Abstract
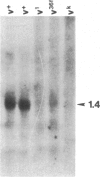
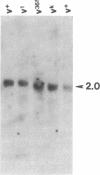
We have cloned vermilion (v), one of the genes required for brown eye pigment synthesis in Drosophila, using a mutant (vH2a) "tagged" with the transposon P factor. Mutations that disrupt v gene expression are clustered within approximately 2 kilobases of DNA. A 1.4-kilobase transcript, homologous to this same region, is present in v+ RNA but absent in RNA from several v mutants. The spontaneous v alleles that are suppressed by the suppressor of sable [su(s)] are apparently identical insertions of 412, a copia-like transposable element. Preliminary evidence suggests that su(s)-suppressible alleles at other loci may also be 412 insertions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baillie D. L., Chovnick A. Studies on the genetic control of tryptophan pyrrolase in Drosophila melanogaster. Mol Gen Genet. 1971;112(4):341–353. doi: 10.1007/BF00334435. [DOI] [PubMed] [Google Scholar]
- Barish N, Fox A S. Immunogenetic Studies of Pseudoallelism in Drosophila Melanogaster. II. Antigenic Effects of the Vermilion Pseudoalleles. Genetics. 1956 Jan;41(1):45–57. doi: 10.1093/genetics/41.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Brittnacher J. G., Ganetzky B. On the Components of Segregation Distortion in DROSOPHILA MELANOGASTER. II. Deletion Mapping and Dosage Analysis of the SD Locus. Genetics. 1983 Apr;103(4):659–673. doi: 10.1093/genetics/103.4.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brookfield J. F., Montgomery E., Langley C. H. Apparent absence of transposable elements related to the P elements of D. melanogaster in other species of Drosophila. 1984 Jul 26-Aug 1Nature. 310(5975):330–332. doi: 10.1038/310330a0. [DOI] [PubMed] [Google Scholar]
- Corces V., Pellicer A., Axel R., Meselson M. Integration, transcription, and control of a Drosophila heat shock gene in mouse cells. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7038–7042. doi: 10.1073/pnas.78.11.7038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnegan D. J., Rubin G. M., Young M. W., Hogness D. S. Repeated gene families in Drosophila melanogaster. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1053–1063. doi: 10.1101/sqb.1978.042.01.106. [DOI] [PubMed] [Google Scholar]
- Germeraad S. Genetic transformation in Drosophila by microinjection of DNA. Nature. 1976 Jul 15;262(5565):229–231. doi: 10.1038/262229a0. [DOI] [PubMed] [Google Scholar]
- Green M. M. Mutant Isoalleles at the Vermilion Locus in Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1952 Apr;38(4):300–305. doi: 10.1073/pnas.38.4.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. PHENOTYPIC VARIATION AND PSEUDO-ALLELISM AT THE FORKED LOCUS IN Drosophila Melanogaster. Proc Natl Acad Sci U S A. 1955 Jun 15;41(6):375–379. doi: 10.1073/pnas.41.6.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. M. PSEUDO-ALLELISM AT THE VERMILION LOCUS IN DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1954 Feb;40(2):92–99. doi: 10.1073/pnas.40.2.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Jacobson K. B., Yim J. J., Grell E. H., Wobbe C. R. Mechanism of suppression in Drosophila: evidence for a macromolecule produced by the su(s)+ locus that inhibits sepiapterin synthase. Cell. 1982 Oct;30(3):817–823. doi: 10.1016/0092-8674(82)90286-0. [DOI] [PubMed] [Google Scholar]
- KAUFMAN S. Studies on tryptophan pyrrolase in Drosophila melanogaster. Genetics. 1962 Jul;47:807–817. doi: 10.1093/genetics/47.7.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubli E. The genetics of transfer RNA in Drosophila. Adv Genet. 1982;21:123–172. doi: 10.1016/s0065-2660(08)60298-9. [DOI] [PubMed] [Google Scholar]
- Langer-Safer P. R., Levine M., Ward D. C. Immunological method for mapping genes on Drosophila polytene chromosomes. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4381–4385. doi: 10.1073/pnas.79.14.4381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre G., Jr Salivary chromosome bands and the frequency of crossing over in Drosophila melanogaster. Genetics. 1971 Apr;67(4):497–513. doi: 10.1093/genetics/67.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levis R., O'Hare K., Rubin G. M. Effects of transposable element insertions on RNA encoded by the white gene of Drosophila. Cell. 1984 Sep;38(2):471–481. doi: 10.1016/0092-8674(84)90502-6. [DOI] [PubMed] [Google Scholar]
- Marzluf G. A. Enzymatic studies with the suppressor of vermilion of Drosophila melanogaster. Genetics. 1965 Sep;52(3):503–512. doi: 10.1093/genetics/52.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerowitz E. M., Hogness D. S. Molecular organization of a Drosophila puff site that responds to ecdysone. Cell. 1982 Jan;28(1):165–176. doi: 10.1016/0092-8674(82)90386-5. [DOI] [PubMed] [Google Scholar]
- Modolell J., Bender W., Meselson M. Drosophila melanogaster mutations suppressible by the suppressor of Hairy-wing are insertions of a 7.3-kilobase mobile element. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1678–1682. doi: 10.1073/pnas.80.6.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hare K., Rubin G. M. Structures of P transposable elements and their sites of insertion and excision in the Drosophila melanogaster genome. Cell. 1983 Aug;34(1):25–35. doi: 10.1016/0092-8674(83)90133-2. [DOI] [PubMed] [Google Scholar]
- Parkhurst S. M., Corces V. G. Forked, gypsys, and suppressors in Drosophila. Cell. 1985 Jun;41(2):429–437. doi: 10.1016/s0092-8674(85)80016-7. [DOI] [PubMed] [Google Scholar]
- Pirrotta V., Bröckl C. Transcription of the Drosophila white locus and some of its mutants. EMBO J. 1984 Mar;3(3):563–568. doi: 10.1002/j.1460-2075.1984.tb01847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poillon W. N., Maeno H., Koike K., Feigelson P. Tryptophan oxygenase of Pseudomonas acidovorans. Purification, composition, and subunit structure. J Biol Chem. 1969 Jul 10;244(13):3447–3456. [PubMed] [Google Scholar]
- RIZKI T. M. MUTANT GENES REGULATING THE INDUCIBILITY OF KYNURENINE SYNTHESIS. J Cell Biol. 1964 May;21:203–211. doi: 10.1083/jcb.21.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rizki T. M., Rizki R. M. Allele specific patterns of suppression of the vermillion locus in Drosophila melanogaster. Genetics. 1968 Aug;59(4):477–485. doi: 10.1093/genetics/59.4.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin G. M., Kidwell M. G., Bingham P. M. The molecular basis of P-M hybrid dysgenesis: the nature of induced mutations. Cell. 1982 Jul;29(3):987–994. doi: 10.1016/0092-8674(82)90462-7. [DOI] [PubMed] [Google Scholar]
- Scherer G., Tschudi C., Perera J., Delius H., Pirrotta V. B104, a new dispersed repeated gene family in Drosophila melanogaster and its analogies with retroviruses. J Mol Biol. 1982 May 25;157(3):435–451. doi: 10.1016/0022-2836(82)90470-3. [DOI] [PubMed] [Google Scholar]
- Schutz G., Feigelson P. Purification and properties of rat liver tryptophan oxygenase. J Biol Chem. 1972 Sep 10;247(17):5327–5332. [PubMed] [Google Scholar]
- Searles L. L., Jokerst R. S., Bingham P. M., Voelker R. A., Greenleaf A. L. Molecular cloning of sequences from a Drosophila RNA polymerase II locus by P element transposon tagging. Cell. 1982 Dec;31(3 Pt 2):585–592. doi: 10.1016/0092-8674(82)90314-2. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sweet H. O. Dilute suppressor, a new suppressor gene in the house mouse. J Hered. 1983 Jul-Aug;74(4):305–306. doi: 10.1093/oxfordjournals.jhered.a109794. [DOI] [PubMed] [Google Scholar]
- Tartof K. D. Interacting gene systems: I. The regulation of tryptophan pyrrolase by the vermilion-suppressor of vermilion system in Drosophila. Genetics. 1969 Jul;62(3):781–795. [PMC free article] [PubMed] [Google Scholar]
- Thireos G., Griffin-Shea R., Kafatos F. C. Untranslated mRNA for a chorion protein of Drosophila melanogaster accumulates transiently at the onset of specific gene amplification. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5789–5793. doi: 10.1073/pnas.77.10.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will B. M., Bayev A. A., Finnegan D. J. Nucleotide sequence of terminal repeats of 412 transposable elements of Drosophila melanogaster. A similarity to proviral long terminal repeats and its implications for the mechanism of transposition. J Mol Biol. 1981 Dec 25;153(4):897–915. doi: 10.1016/0022-2836(81)90458-7. [DOI] [PubMed] [Google Scholar]
- Winston F., Durbin K. J., Fink G. R. The SPT3 gene is required for normal transcription of Ty elements in S. cerevisiae. Cell. 1984 Dec;39(3 Pt 2):675–682. doi: 10.1016/0092-8674(84)90474-4. [DOI] [PubMed] [Google Scholar]
- Wright T. R., Hodgetts R. B., Sherald A. F. The genetics of dopa decarboxylase in Drosophila melanogaster. I. Isolation and characterization of deficiencies that delete the dopa-decarboxylase-dosage-sensitive region and the alpha-methyl-dopa-hypersensitive locus. Genetics. 1976 Oct;84(2):267–285. doi: 10.1093/genetics/84.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young M. W., Schwartz H. E. Nomadic gene families in Drosophila. Cold Spring Harb Symp Quant Biol. 1981;45(Pt 2):629–640. doi: 10.1101/sqb.1981.045.01.081. [DOI] [PubMed] [Google Scholar]