Abstract
Most biological processes are governed by multiprotein complexes rather than individual proteins. Identification of protein complexes therefore is becoming increasingly important to gain a molecular understanding of cells and organisms. Mass spectrometry–based proteomics combined with affinity-tag-based protein purification is one of the most effective strategies to isolate and identify protein complexes. The development of tandem-affinity purification approaches has revolutionized proteomics experiments. These two-step affinity purification strategies allow rapid, effective purification of protein complexes and, at the same time, minimize background. Identification of even very low-abundant protein complexes with modern sensitive mass spectrometers has become routine. Here, we describe two general strategies for tandem-affinity purification followed by mass spectrometric identification of protein complexes.
Keywords: tandem-affinity purification (TAP), His-Bio tag, mass spectrometry, protein complex identification, in-vivo cross-linking, MudPIT
1 Introduction
Tandem-affinity purification combined with mass spectrometric protein identification is a powerful approach to determine the composition of biologically relevant protein complexes. Typically, a protein of interest fused to a tandem-affinity purification tag (TAP-tag) is expressed in cells and used as a bait to purify protein complexes that assemble on the TAP-tagged protein in vivo. Two-step purification based on high-affinity interactions of the TAP-tag with tag-specific binding resins result in highly purified protein complexes that can be analyzed by mass spectrometry. A number of tandem-affinity tags have been developed [1–6]. Here, we describe the procedures for the isolation of protein complexes under native purification conditions using the original TAP-tag developed by the Seraphin group [1, 7] (Fig. 21.1) and a combination of in-vivo cross linking and tandem-affinity purification under denaturing conditions using the HB tandem affinity tag [6, 8, 9] (Fig. 21.2). Native purification conditions using the Seraphin TAP-tag is the method of choice to identify relatively stable protein complexes, whereas transient or weak interactions can be captured by in-vivo cross-linking followed by tandem affinity purification using HB-tagged bait proteins.
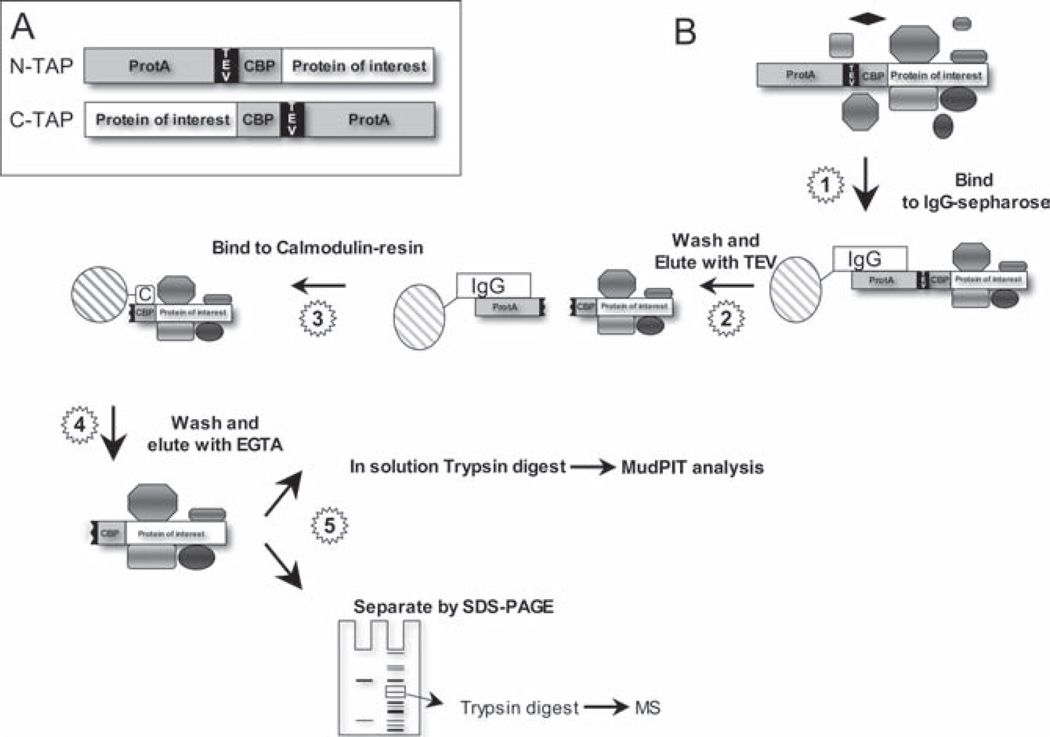
Fig. 21.1.
Tandem affinity purification using the protA/CBP tag: (A) N-TAP for N-terminal tagging and C-TAP for C-terminal tagging of proteins. The IgG-interacting domain of protein A (protA) and the calmodulin binding peptide (CBP) are separated by a TEV protease recognition site. Note the different tag configurations of N-TAP and C-TAP. (B) Protein complexes assembled on the tagged bait protein are first purified on IgG sepharose, eluted by site-specific proteolysis with TEV, and further purified on calmodulin beads. The purified protein complex is eluted with EGTA and processed for mass spectrometric analysis (MS). MS analysis (1D-LC-MS/MS) of individual protein bands can be performed after separation by SDS-PAGE and “in-gel” tryptic digest. Alternatively, the entire protein mixture can be analyzed directly by “in-solution” digest followed by 2D-LC-MS/MS (MudPIT)
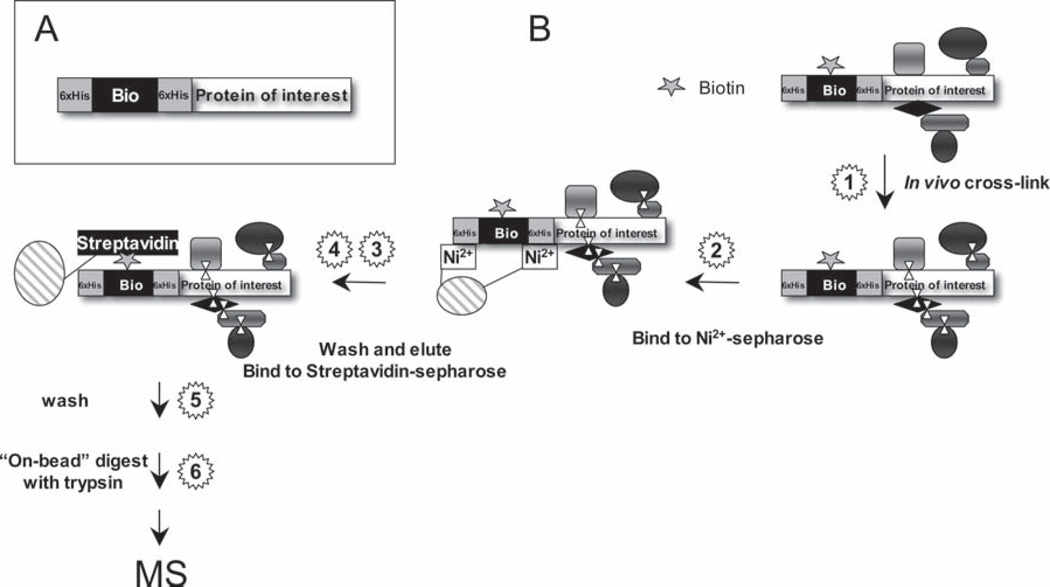
Fig. 21.2.
HBH-tag based tandem affinity purification combined with in-vivo cross-linking: (A) The HBH-tag consists of a bacterially derived in-vivo biotinylation signaling peptide (Bio), flanked by hexahistidine motifs (6xHis). The biotinylation signal peptide induces attachment of biotin in vivo and allows purification of HBH-tagged proteins on streptavidin resins. (B) Individual components of protein complexes can be covalently linked to each other by in-vivo cross-linking to preserve their composition during the purification procedure. Complexes are sequentially purified on Ni2+ sepharose and streptavidin sepharose. Both purification steps are performed under fully denaturing conditions to minimize background binding. Because of the irreversible nature of the streptavidin-biotin interaction, “on-bead” tryptic digest is used to elute peptides of the bound protein complex from the streptavidin sepharose for mass spectrometric identification
The seraphin TAP tag (protA/CBP tag) consists of the immunoglobulin interacting domain of protein A and a calmodulin-binding peptide (CBP) (Fig. 21.1). The two high-affinity binding domains of the protA/CBP tag are separated by a short recognition sequence for the site-specific tobacco-etch virus protease (TEV protease). The TEV site allows proteolytic elution of the protein complex from IgG-sepharose after the first affinity-purification step, which is based on the protA/IgG-sepharose interaction. The eluted protein complex is further purified by binding to a calmodulin affinity resin, eluted with EGTA and processed for mass spectrometric (MS) analyses (Fig. 21.1).
For best sensitivity, MS analysis is done in solution without further separation of the purified protein complexes by SDS-PAGE. As “in-solution” processing typically results in relatively complex peptide mixtures, multidimensional liquid chromatography combined with tandem MS is required for optimal results. This strategy is often referred to as MudPIT (multidimensional protein identification technique) [10, 11]. Alternatively, purified protein complexes can be separated into their subunits by SDS-PAGE and individual, stained protein bands can be excised from the gel and subjected to “in-gel” analyses by MS (Fig. 21.1). The “in-gel” strategy substantially reduces the complexity of the samples, which simplifies MS analyses, but decreases sensitivity due to poor recovery of low-abundant proteins from the gel matrix. Therefore, MudPIT analysis is the method of choice when comprehensive identification of protein complex components is important. The “in-gel” approach is well suited for identification of major subunits of protein complexes.
The protA/CBP tag is very effective for purification of relatively stable associated protein complexes. However, weak or transient interactors often are lost during the purification. Loosely associated components of protein complexes can be covalently attached to each other by in-vivo cross-linking. Typically, cells are treated with cell permeable cross-linking agents, such as formaldehyde, to covalently link protein complexes as they exist in vivo. The challenge is to minimize the background associated with the subsequent tandem-affinity purification as the cross-linking step can amplify nonspecific purification. The use of HBH-tagged bait proteins can reduce this problem, because it tolerates completely denaturing purification conditions that prevent nonspecific interactions [6, 8]. Since protein complexes were covalently linked by in-vivo cross-linking, the denaturing conditions cannot dissociate the components. The HBH-tag is a derivative of the HB-tag series and consists of a bacterially derived biotinylation signal flanked by hexahistidine motifs [6, 8, 9]. The biotinylation signal induces the attachment of biotin to a specific lysine residue in the tag in vivo [12]. HBH-tagged proteins can be sequentially purified on Ni2+ chelate resins and streptavidin sepharose (Fig. 21.2). Importantly, both purification steps tolerate highly denaturing conditions, such as 8 M urea or 6 M guanidinium [6, 8]. The high-affinity interaction between the biotin attached to the HBH-tag and streptavidin prevents efficient elution [13]. “On-bead” tryptic digest therefore is used to release peptides from the purified, cross-linked protein complex for MudPIT analysis.
2 Materials
2.1 Yeast Cell Culture and Lysis
YEPD medium: 2% (w/v) Bacto peptone, 1% (w/v) yeast extract, 2% (w/v) dextrose, 0.006% (w/v) adenosine and uracil (Sigma-Aldrich, St. Louis, MO).
Glass beads, 0.5 mm (Biospec Products, Inc., Bartlesville, OK).
Antifoam A (catalogue number A-5758; Sigma-Aldrich).
0.8-µm nitrocellulose membrane (Millipore, Billerica, MA).
2.2 Mammalian Cell Culture and Lysis
Dulbeco’s Modified Eagle’s Medium (DMEM; Mediatech, Herndon, VA) supplemented with 10% (v/v) fetal bovine serum (FBS, GIBCO, Bethesda, MA), 1% penicillin/streptomycin (GIBCO, Bethesda, MA).
Formaldehyde (catalogue number F-8775; Sigma).
Antibiotics according to selection marker.
10X PBS buffer: 0.58 M Na2HPO4, 0.17 M NaH2PO4, 0.68 M NaCl, pH 7.4.
Cell scraper for cell harvesting.
18G, 21G, and 27G needles to shear DNA (Becton Dickinson, NJ).
2.3 Native Purification Using the ProtA/CBP TAP Tag
Lysis buffer : 150 mM of NaCl, 50 mM of molanty? Tris-HCl pH 8.0, 5 mM of EDTA, 10% (v/v) glycerol, 0.2% (v/v) Nonidet P-40, 1 mM of NaF. Immediately prior to use, add (final concentrations): 1 mM phenylmethylsulfonyl fluoride (PMSF) and 1 µg/mL each aprotinin, leupeptin, pepstatin.
TEV cleavage buffer: 150 mM of NaCl, 10 mM of Tris-HCl pH 8.0, 0.5 mM of EDTA, 0.1% (v/v) Nonidet P-40. Add dithiothreitol (DTT) to 1 mM just before use.
Calmodulin binding buffer : 150 mM NaCl, 10 mM Tris-HCl pH 8.0, 1 mM MgCl2, 1 mM imidazole, 0.1% (v/v) Nonidt P-40. Add CaCl2 to 2 mM and β-mercaptoethanol to 10 mM just before use.
Calmodulin elution buffer : 150 mM NaCl, 10 mM Tris-HCl pH 8.0. Add EGTA and β-mercaptoethanol (both to 10 mM) just before use.
AcTEV Protease; 10 units/µL (Invitrogen, Carlsbad, CA).
IgG Sepharose™ 6 Fast Flow (GE Healthcare, Uppsala, Sweden).
Calmodulin affinity resin (Stratagene, La Jolla, CA).
Poly-Prep® chromatography columns, 2-mL size (Bio-Rad, Hercules, CA).
PMSF: 0.5 M–1 M solution in isopropanol; store at ≤4 °C.
2.4 Purification After In-Vivo Cross-Linking Using the HBH Affinity Tag
Check the pH of all buffers before use and readjust them accordingly.
Buffer A-8: 8 M urea, 300 mM NaCl, 50 mM sodium phosphate buffer pH 8.0 (0.68 mL of 0.5 M NaH2PO4 and 9.32 mL of 0.5 M Na2HPO4 in 100 mL), 0.5% (v/v) Nonidet P-40, pH 8.0.
Buffer A-6.3: 8 M urea, 300 mM NaCl, 50 mM sodium phosphate buffer pH 6.3 (8.22 mL of 0.5 M NaH2PO4 and 1.78 mL of 0.5 M Na2HPO4 in 100 mL), 0.5% (v/v) Nonidet P-40, pH 6.3.
Buffer A-6.3-imidazole: same as buffer A-6.3 but also containing 10 mM of imidazole, pH 6.3.
Buffer B: 8 M urea, 200 mM NaCl, 50 mM sodium phosphate buffer (9.21 mL of 0.5 M NaH2PO4 and 0.79 mL of 0.5 M Na2HPO4 in 100 mL), 2% (w/v) SDS, 10 mM of EDTA, 100 mM of Tris, adjust pH of final solution to 4.3.
Buffer C: 8 M urea, 0.2 M NaCl, 0.2% (w/v) SDS, 100 mM Tris-HCl, pH 8.0.
Buffer D: 8 M urea, 0.2 M NaCl, 100 of mM Tris-HCl, pH 8.0.
Ni2+ Sepharose™ 6 fast flow beads (GE Healthcare).
Immobilized streptavidin beads (Pierce, Rockford, IL).
Poly-Prep® chromatography columns (Bio-Rad, Hercules, CA).
50 mM NH4HCO3 buffer.
Trypsin (Promega, Madison, WI).
HPLC-grade H2O.
PMSF, 0.5 M–1 M solution in isopropanol, stored at ≤4 °C.
2.5 Western Blot Analysis
4X SDS sample buffer: 250 mM Tris-HCl, pH 6.8, 8% (w/v) SDS, 300 mM DTT, 30% (v/v) glycerol, 0.02% (w/v) bromophenol blue.
Antibodies: For detection of the protA/CBP tag, peroxidase antiperoxidase complex (catalogue number P1291; Sigma-Aldrich), 1:2000 in blocking buffer and anti-CBP antibody (catalogue number 07-482; Millipore), 1:2000 in blocking buffer. For detection of the HBH-tag, RGS-His antibody (catalogue number 34610; Qiagen, Valencia, CA), 1:2000 in blocking buffer or horse-radish peroxidase conjugated streptavidin (catalogue number PI21126; Fisher, Pittsburgh), 1:10.000 in TBS-T buffer.
10X TBS-T (Tris-buffered saline with Tween), 10X stock: 1.37 M NaCl, 27 mM KCl, 250 mM Tris-HCl, pH 7.4, 1% (v/v) Tween 20.
Blocking buffer: 5% (w/v) nonfat dry milk in 1X TBS-T.
2.6 Sample Preparation for “In-Gel” Identification
NuPAGE Bis-Tris gel 4-12% with MES running buffer (Invitrogen) or similar.
SilverSNAP stain for mass spectrometry (Pierce) or similar.
25 mM NH4HCO3 (100 mg/50 mL) in H2O.
25 mM NH4HCO3 in 50% (v/v) acetonitrile (ACN), 50% H2O.
50% (v/v) ACN, 5% (v/v) formic acid (FA) (Sigma-Aldrich).
10 ng/µL of trypsin (Promega) in 25 mM NH4HCO3 in H2O (see Note 1).
10 mM DTT in 25 mM NH4HCO3 (1.5 mg/mL) (see Note 2).
55 mM iodoacetamide in 25 mM NH4HCO3 (10 mg/mL) (see Note 2).
0.65-mL siliconized tubes (Fisher).
SpeedVac (GMI, Ramsey, MN).
2.7 Sample Preparation for MudPIT Analysis
50 mM of NH4HCO3.
0.4 µg/µL trypsin (Promega) in 1 mM trifluoroacetic acid (TFA).
Lys-C protease, 1 µg/µL in water (Wako Chemical, Japan).
Strong cation exchange PolySULFOETHYL column (The Nest Group, Southborough, MA).
Buffer C18-A: 2% (v/v) ACN, 98% (v/v) H2O, 0.1 % (v/v) FA.
Buffer C18-B: 98% (v/v) ACN, 2% (v/v) H2O, 0.1 % (v/v) FA.
Buffer SCX-A: 5 mM of KH2PO4, 30% (v/v) ACN, 0.1% (v/v) FA, adjusted to pH 2.7 with FA.
Buffer SCX-B: 5 mM of KH2PO4, 350 mM of KCl, 30% (v/v) ACN, 0.1% (v/v) FA, adjusted to pH 2.7 with FA.
0.1% (v/v) TFA.
Vivapure C-18 microcolumn (Sartorius, Göttingen, Germany).
Pepmap C18 capillary column (Dionex, Bannockburn, IL) (length: 15 cm, ID 75 µm), capillary columns from another vender, or self-packed.
500 fmol/µL BSA digest (Michrom Bioresources, Auburn, CA).
3 Methods
3.1 Native Purification Using the ProtA/CBP TAP Tag (Fig. 21.1)
Tandem-affinity purification with the protA/CBP tag very effectively removes the background. However, nonspecific interaction with the purification matrix can never be completely avoided. To distinguish specific interactions from the background, we recommend parallel processing of a sample from cells that do not express the protA/CBP tagged bait protein. Proteins detected in the tagged cell line but not in the untagged cells most likely are in a protein complex with the bait protein.
3.1.1 Growth and Lysis of Yeast Cells Expressing TAP-Tagged Proteins
Grow yeast cells in 1 L of YEPD medium to an Absorbance (600 nm) = 1.5 and collect cells by centrifugation (2500 g, 5 min, 4 °C), or by filtration.
Wash the cell pellet in 40 mL of ice cold lysis buffer (without added aprotenin, leupeptin, or pepstatin), pellet the cells by centrifugation as previously, remove the supernatant, and quickly freeze the pellet and store it at −80 °C (see Note 3).
Break the cells using glass beads or by an alternative yeast cell lysis method (see Note 4).
Separate the lysate from the glass beads. One easy way is to invert the tube, and with a 21G needle, poke a hole at the bottom of the tube. Insert the tube with the hole into a fresh tube and centrifuge carefully for 30 s at 1,000 g. The lysate will pass through the hole in the upper tube and collect in the lower tube, whereas the glass beads remain in the upper tube.
Centrifuge the lysate to remove cell debris (25,000 g, 30 min, 4 °C). Transfer the clarified supernatant to a fresh tube. If the lysate is viscous, use a syringe and pass it once through an 18G needle and twice through a 21G needle to shear the DNA. Save 50 µL of the lysate for analysis. One can expect to obtain between 100 and 250 mg of total protein.
3.1.2 Growth and Lysis of Mammalian Cells Expressing TAP-Tagged Proteins
Grow cells expressing the TAP-tagged protein in DMEM to 90% confluence. Use 5–10 150-mm plates. One 150-mm plate of HeLa cells yields about 2–5 mg of total protein.
Wash cells on plates twice with 5 mL of ice-cold 1X PBS, pH 7.4.
Add 5 mL of ice-cold 1X PBS and detach the cells using a cell scraper. Rinse the plates with 2 mL of ice-cold 1X PBS to increase the quantity of harvested cells. Keep the harvested cells on ice all the time.
Transfer the cells into a tube and centrifuge at 400 g for 3 min at 4 °C. Discard the supernatant and freeze the pellet at −80 °C.
For a total of 10 150-mm plates of cells harvested, add 20 mL of lysis buffer (see Note 3) to the frozen pellet. Resuspend immediately and keep on ice for a few minutes.
At this point the lysate is very viscous, due to the presence of chromosomal DNA. Shear the DNA by passing the lysates several times through a 27G needle, until the viscosity is similar to that of water.
Centrifuge the lysate at 25,000 g for 30 min at 4 °C. Transfer the clarified supernatant to a fresh tube. Save 50 µL of aliquots of the lysate for analysis.
3.1.3 Tandem Affinity Purification Using ProtA/CBP-Tagged Bait Proteins
The optimal amount of IgG beads required to bind most of the protA/CBP-tagged protein complex should be determined. Adding too much IgG beads increases the background, whereas too little IgG resin results in inefficient purification (see Note 5). A good starting point is 150 µL of IgG beads for 100 mg of total protein lysate. All steps are at 4 °C unless otherwise stated.
Wash the IgG beads twice with 1 mL of lysis buffer. Centrifuge at 100 g for 1 min to pellet the beads.
Add the IgG beads to the cell lysates.
Incubate for 2 h at 4 °C.
Centrifuge at 100 g for 1 min to pellet the IgG beads and carefully remove the supernatant without disturbing the beads. Save 50 µL of the supernatant for analysis (this is the IgG-unbound fraction).
Wash the beads three times with 10 mL of ice-cold lysis buffer. Centrifuge and remove supernatant as previously.
Wash twice with 10 mL of ice-cold TEV cleavage buffer.
TEV protease cleavage: add 1 mL of TEV cleavage buffer and 10 µL of acTEV protease.
Incubate for 2 h at room temperature and then overnight at 4 °C. Mix samples gently during the incubation.
Centrifuge at 100 g for 1 min and transfer the supernatant into a new 15 mL tube. Save 50 µL for analysis. Store the IgG beads at −20 °C for analysis.
Add 3 µL of 1 M CaCl2 to the 1 mL of TEV-eluted protein fraction (final concentration 3 mM of CaCl2).
Wash the calmodulin beads three times with 1 mL of calmodulin binding buffer.
Resuspend 50 µL of the calmodulin beads in 1 mL of calmodulin binding buffer and add the bead suspension to the TEV-eluted protein sample (total volume is 2 mL) (see Note 6).
Incubate at 4 °C for 2-4 h with gentle mixing.
Centrifuge at 100 g for 1 min and carefully remove the supernatant. Retain 100 µL for analysis (this is the calmodulin-unbound fraction).
Gently resuspend the beads in 1 mL of calmodulin binding buffer, transfer into a 2 mL Poly-Prep column and allow the column to drain.
Wash the beads four times with 1.5 mL of calmodulin binding buffer at 4 °C.
Elute proteins from the beads with 1 mL of calmodulin elution buffer and collect in a microcentrifuge tube. Save 50 µL for analysis.
Precipitate proteins by adding 335 µL of 100% (v/v) trichloroacetic acid (TCA; final concentration 25%) and incubate on ice for 30 min with occasional vortexing (see Note 7).
Pellet proteins by centrifugation in a microcentifuge at 13,000 g for 30 min at 4 °C.
Wash the pellet three times with 0.5 mL of prechilled (−20 °C) acetone. After each wash, pellet the protein by centrifugation at 13,000 g for 10 min at 4 °C.
Remove the acetone and air dry the pellet (about 5–10 min).
3.1.4 Western Blot Analysis
To ascertain the efficiency of the purification, the small samples collected from the different purification steps should be analyzed by Western blotting.
To evaluate the efficiency of binding to IgG sepharose, analyze 20 µL of the collected lysates and 20 µL of the IgG-unbound fraction (Sect. 3.1.3, step 4.) by Western blotting with peroxidase antiperoxidase complex to detect the protA part of the TAP tag.
To analyze the efficiency of TEV elution, boil the IgG beads collected in Sect. 3.1.3, step 9 in 500 µL of 1X SDS sample buffer for 5–10 min and separate 10 µL of this sample, in addition to 20 µL of the TEV-eluted fraction (collected in step 3.1.3.9.) by SDS-PAGE. Analyze by Western blotting using an anti-CBP antibody (see Note 8).
To verify efficient binding to the calmodulin beads and subsequent elution, compare the following fractions by Western blotting using an anti-CBP antibody: 20 µL of the TEV-eluted fraction (Sect. 3.1.3, step 9), 40 µL calmodulin-unbound fraction (Sect. 3.1.3, step 14), and 20 µL of the calmodulin-elution (Sect. 3.1.3, step 17).
3.2 Purification of HBH-Tagged Proteins after In Vivo Cross-Linking (Fig. 21. 2)
3.2.1 Growth, In-Vivo Cross-Linking, and Lysis of Yeast Cells Expressing HB-Tagged Proteins
Grow yeast cells expressing the HBH-tagged protein of interest in 1 L of YEPD medium to an Absorbance (600 nm) = 1.5.
Add formaldehyde to the medium to a final concentration of 1% (v/v) and incubate at 30 °C for 10 min.
Add 2.5 M glycine to a final concentration of 0.125 M and incubate at 30 °C for 10 min to quench the cross-linking reaction.
Collect cells by centrifugation (2,500 g, 5 min, 4 °C) or filtration through a 0.8-µm nitrocellulose membrane.
Wash the cell pellet in 20 mL of buffer A-8 containing 1 mM PMSF, pellet the cells by centrifugation as previously, remove the supernatant, and quickly freeze the pellet and store it at −80 °C.
Break the cells using glass beads or by an alternative yeast cell lysis method (see Note 4).
Separate the lysate from the glass beads. One easy way is to invert the tube, and with a 21G needle, poke a hole at the bottom of the tube. Insert the tube with the hole into a fresh tube and centrifuge carefully for 30 s at 1,000 g. The lysate passes through the hole in the upper tube and collects in the lower tube, whereas the glass beads remain in the upper tube.
Centrifuge the lysate to remove cell debris (25,000 g, 30 min, 20 °C). Transfer the clarified supernatant to a fresh tube.
3.2.2 Growth, In-Vivo Cross-Linking, and Lysis of Mammalian Cells Expressing HBH-Tagged Protein
Grow cells expressing the HBH-tag in DMEM to 90% confluency (one 150-mm plate of HeLa cells yields about 2 mg of total protein).
Add formaldehyde to a final concentration of 1% (v/v) and incubate at 37 °C for 10 min.
Quench the cross-linking reaction by adding glycine to a final concentration of 0.125 M and incubate at 37 °C for 10 min.
Wash the cells on the plates twice with 5 mL of ice-cold 1X PBS, pH 7.4.
Add 5 mL of ice-cold 1X PBS and detach cells from the plate with a cell scraper. Rinse the plate with an additional 2 mL of ice-cold 1X PBS to increase the yield of harvested cells.
Transfer the cells into a tube and centrifuge at 100 g for 3 min at 4 °C.
Discard the supernatant, for each 150 mm plate of lysed cells add 2 mL of buffer A-8 containing 1 mM PMSF and resuspend the cells.
Shear the DNA with a 27G needle, until the viscosity is similar to that of water.
Centrifuge the lysate (25,000 g, 30 min, 20 °C). Transfer the clarified supernatant to a fresh tube. Save 50 µL lysates for analysis.
3.2.3 Tandem-Affinity Purification of HBH-Tagged Proteins
To distinguish specific interactions from the background, we recommend parallel processing of a sample from cells that do not express the HBH-tagged bait protein. Proteins detected in the tagged cell line but not in the untagged cells most likely are in a protein complex with the bait protein.
Use 70 µL of Ni2+ sepharose beads for each 1 mg of protein lysate. Wash the beads three times with at least five bead volumes of buffer A-8 (without PMSF). Pellet the beads by centrifugation at 100 g for 1 min, and remove the supernatant.
Add Ni2+ sepharose beads to a lysate, followed by imidazole to a final concentration of 10 mM to reduce nonspecific binding.
Incubate on a rocking platform at room temperature for 4 h or overnight.
Pellet the beads by centrifugation at 100 g for 1 min, and remove the supernatant. Save 50 µL for analysis (Ni-unbound fraction).
Wash the Ni2+ sepharose beads sequentially with 20 bead volumes of buffer A-8, buffer A-6.3, and buffer A-6.3-imidazole, respectively.
Elute HBH- tagged proteins twice with five bead volumes of buffer B. Incubate for at least 10 min at room temperature for each elution step, and pool the eluates. Save 50 µL for analysis (Ni2+ eluate).
Adjust the pH of the eluate to pH 8.0 (add ~25 µL of 1 M of NaOH to each 1 mL of eluate).
Prepare streptavidin sepharose by washing with 2X 3 mL of buffer C. Use 15 µL of beads for each 1 mg protein in the whole cell lysate.
Incubate the Ni2+ sepharose eluate with streptavidin beads in a Poly-Prep chromatography column on a rocking platform overnight at room temperature.
Drain the column. Save 50 µL of the flow-through for analysis (streptavidin unbound fraction).
Wash the streptavidin beads (in the Poly-Prep chromatography column) sequentially with 2X 25 bead volumes of buffer-C and buffer-D, respectively.
Add 3 mL of 50 mM NH4HCO3, pH 8.0 and allow the column to drain.
Repeat step 12 once more.
Add NH4HCO3 buffer (approximately 50% of the bead volume) for trypsinization.
3.2.4 “On-Bead” Digestion for Mass Spectrometric Analysis
Dissolve 20 µg of trypsin in 50 µL of 1 mM TFA in the original glass tube.
Incubate the sample (from Sect. 3.2.3, step 14) with trypsin at 37 °C for 12–16 h on a rocking platform. Use 1 µg of trypsin for every 2 mg of whole cell lysate used in the first purification step (see Note 9).
Carefully collect the supernatant and add FA to a final concentration of 1% (v/v).
Reextract tryptic peptides from the beads two or three times by adding approximately 50% of the bead volume of 25% (v/v) ACN, 0.1% (v/v) FA to the beads.
Pool the extracted peptides and concentrate to about 5 µL using a SpeedVac.
Add 100 µL of H2O and concentrate (SpeedVac) to about 5 µL. Repeat this step once more.
Acidify by adding 100 µL of 0.1% (v/v) FA.
Samples can be stored frozen at this step or used immediately for 1-D- (Sect. 3.3.3) or 2-D-MS analysis (Sect. 3.4.1).
3.2.5 Western Blot Analysis of Purification
To ascertain the efficiency of the purification, the small samples collected from the different purification steps should be analyzed by Western blotting.
To evaluate the efficiency of binding to Ni2+ sepharose and the extent of cross-linking, analyze 20 µL of the collected whole cell lysates and 20 µL of the Ni-unbound fraction (Sect. 3.2.3, step 4) by Western blotting with the anti-RGS-His antibody.
To analyze the efficiency of binding to streptavidin, use 10 µl of the Ni2+ eluate (Sect. 3.2.3, step 6) and 10 µL of the streptavidin unbound fraction (Sect. 3.2.3, step 10) for Western blot analysis with the anti-RGS-His antibody or horseradish- peroxidase-conjugated streptavidin (see Note 10).
3.3 Mass Spectrometric Identification of Proteins after Separation by SDS-PAGE
3.3.1 Separation by SDS-PAGE
Components of protein complexes can have a wide range of molecular weights. We therefore recommend the use of a gradient gel for separation of samples by SDS-PAGE. Many manufacturers offer precast gradient gels compatible with subsequent mass spectrometric analyses. The NuPAGE Bis-Tris gel mentioned in this section is only one of many options. To identify protein bands after separation, silver staining offers the best sensitivity. Not all silver staining protocols are compatible with mass spectrometric analysis, but a number of manufacturers offer mass spectrometry-compatible silver staining kits. The SilverSNAP kit is one of several choices.
Separate samples from Sect. 3.1.3, step 21 on a 4–12% NuPAGE Bis-Tris gel (or similar) according to the suppliers instructions. Samples should be thoroughly dissolved in the gel-loading buffer supplied with the gels. Load the purified sample from the protA/CBP-tagged cell line and the control sample (untagged cell line).
Stain the gel with SilverSNAP stain or similar. Protein bands present in the tagged sample and absent in the untagged control sample can be excised and analyzed.
3.3.2 Excision and “In-Gel” Digestion of Protein Bands
Chop each gel slice into small pieces (1 mm2) and place into a 0.65-mL siliconized tube.
Add 100 µL (or enough to cover) of 25 mM NH4HCO3, 50% (v/v) ACN and vortex for 10 min. Pellet the gel pieces by centrifugation at 16,000 g for 30 s.
Using a gel-loading micropipette tip, extract the supernatant and discard.
Repeat steps 2 and 3 twice more.
-
Dry (SpeedVac) the gel pieces to complete dryness (approximately 20 min).
For low level proteins (<1 pmol), especially those separated by 1D SDS-PAGE, reduction and alkylation (steps 6–11) is recommended. Otherwise, go directly to step 12.
Add 25 µL (or enough to cover) of 10 mM DTT in 25 mM NH4HCO3 to the dried gel pieces.
Vortex and centrifuge at 16,000 g for 30 s. Allow the reduction reaction to proceed at 56 °C for 1 h.
Remove the supernatant and add 25 µL (or enough to cover) of 55 mM iodoacetamide to the gel pieces. Vortex and centrifuge at 16,000 g for 30 s. Allow the alkylation reaction to proceed in the dark for 45 min at room temperature. Occasionally vortex and centrifuge at 16,000 g for 30 s.
Discard the supernatant. Wash the gel pieces with ~100 µL of NH4HCO3, Vortex for 10 min and centrifuge at 16,000 g for 30 s.
Discard the supernatant and dehydrate the gel pieces with ~100 µL (or enough to cover) of 25 mM NH4HCO3, 50% (v/v) ACN, vortex for 5 min and centrifuge at 16,000 g for 30 s. Repeat once more.
Completely dry the gel pieces in a SpeedVac (approximately 20 min)
Add 5–10 µL of trypsin (10 ng/µL) to the dried gel pieces and allow the gel to rehydrate for a few min.
Add 25 µL of 25 mM NH4HCO3 (or sufficient volume to cover the gel pieces), vortex for 5 min, centrifuge at 16,000 g for 30 s and incubate at 37 °C for 4 h, to overnight.
Centrifuge at 16,000 g for 30 s. Add 10 µL of H2O, vortex for 10 min and centrifuge at 16,000 g for 30 s.
Transfer the digest solution (aqueous extraction) into a new 0.65-mL siliconized tube.
Add 30 µL (enough to cover) of 50% (v/v) ACN, 5% (v/v) formic acid to the gel pieces, vortex for 10 min and centrifuge at 16,000 g for 30 s. Transfer the supernatant to the same extraction tube as in step 15.
Repeat step 16 once more.
Add 10 µL of 100% (v/v) ACN to the gel pieces, vortex for 5 min and centrifuge at 16,000 g for 30 s. Transfer the supernatant to the same extraction tube as in step 15.
Centrifuge the extracted digest (extraction tube from step 15) at 16,000 g for 30 s and reduce the volume to 5–10 µL in a SpeedVac.
Add 5–10 µL of 0.1% (v/v) FA to the tube before mass spectrometric analysis. Samples can be directly analyzed by LC-MS/MS or analyzed by MALDI after desalting.
3.3.3 Mass Spectrometry
Condition the Pepmap C18 column sequentially in buffer C18-B for 90 min and buffer C18-A for 30 min. Adjust the column flow rate to 250 nL/min.
Inject 1 µL of 500 fmol/µL BSA digest and separate the sample at the following gradient using buffers C18-A and C18-B: 0% buffer C18-B for 5 min, 0–35% buffer C18-B for 30 min, 80% buffer C18-B for 5 min, and 0% buffer C18-B for 30 min. Inject 1 µL of 10–50 ftmol/µL BSA digest using the same gradient. The column is now ready to be used.
The protein digest from Sect. 3.3.2, step 20 can be directly injected onto the column for LC-MS/MS analysis.
3.4 Identification of Proteins by MudPIT Analysis
3.4.1 Protease Digestion of Purified Protein Complexes
“On-bead” trypsin digestion of protein complexes purified with HBH-tagged baits, after in-vivo cross-linking, is described in Sect. 3.2.4. Samples prepared in that section (step 8) can be directly analyzed by 1-D-MS/MS, as described in Sect. 3.3.3, step 3, or by 2-D-MS/MS (Sect. 3.4.2). The following steps describe protease digestion of TCA-precipitated affinity-purified proteins as generated in Sect. 3.1.3, step 21.
Dissolve the protein pellet from Sect. 3.1.3, step 21 in 100 µL of 8 M urea, 50 mM NH4HCO3.
Add 2% (enzyme weight/protein weight) Lys-C and digest at 37 °C for 4 h.
Add 440 µL of 50 mM NH4HCO3 to dilute the urea concentration to ≤1.5 M. Add 1% trypsin (enzyme weight/protein weight) and digest at 37 °C for 8 h or overnight.
Reduce the volume to approximately 10 µL in a SpeedVac.
Add 100 µL of 0.1% (v/v) FA and proceed with 1-D-MS/MS Sect. 3.3.3, step 3), or 2-D-MS/MS (Sect. 3.4.2) or store the sample frozen at −20 °C.
3.4.2 Peptide Separation by Ion-Exchange Chromatography
Separate samples (generated at Sect. 3.2.4, step 8, or at Sect. 3.4.1, step 5) on a strong cation exchange column with the following gradient: 0–5% buffer SCX-B for 2 min, 5–35% buffer SCX-B for 30 min, 35–100% buffer SCX-B for 10 min. Collect the flow-through and each peak fraction.
Concentrate each fraction to about 5–10 µL in a SpeedVac.
Add 180 µL of 0.1% (v/v) TFA to each fraction.
Desalt the samples using Vivapure C-18 microcolumns according to the manufacturer’s instructions.
After desalting, concentrate samples to 1–2 µL (using a SpeedVac) and add 10 µL of 0.1% (v/v) FA to each sample for LC-MS/MS analysis.
3.4.3 LC-MS/MS Analysis
Each desalted fraction from Sect. 3.4.2, step 5 can be analyzed by 1-D-LC-MS/MS (as described in Sect. 3.3.3). As the nongel purified tryptic peptides separated by ion exchange chromatography are more complex than the “in-gel” digestion samples, the gradient should be modified as follows: 0% buffer C18-B for 5 min, 0–35% buffer C18-B for 80 min, 80% buffer C18-B for 5 min, and 0% buffer C18-B for 30 min.
Automatically submit the acquired LC-MS/MS data to commercially available search engines, such as MASCOT, Protein Prospector, or SEQUEST for database searching, protein identification, and characterization of posttranslational modifications.
Acknowledgments
We thank Christian Tagwerker and Cortnie Guerrero for their contributions in developing the protocols described here and Karin Flick for the figure design. Work in the laboratory of P. Kaiser is supported by grants from NIH (RO1GM-66164, R21CA113823) and the California Breast Cancer Research Program (11NB-0177). L. Huang acknowledges support from NIH (GM074830) and the DOD (PC-041126). D. Meierhofer is supported by an Erwin Schroedinger Fellowship (FWF J2665).
Footnotes
Diluted trypsin solution should be prepared immediately before the digestion.
Although this step is optional, if performed, DTT and iodoacetamide solutions need to be freshly prepared for reduction and alkylation of cysteines.
It is important to test the extraction of the tagged protein on a small sample first. Some proteins, especially DNA-bound proteins, require higher salt concentrations for efficient extraction. High salt concentrations can disrupt protein complexes, and therefore salt concentrations in the lysis buffer should not exceed that required for extraction of the tagged protein. To determine the best salt concentration, prepare several small aliquots. Extract one sample with lysis buffer containing 2% (w/v) SDS, which serves as a control sample for complete extraction. Extract the other samples with lysis buffer containing increasing amounts of NaCl (e.g., 150, 200, 300, 400, and 500 mM). Analyze all the samples by Western blotting using the peroxidase antiperoxidase complex to compare the extraction efficiencies of each salt concentration. In some cases, increasing the concentration of the detergent in the lysis buffer can improve extraction.
A number of methods exist for breaking yeast cells. Very reliable results are achieved when cells are broken with a FastPrep™ FP120 (Qbiogene, Carlsbad, CA) at setting 4.5, for four times for 30 s each at 4 °C, with a 1 min break on ice between the runs. Alternatively, cells can be vortexed with glass beads five times for 1 min at 4 °C, with a 1 min break on ice between the runs. In either case, add two or three pellet volumes of lysis buffer, approximately 1 µL of antifoam A for each 200 µL of lysis buffer and glass beads until the beads reach the top level of the lysis buffer. Cells also can be lysed with a French pressure cell at maximum pressure or by grinding the pellet in liquid nitrogen.
Use a small amount of the cell lysate and incubate with different quantities of IgG beads at 4 °C for 2 h. Pellet the beads by centrifugation at 100 g for 1 min. Use 10 µL of the supernatant (lysate after binding to IgG beads) and analyze (along with the equivalent quantity of total lysate) by Western blotting using the peroxidase antiperoxidase complex. Efficient binding of the protA/CBP-tagged protein to the IgG resin is evident by significantly reduced levels of the protA/ CBP-tagged protein in the supernatant fraction. Choose the quantity of IgG beads that shows efficient binding, although not necessarily the most efficient binding, as it is important to keep in mind that adding too much IgG resin will increase nonspecific binding.
The amount of calmodulin resin required varies depending on the amount of purified tagged protein. Too much resin increases the background and too little resin results in inefficient recovery of the tagged protein. The suggested bead volume should be increased if the analysis of the purification steps suggests inefficient recovery of the tagged protein in this step.
If the sample is to be analyzed by MudPIT, in addition to separation by SDS-PAGE and “in-gel” digestion, split the eluted fraction into 2X 500 µL aliquots, and precipitate each with TCA (25% final concentration).
TEV cleavage results in a reduction of the molecular weight of the protA/CBP-tagged protein because the protA part of the TAP tag is removed by TEV cleavage (Fig. 21.1).
The amount of trypsin suggested is a rough estimate. To determine the amount of trypsin required more accurately, measure the protein concentration in the Ni2+ eluate (Sect. 3.2, step 6) and in the streptavidin unbound fraction (Sect. 3.2.3, step 10). The difference inprotein concentration in these two fractions is a good approximation of the amount of protein bound to the streptavidin beads. For every 1 µg of protein bound to the streptavidin beads, use between 0.01 and 0.02 µg of trypsin (the ratio of protein/trypsin is between 1/100 and 1/50).
The RGS-His antibody detects HBH-tagged proteins and can be used to measure the efficiency of the purification steps. Horseradish-peroxidase-conjugated streptavidin can be used to analyze the purification after elution from the Ni2+ sepharose. However, it is not very useful for analysis of the first purification step because all eukaryotic cells express between four and six endogenous biotinylated proteins. The endogenous biotinylated proteins are relatively abundant and can complicate interpretation of the Western blot results. However, endogenous biotinylated proteins are lost during the first purification step and horseradish-peroxidase-conjugated streptavidin is useful for detection of HBH-tagged proteins after the first purification step, providing important information about the efficiency of biotinylating the HBH-tagged bait protein.
References
- 1.Rigaut G, Shevchenko A, Rutz B, Wilm M, Mann M, Seraphin B. A generic protein purification method for protein complex characterization and proteome exploration. Nat Biotechnol. 1999;17:1030–1032. doi: 10.1038/13732. [DOI] [PubMed] [Google Scholar]
- 2.Cheeseman IM, Brew C, Wolyniak M, Desai A, Anderson S, Muster N, Yates JR, Huffaker TC, Drubin DG, Barnes G. Implication of a novel multiprotein Dam1p complex in outer kinetochore function. J Cell Biol. 2001;155:1137–1145. doi: 10.1083/jcb.200109063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Denison C, Rudner AD, Gerber SA, Bakalarski CE, Moazed D, Gygi SP. A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol Cell Proteomics. 2004 doi: 10.1074/mcp.M400154-MCP200. [DOI] [PubMed] [Google Scholar]
- 4.Graumann J, Dunipace LA, Seol JH, McDonald WH, Yates JR, III, Wold BJ, Deshaies RJ. Applicability of tandem affinity purification MudPIT to pathway proteomics in yeast. Mol Cell Proteomics. 2004;3:226–237. doi: 10.1074/mcp.M300099-MCP200. [DOI] [PubMed] [Google Scholar]
- 5.Hannich JT, Lewis A, Kroetz MB, Li SJ, Heide H, Emili A, Hochstrasser M. Defining the SUMO-modified proteome by multiple approaches in Saccharomyces cerevisiae. J Biol Chem. 2005;280:4102–4110. doi: 10.1074/jbc.M413209200. [DOI] [PubMed] [Google Scholar]
- 6.Tagwerker C, Flick K, Cu M, Guerrero C, Dou Y, Auer B, Baldi P, Huang L, Kaiser P. A tandem affinity tag for two-step purification under fully denaturing conditions: Application in ubiquitin profiling and protein complex identification combined with in vivo cross-linking. Mol Cell Proteomics. 2006;5:737–748. doi: 10.1074/mcp.M500368-MCP200. [DOI] [PubMed] [Google Scholar]
- 7.Puig O, Caspary F, Rigaut G, Rutz B, Bouveret E, Bragado-Nilsson E, Wilm M, Seraphin B. The tandem affinity purification (TAP) method: a general procedure of protein complex purification. Methods. 2001;24:218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 8.Guerrero C, Tagwerker C, Kaiser P, Huang L. An integrated mass spectrometry-based proteomic approach: quantitative analysis of tandem affinity-purified in vivo cross-linked protein complexes (QTAX) to decipher the 26 S proteasome-interacting network. Mol Cell Proteomics. 2006;5:366–378. doi: 10.1074/mcp.M500303-MCP200. [DOI] [PubMed] [Google Scholar]
- 9.Tagwerker C, Zhang H, Wang X, Larsen LS, Lathrop RH, Hatfield GW, Auer B, Huang L, Kaiser P. HB tag modules for PCR-based gene tagging and tandem affinity purification in Saccharomyces cerevisiae. Yeast. 2006;23:623–632. doi: 10.1002/yea.1380. [DOI] [PubMed] [Google Scholar]
- 10.Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., III Direct analysis of protein complexes using mass spectrometry. Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- 11.Washburn MP, Wolters D, Yates JR., III Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat Biotechnol. 2001;19:242–247. doi: 10.1038/85686. [DOI] [PubMed] [Google Scholar]
- 12.Cronan JE., Jr Biotination of proteins in vivo. A post-translational modification to label, purify, and study proteins. J Biol Chem. 1990;265:10327–10333. [PubMed] [Google Scholar]
- 13.Savage D, Mattson G, Desa S, Niedlander G. Avidin-biotin chemistry: A handbook. Rockford, IL.: Pierce Chemical; 1994. [Google Scholar]