] [PubMed] [Google Scholar]
- 87. Sofuoglu M, Brown S, Babb DA, et al. Carvedilol affects the physiological and behavioral response to smoked cocaine in humans. Drug Alcohol Depend. 2000;60:69–76 [DOI] [PubMed] [Google Scholar]
- 88. Hollander JE, Carter WA, Hoffman RS. Use of phentolamine for cocaine-induced myocardial ischemia [letter]. N Engl J Med. 1992;327(5):361. [DOI] [PubMed] [Google Scholar]
- 89. Chan GM, Sharma R, Price D, Hoffman RS, Nelson LS. Phentolamine therapy for cocaine-association acute coronary syndrome (CAACS). J Med Toxicol. 2006;2(3):108–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lange RA, Cigarroa RG, Yancy CW, Jr, et al. Cocaine-induced coronary-artery vasoconstriction. N Engl J Med. 1989;321(23):1557–1562 [DOI] [PubMed] [Google Scholar]
- 91. Menon DV, Wang Z, Fadel PJ, et al. Central sympatholysis as a novel countermeasure for cocaine-induced sympathetic activation and vasoconstriction in humans. J Am Coll Cardiol. 2007;50(7):626–633 [DOI] [PubMed] [Google Scholar]
- 92. Hollander JE, Burstein JL, Hoffman RS, Shih RD, Wilson LD; Cocaine Associated Myocardial Infarction (CAMI) Study Group Cocaine-associated myocardial infarction: clinical safety of thrombolytic therapy. Chest. 1995;107(5):1237–1241 [DOI] [PubMed] [Google Scholar]
- 93. Hollander JE, Wilson LD, Leo PJ, Shih RD. Complications from the use of thrombolytic agents in patients with cocaine associated chest pain. J Emerg Med. 1996;14(6):731–736 [DOI] [PubMed] [Google Scholar]
- 94. Singh S, Arora R, Khraisat A, et al. Increased incidence of in-stent thrombosis related to cocaine use: case series and review of literature. J Cardiovasc Pharmacol Ther. 2007;12(4):298–303 [DOI] [PubMed] [Google Scholar]
- 95. Karlsson G, Rehman J, Kalaria V, Breall JA. Increased incidence of stent thrombosis in patients with cocaine use. Catheter Cardiovasc Interv. 2007;69(7):955–958 [DOI] [PubMed] [Google Scholar]
- 96. King SB, III, Smith SC, Jr, Hirshfeld JW, Jr, et al. 2007 Focused Update of the ACC/AHA/SCAI 2005 Guideline Update for Percutaneous Coronary Intervention: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2008;117:261–295 [DOI] [PubMed] [Google Scholar]