Abstract
Interleukin- (IL-) 10, anti-inflammatory cytokine, is known to inhibit the protective immune responses against malaria parasites and to be involved in exacerbating parasitemia during Plasmodium infection. In contrast, IL-10 is regarded as necessary for suppressing severe pathology during Plasmodium infection. Here, we summarize the role of IL-10 during murine malaria infection, focusing especially on coinfection with lethal and nonlethal strains of malaria parasites. Recent studies have demonstrated that the major sources of IL-10 are subpopulations of CD4+ T cells in humans and mice infected with Plasmodium. We also discuss the influence of innate immunity on the induction of CD4+ T cells during murine malaria coinfection.
1. Introduction
Malaria, caused by protozoan parasites of the genus Plasmodium, is the major parasitic disease in tropical and subtropical regions, including parts of the Americas, Asia, and Africa. With more than 200–300 million clinical cases globally and approximately 1 million deaths per year, malaria represents the most important infectious disease worldwide. Four species of Plasmodium infect humans: P. falciparum, P. vivax, P. malariae, and P. ovale.
Human malarial parasites develop through two stages in humans: a liver stage and a blood stage. The asexual blood stage of the parasite is the cause of malarial pathologies. Therefore, it is important to prevent the replication of this stage of parasite. Particularly, P. falciparum causes severe pathologies such as cerebral malaria, severe anemia, and respiratory injury in the blood stage. It is necessary to understand the mechanism of protective immunity against the blood stage of the parasite during malaria infection. Nevertheless, it is difficult to investigate the human in vivo immune response against malaria parasite for many reasons. Consequently, murine malaria models with P. berghei, P. yoelii, and P. chabaudi have been used to elucidate the immune interaction in hosts and to demonstrate many factors associated with malarial defense mechanisms [1].
2. Parasite Killing: The Role of Proinflammatory Cytokines during Human and Murine Malaria Infection
Interferon- (IFN-) γ and Interleukin- (IL-) 12 play a crucial role in the clearance of intracellular pathogens [2–5]. Low levels of IFN-γ and IL-12 production have been observed in young African children with severe anemia during infection with P. falciparum [6]. The IFN-γ-mediated responses have been shown to be involved in protection against infection with P. falciparum [7]. In murine malaria, IFN-γ produced by CD4+ T cells has been shown to play a pivotal role in protective immunity against P. chabaudi (Pc) AS [8], nonlethal P. berghei (Pb) XAT [9], and P. yoelii (Py) 17XNL [10] infection. Actually, IFN-γ-depleted mice infected with murine malaria parasites show high levels of parasitemia and eventually die. IL-12 is a necessary factor for clearance of nonlethal Pc AS [11], Pb XAT [12], and Py 17XNL [13], suggesting that IL-12 plays an important role in protective immunity via IFN-γ production in murine malaria. Production of IFN-γ and IL-12 is suppressed by anti-inflammatory cytokines such as IL-10. It is possible that enhancement of IL-10 production contributes to suppression of parasite killing, considering that IL-10 plays a detrimental role during P. falciparum infection.
3. Source and Biological Effect of IL-10
IL-10, an anti-inflammatory cytokine, plays an important role in regulating immune responses in hosts, as does TGF-β. The major source of IL-10 is known to be T cell subsets including Th1 cells, Th2 cells, Tr1 cells (CD25+Foxp3−), and regulatory T (Treg) cells (CD25+Foxp3+). In antigen-primed T cells, Th2 cells were originally believed to be the major source of IL-10. Stimulation of Th1 cells with IL-27 upregulates IL-10 production and enhances IFN-γ expression slightly [14, 15]. Tr1 were identified as a subset of CD4+ cells that produce high levels of IL-10, low levels of IL-2, but not IL-4. They develop from naïve T cells under the influence of IL-27 [15–17]. IL-10 is also produced by naturally occurring Treg cells [18]. TGF-β induces the expression of IL-10 [19]. Moreover, IL-2, an important activator of suppressive activity by Treg cells, enhances IL-10 production [20, 21]. Today, it is known that the source of IL-10 is not only T cell subsets but also almost all leukocytes [22–25].
Apparently, monocytes/macrophages are the main target cells of inhibitory IL-10 effects [26]: IL-10 inhibits the release of proinflammatory mediators from monocytes/macrophages, and thereby inhibits the LPS- and IFN-γ-induced secretion of TNF-α, IL-1β, IL-6, IL-8, G-CSF, and GM-CSF [27, 28]. Furthermore, IL-10 inhibits the antigen presentation of monocytes/macrophages. Moreover, the IL-10-induced inhibition of IL-12 synthesis in antigen-presenting cells results in reduced IFN-γ production in T cells [29]. Actually, IL-10 inhibits both the proliferation and the cytokine synthesis of CD4+ T cells, including the production of IL-2 and IFN-γ by Th1 and of IL-4 and IL-5 by Th2 [30, 31].
4. Detrimental Effect of IL-10 on the Outcome of Human and Murine Malaria Infection
High levels of IL-10 and TNF in plasma have been characteristic of young African children with malarial anemia and high levels of parasitemia [32–39]. In common IL-10 promoter variants, the -1082A/-819T/-592A (ATA) haplotype has been associated with increased susceptibility to severe anemia [39]. Their IL-10 : IL-12 ratio was higher than that in the non-ATA haplotype. On the other hand, the -1082G/-819C/-592C (GCC) haplotype has been associated with protection against severe anemia [39]. The IL-10 : IL-12 ratio in the GCC haplotype was lower than that in the ATA haplotype. These findings suggest that a high IL-10 : IL-12 ratio is associated with the downregulation of IFN-γ production and that it causes development of severe anemia during P. falciparum infection.
Lethal Py 17XL-infected mice show higher levels of IL-10 and TGF-β production than nonlethal Py 17XNL-infected mice early in infection [40, 41]. High levels of IL-10 and TGF-β are associated with inhibition of proinflammatory response, resulting in high levels of parasitemia, severe anemia by which RBCs ruptured, causing parasite replication and the death of infected mice. Depletion or deficiency of IL-10 [40, 42], or the blockade of IL-10 receptor [41] regulates parasitemia during lethal Py 17XL infection and prolongs survival of infected mice. Couper et al. [42] reported that the major source of IL-10 in lethal Py 17XL-infected mice is CD4+ Tr1 cells, just as it is in toxoplasmosis [43] and cutaneous leishmaniasis [44] (Figure 1).
Figure 1.
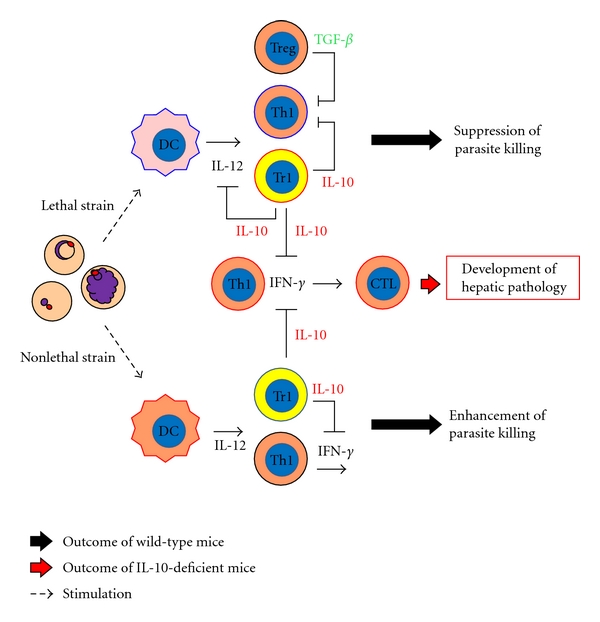
Scheme of immune responses during lethal P. yoelii 17XL and nonlethal P. yoelii 17XL infection: DC: dendritic cell; Th: helper T cell (CD4+ T cells); Tr1, IL-10-producing CD4+ T cells; Treg, regulatory T cells; CTL, cytotoxic T cells (CD8+ T cells). In a lethal P. yoelii 17XL infection, Tr1 are induced. IL-10 inhibits proinflammatory cytokine production for parasite killing, producing high levels of parasitemia and the death of mice. Tr1 are also induced in nonlethal Py 17XNL as well as lethal Py 17XL infection. However, parasite killing occurs during nonlethal Py 17XNL infection. When IL-10 is ineffective (depletion or deficiency of IL-10 or the blockade of IL-10 receptor) in mice infected with nonlethal Py 17XNL or lethal Py 17XL, excessive inflammation is induced in association with the development of hepatic pathology.
In nonlethal Py 17XNL-infected mice, the production of IL-10 and TGF-β is induced in the late phase of infection [41]. The population of CD4+ Tr1 cells has been shown to be the major source of IL-10 in nonlethal Py 17XNL as well as lethal Py 17XL infection. Moreover, IL-10-deficient mice show marked suppression of the replication of parasites compared with that in wild-type mice [42] (Figure 1). These findings suggest that enhanced-IL-10 production suppresses inflammatory response against malaria parasites, resulting in high levels of parasitemia and anemia by replication of parasites in infected mice. Results show that IL-10 plays a detrimental role during human and murine malaria infection.
5. Role of Anti-Inflammatory Cytokines during Murine Malaria Infection
Reportedly, a low IL-10/TNF ratio is associated with severe malarial anemia [36–38]. These results suggest that low levels of IL-10 production are associated with enhancement of TNF production, followed by increased IFN-γ production. The enhancement of TNF production might be associated with the aggravation of disease severity, such as severe anemia, by which phagocytosis of uninfected RBC occurs [45], or dyserythropoiesis [46]. Moreover, results obtained using mouse models have suggested that IL-10 plays a protective role in the host during murine malaria infection. Although IL-10-deficient mice show lower levels of parasitemia than wild-type mice do during murine malaria infection, they indicate severe diseases such as hepatic pathology [42, 47, 48] and cerebral pathology [49, 50]. Actually, inflammation, which is involved in parasite killing, is upregulated in IL-10-deficient mice, but excessive inflammation, such as the increase of IFN-γ production, also presents the risk of developing hepatic pathology and/or cerebral pathology. Therefore, it seems that IL-10 might be necessary for suppression of hepatic pathology and cerebral pathology in the host during infection.
B6 mice infected with Pb NK65 display hepatic pathology and die within 2 weeks. The development of severe hepatic pathology is involved in IL-12 [11], IFN-γ, and CD8+ T cells [9]. The IL-12 production is induced through a MyD88-dependent pathway in DCs or macrophage and engenders hepatic pathology in a perforin/granzyme-dependent manner during Pb NK65 infection [51]. Coinfection with nonlethal Pb XAT or Py 17XNL prevents the development of hepatic pathology caused by Pb NK65 infection and prolongs survival of mice [47]. In fact, IL-10 KO mice coinfected with nonlethal Pb XAT or Py 17XNL showed severe hepatic pathology, suggesting that IL-10 is involved in suppression of disease severity during coinfection [47] (Figure 2(b)). During lethal Py 17XL or nonlethal Py 17XNL infection, IL-10, which is derived from CD4+ Tr1 cells, is also necessary for the prevention of hepatic pathology [42]. Nevertheless, it remains unclear whether IFN-γ and CD8+ T cells are associated with development of hepatic pathology in mice infected with Py 17XL or Py 17XNL (Figure 1).
Figure 2.
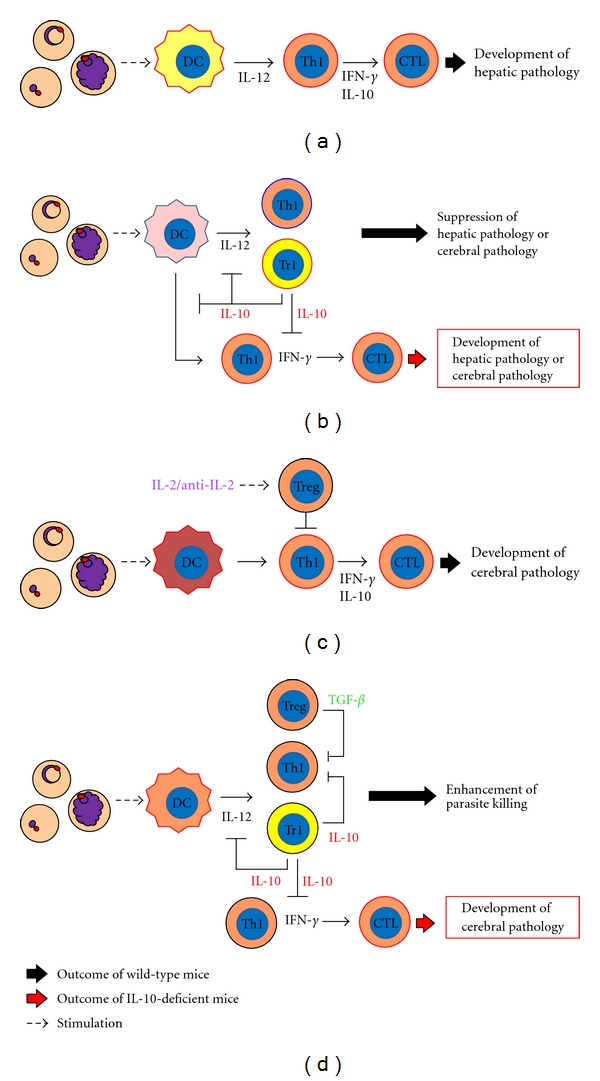
Role of anti-inflammatory responses during murine malaria infection. (a) Immune responses during lethal P. berghei NK65 infection. (b) Possible mechanism by which development of disease severity such as hepatic or cerebral pathology is suppressed by coinfection with nonlethal murine malaria parasites. (c) Immune responses during lethal P. berghei ANKA infection. (d) Immune responses during P. chabaudi AS infection. (a) and (c) A type of DC activated by lethal P. berghei NK65 or P. berghei ANKA might induce pathological Th1 and CTL. The pathological Th1 and CTL are involved in excessive inflammation and the development of severe pathology, such as hepatic pathology (P. berghei NK65) or experimental cerebral malaria (ECM) (P. berghei ANKA). (b) A type of DC activated by nonlethal malaria parasites before activation by lethal parasites might expand Tr1, but not pathological Th1, and might subsequently suppress severe disease. IL-10, which might be produced by Tr1, inhibits proinflammatory cytokine production and expansion of pathological Th1 during coinfection. Therefore, coinfected IL-10 deficient mice develop severe pathology, such as hepatic pathology or ECM. (d) In P. chabaudi AS infection, IL-10 from Tr1 and Treg are associated with suppression of proinflammatory cytokine production and expansion of pathological Th1. A deficiency of IL-10 or TGF-β contributes to development of ECM.
Mice infected with Pb ANKA show similar features to human cerebral malaria (CM) regarding neurologic signs and histopathological findings, considering that Pb ANKA infection in mice might be an experimental model of CM (ECM) [52, 53]. Proinflammatory cytokines, such as IFN-γ and lymphotoxin-α, are known to accelerate ECM development [54] (Figure 2(c)). In contrast, anti-inflammatory cytokines, such as IL-10, prevent ECM development [55, 56]. However, it remains unclear how IL-10 suppresses ECM development, because high levels of IL-10 production were observed in spleen [57] and plasma [58] of mice singly infected with Pb ANKA.
The ECM development is suppressed by the simultaneous presence of murine AIDS during Pb ANKA infection [55]. Results demonstrated that murine AIDS-mediated protection of ECM is dependent on IL-10, which is produced by splenic CD4+ T cells, with the use of anti-IL-10 mAb. It is particularly interesting that coinfection with parasites such as nonlethal Pb XAT [50] or Filaria [59] has also been shown to prevent ECM development. The suppressive effect of coinfection with nonlethal Pb XAT or Filaria on ECM during Pb ANKA infection was abrogated in IL-10 KO mice [50, 59], suggesting that IL-10 plays a crucial role in the suppression of ECM during coinfection with other parasites (Figure 2(b)).
In contrast to coinfection with nonlethal Pb XAT, the suppressive effect of coinfection with nonlethal Py 17XNL on ECM during Pb ANKA infection is independent of IL-10 [50, 60]. A recent study demonstrated that Treg cells, which are expanded by IL-2/anti-IL-2 complexes, suppress the recruitment of pathogenic CD4+ and CD8+ T cells to brains and protect mice from developing ECM during Pb ANKA [61]. The IL-2/anti-IL-2 complexes enhanced the levels of Foxp3 and CTLA-4 expression and increased the levels of IL-10 production from Treg cells during Pb ANKA infection. However, the suppression of ECM by Treg cells was dependent on CTLA-4 but not on IL-10 [61] (Figure 2(c)). The suppressive effect of coinfection with nonlethal Py 17XNL on ECM during Pb ANKA infection was not reversible by depleting antibodies against CD25+-bearing CD4+ T cells or CTLA4+-bearing CD4+ T cells [60]. A key factor that has a suppressive effect on ECM by coinfection with nonlethal Py 17XNL has not yet been discovered.
Development of severe disease such as hepatic pathology and cerebral pathology generally involves excessive inflammation in murine malaria parasites. Little is known about the differences between the developmental mechanisms of hepatic pathology and cerebral pathology during Pb NK65 and Pb ANKA infection, respectively. However, IL-10 can downregulate excessive inflammation during Pb NK65 or Pb ANKA infection. It is associated with the suppression of hepatic pathology and cerebral pathology. Results show that IL-10 plays a protective role in the host during P. falciparum infection.
6. A Different Type of DC Induced by Lethal and Nonlethal Murine Malaria Infection
How are different subsets of CD4+ T cells, such as pathological CD4+ T cells [62, 63], IL-10-producing CD4+ T cells [42], and Treg cells [64], induced between lethal and nonlethal murine malaria infection? The development of CM is inhibited completely by the simultaneous presence of nonlethal Py 17XNL [60], lethal Pb K173 [57], and nonlethal Pb XAT [50]. However, protection from CM was not induced in mice when they were infected with Py 17XNL on day 4 after Pb ANKA infection [60]. Similarly, coinfection with Pb XAT on day 1 or day 3 after infection with Pb ANKA failed to protect mice from cerebral malaria (Niikura et al. unpublished data). In simultaneous infection with Pb ANKA and Pb K173, suppression of ECM was associated with the induction of cytokines such as IFN-γ, IL-10, and IL-12 on day 1 after infection [57]. These findings suggest that the presence of other parasites might modulate some key factors/cells that are involved in innate immunity in early infection with Pb ANKA. Actually, DCs are important for initiating immune responses against malaria parasites. It is possible that immune responses induced by DCs produce protective and pathological effects, respectively, when mice are infected with nonlethal and lethal parasites. Therefore, DCs might contribute to the determination of the virulence of malaria parasites. In coinfection, a type of DC activated by nonlethal malaria parasites before activation by lethal parasites might fail to expand pathological CD4+ T cells and subsequently fail to suppress severe disease.
Wykes et al. [13] showed that although DCs from mice infected with nonlethal Py 17XNL were fully functional, DCs from mice infected with lethal Py YM were unable to produce IL-12 or present antigens to T cells. Apparently, lethal malaria causes a failure of DC function, resulting in the suppression of Th1 immune responses (Figure 1). Similar to lethal Py 17XL infected mice, it is possible that mice infected with lethal Py YM induce IL-10-producing CD4+ T cells. Although IL-10 might inhibit the DC function, such as antigen presentation and release of proinflammatory cytokines, little is known about whether IL-10 associates with a different type of DC induced between lethal and nonlethal murine malaria infection. Toll-like receptors (TLRs) play an important role in the innate immune system against pathogens [65]. Therefore, TLRs might be associated with disease severity during malaria infection. During lethal Py 17XL infection, TLR9 signaling in DCs is known to be crucial for the activation of Treg cells that suppress Th1 immune responses, causing high levels of parasitemia [64]. In contrast, MyD88, but not TLR signaling, has been shown to be necessary for elimination of parasites in mice infected with nonlethal Py 17XNL [66]. Accordingly, a different type of DC induced between lethal and nonlethal murine malaria infection might induce different subsets of CD4+ T cells, such as IL-10-producing CD4+ T cells or Treg cells.
7. Is IL-10 Necessary for Host Protection against Murine Malaria Parasites?
Although IL-10-deficient mice suppressed an increase of parasitemia during coinfection with lethal and nonlethal parasites, mice were unable to eliminate parasites completely and eventually died [47, 50]. These results suggest that the lethal strains of malaria parasites may modulate the induction of adaptive immunity independent of IL-10. Millington et al. [67] demonstrated that Plasmodium infection inhibits the induction of adaptive immunity to heterologous antigens by modulating DC function. According to their paper, hemozoin (HZ), rather than infected RBC membranes, was a key factor involved in the suppression of murine DC function. On the other hand, it has been shown that repeated stimulation through TLR9, which is the receptor for HZ, engenders tolerance to signaling through TLR4 [68].
In fact, HZ activates DCs through the TLR9-MyD88 pathway [69]. A recent study has demonstrated that parasite protein-DNA complex, but not HZ, plays a crucial role in TLR9-mediated activation of DCs during infection [70]. Stimulation through TLR9 might be associated with development of severe hepatic pathology, because MyD88 pathway, which is activated by TLR9 stimulation, is known to be involved in severe hepatic pathology caused by Pb NK65 [51]. Coban et al. [71] and Griffith et al. [72] reported that the TLRs-MyD88 signaling pathway might play a critical role in ECM during lethal Pb ANKA infection. It has been shown that ECM is prevented by oral treatment with E6446, which is a synthetic antagonist of nucleic acid-sensing TLRs [73]. In contrast, it is demonstrated that murine cerebral malaria development is independent of Toll-like receptor signaling [74, 75]. It remains controversial whether TLRs-MyD88 signaling pathway is associated with ECM development.
In summary, IL-10 is necessary for suppression of hepatic pathology or ECM in the host although IL-10 entails a risk of downregulation of protective immunity against malaria parasites. CD4+ T cells of different kinds, such as pathological CD4+ T cells, IL-10-producing CD4+ T cells, or Treg cells, are induced during different kinds of Plasmodium spp infection. To induce a more effective immune response in host defense against Plasmodium spp, it is necessary to elucidate the interaction of innate and acquired immune cells such as DCs, αβ T cells, and γδ T cells.
Acknowledgments
This work was supported by Grant-in-Aid no. 20590428 for Scientific Research (C) to F. Kobayashi and Grant-in-Aid no. 21790410 for young Scientists (B) to M. Niikura from the Japan Society for the Promotion of Science.
References
- 1.Hernandez-Valladares M, Naessens J, Iraqi FA. Genetic resistance to malaria in mouse models. Trends in Parasitology. 2005;21(8):352–355. doi: 10.1016/j.pt.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Stevenson MM, Tam MF, Belosevic M, Van Der Meide PH, Podoba JE. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infection and Immunity. 1990;58(10):3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sypek JP, Chung CL, Mayor SEH, et al. Resolution of cutaneous leishmaniasis: interleukin 12 initiates a protective T helper type 1 immune response. Journal of Experimental Medicine. 1993;177(6):1797–1802. doi: 10.1084/jem.177.6.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gazzinelli RT, Hieny S, Wynn TA, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell- deficient hosts. Proceedings of the National Academy of Sciences of the United States of America. 1993;90(13):6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gazzinelli RT, Wysocka M, Hayashi S, et al. Parasite-induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. Journal of Immunology. 1994;153(6):2533–2543. [PubMed] [Google Scholar]
- 6.Luty AJF, Perkins DJ, Lell B, et al. Low interleukin-12 activity in severe Plasmodium falciparum malaria. Infection and Immunity. 2000;68(7):3909–3915. doi: 10.1128/iai.68.7.3909-3915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luty AJF, Lell B, Schmidt-Ott R, et al. Interferon-γ responses are associated with resistance to reinfection with Plasmodium falciparum in young African children. Journal of Infectious Diseases. 1999;179(4):980–988. doi: 10.1086/314689. [DOI] [PubMed] [Google Scholar]
- 8.Clark IA, Hunt NH, Butcher GA, Cowden WB. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-γ or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. Journal of Immunology. 1987;139(10):3493–3496. [PubMed] [Google Scholar]
- 9.Waqki S, Uehara S, Kanbe K, Ono K, Suzuki M, Nariuchi H. The role of T cells in pathogenesis and protective immunity to murine malaria. Immunology. 1992;75(4):646–651. [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi F, Morii T, Matsui T, et al. Production of interleukin 10 during malaria caused by lethal and nonlethal variants of Plasmodium yoelii yoelii. Parasitology Research. 1996;82(5):385–391. doi: 10.1007/s004360050133. [DOI] [PubMed] [Google Scholar]
- 11.Su Z, Stevenson MM. IL-12 is required for antibody-mediated protective immunity against blood-stage Plasmodium chabaudi AS malaria infection in mice. Journal of Immunology. 2002;168(3):1348–1355. doi: 10.4049/jimmunol.168.3.1348. [DOI] [PubMed] [Google Scholar]
- 12.Yoshimoto T, Yoneto T, Waki S, Nariuchi H. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. Journal of Infectious Diseases. 1998;177(6):1674–1681. doi: 10.1086/515301. [DOI] [PubMed] [Google Scholar]
- 13.Wykes MN, Liu XQ, Beattie L, et al. Plasmodium strain determines dendritic cell function essential for survival from malaria. PLoS Pathogens. 2007;3(7) doi: 10.1371/journal.ppat.0030096. Article ID e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anderson CF, Stumhofer JS, Hunter CA, Sacks D. IL-27 regulates IL-10 and IL-17 from CD4+ cells in nonhealing Leishmania major infection. Journal of Immunology. 2009;183(7):4619–4627. doi: 10.4049/jimmunol.0804024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stumhofer JS, Silver JS, Laurence A, et al. Interleukins 27 and 6 induce STAT3-mediated T cell production of interleukin 10. Nature Immunology. 2007;8(12):1363–1371. doi: 10.1038/ni1537. [DOI] [PubMed] [Google Scholar]
- 16.Batten M, Kljavin NM, Li J, Walter MJ, De Sauvage FJ, Ghilardi N. Cutting edge: IL-27 is a potent inducer of IL-10 but not FoxP3 in murine T cells. Journal of Immunology. 2008;180(5):2752–2756. doi: 10.4049/jimmunol.180.5.2752. [DOI] [PubMed] [Google Scholar]
- 17.Murugaiyan G, Mittal A, Lopez-Diego R, Maier LM, Anderson DE, Weiner HL. IL-27 is a key regulator of IL-10 and IL-17 production by human CD+ T cells. Journal of Immunology. 2009;183(4):2435–2443. doi: 10.4049/jimmunol.0900568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard CL, Harrington LE, Janowski KM, et al. Regulatory T cells expressing interleukin 10 develop from Foxp3+ and Foxp3− precursor cells in the absence of interleukin 10. Nature Immunology. 2007;8(9):931–941. doi: 10.1038/ni1504. [DOI] [PubMed] [Google Scholar]
- 19.Maynard CL, Hatton RD, Helms WS, Oliver JR, Stephensen CB, Weaver CT. Contrasting roles for all-trans retinoic acid in TGF-β-mediated induction of Foxp3 and Il10 genes in developing regulatory T cells. Journal of Experimental Medicine. 2009;206(2):343–357. doi: 10.1084/jem.20080950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brandenburg S, Takahashi T, de la Rosa M, et al. IL-2 induces in vivo suppression by CD4+CD25+Foxp3+ regulatory T cells. European Journal of Immunology. 2008;38(6):1643–1653. doi: 10.1002/eji.200737791. [DOI] [PubMed] [Google Scholar]
- 21.Tsuji-Takayama K, Suzuki M, Yamamoto M, et al. The production of IL-10 by human regulatory T cells is enhanced by IL-2 through a STAT5-responsive intronic enhancer in the IL-10 locus. Journal of Immunology. 2008;181(6):3897–3905. doi: 10.4049/jimmunol.181.6.3897. [DOI] [PubMed] [Google Scholar]
- 22.Murai M, Turovskaya O, Kim G, et al. Interleukin 10 acts on regulatory T cells to maintain expression of the transcription factor Foxp3 and suppressive function in mice with colitis. Nature immunology. 2009;10(11):1178–1184. doi: 10.1038/ni.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roers A, Siewe L, Strittmatter E, et al. T cell-specific inactivation of the interleukin 10 gene in mice results in enhanced T cell responses but normal innate responses to lipopolysaccharide or skin irritation. Journal of Experimental Medicine. 2004;200(10):1289–1297. doi: 10.1084/jem.20041789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seki S, Osada SI, Ono S, et al. Role of liver NK cells and peritoneal macrophages in gamma interferon and interleukin, 10 production in experimental bacterial peritonitis in mice. Infection and Immunity. 1998;66(11):5286–5294. doi: 10.1128/iai.66.11.5286-5294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siewe L, Bollati-Fogolin M, Wickenhauser C, Krieg T, Müller W, Roers A. Interleukin-10 derived from macrophages and/or neutrophils regulates the inflammatory response to LPS but not the response to CpG DNA. European Journal of Immunology. 2006;36(12):3248–3255. doi: 10.1002/eji.200636012. [DOI] [PubMed] [Google Scholar]
- 26.Sabat R, Grütz G, Warszawska K, et al. Biology of interleukin-10. Cytokine and Growth Factor Reviews. 2010;21(5):331–344. doi: 10.1016/j.cytogfr.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 27.De Waal Malefyt R, Abrams J, Bennett B, Figdor CG, De Vries JE. Interleukin 10(IL-10) inhibits cytokine synthesis by human monocytes: an autoregulatory role of IL-10 produced by monocytes. Journal of Experimental Medicine. 1991;174(5):1209–1220. doi: 10.1084/jem.174.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fiorentino DF, Zlotnik A, Mosmann TR, Howard M, O’Garra A. IL-10 inhibits cytokine production by activated macrophages. Journal of Immunology. 1991;147(11):3815–3822. [PubMed] [Google Scholar]
- 29.D’Andrea A, Aste-Amezaga M, Valiante NM, Ma X, Kubin M, Trinchieri G. Interleukin 10 (IL-10) inhibits human lymphocyte interferon γ-production by suppressing natural killer cell stimulatory factor/IL-12 synthesis in accessory cells. Journal of Experimental Medicine. 1993;178(3):1041–1048. doi: 10.1084/jem.178.3.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Del Prete G, De Carli M, Almerigogna F, Giudizi MG, Biagiotti R, Romagnani S. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) T cell clones and inhibits their antigen-specific proliferation and cytokine production. Journal of Immunology. 1993;150(2):353–360. [PubMed] [Google Scholar]
- 31.Groux H, Bigler M, De Vries JE, Roncarolo MG. Interleukin-10 induces a long-term antigen-specific anergic state in human CD4+ T cells. Journal of Experimental Medicine. 1996;184(1):19–29. doi: 10.1084/jem.184.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gopinathan VP, Subramanian AR. Vivax and falciparum malaria seen at an Indian service hospital. Journal of Tropical Medicine and Hygiene. 1986;89(2):51–55. [PubMed] [Google Scholar]
- 33.Grau GE, Taylor TE, Molyneux ME, et al. Tumor necrosis factor and disease severity in children with falciparum malaria. The New England Journal of Medicine. 1989;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- 34.Kwiatkowski D, Hill AVS, Sambou I, et al. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. The Lancet. 1990;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- 35.Shaffer N, Grau GE, Hedberg K, et al. Tumor necrosis factor and severe malaria. Journal of Infectious Diseases. 1991;163(1):96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- 36.Mordmüller BG, Metzger WG, Juillard P, et al. Tumor necrosis factor in Plasmodium falciparum malaria: high plasma level is associated with fever, but high production capacity is associated with rapid fever clearance. European Cytokine Network. 1997;8(1):29–35. [PubMed] [Google Scholar]
- 37.Kurtzhals JAL, Adabayeri V, Goka BQ, et al. Low plasma concentrations of interleukin-10 in severe malarial anaemia compared with cerebral and uncomplicated malaria. The Lancet. 1998;351(9118):1768–1772. doi: 10.1016/S0140-6736(97)09439-7. [DOI] [PubMed] [Google Scholar]
- 38.Othoro C, Lal AA, Nahlen B, Koech D, Orago ASS, Udhayakumar V. A low interleukin-10 tumor necrosis factor-α ratio is associated with malaria anemia in children residing in a holoendemic malaria region in western Kenya. Journal of Infectious Diseases. 1999;179(1):279–282. doi: 10.1086/314548. [DOI] [PubMed] [Google Scholar]
- 39.Ouma C, Davenport GC, Were T, et al. Haplotypes of IL-10 promoter variants are associated with susceptibility to severe malarial anemia and functional changes in IL-10 production. Human Genetics. 2008;124(5):515–524. doi: 10.1007/s00439-008-0578-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobayashi F, Ishida H, Matsui T, Tsuji M. Effects of in vivo administration of anti-IL-10 or anti-IFN-γ monoclonal antibody on the host defense mechanism against Plasmodium yoelii yoelii infection. Journal of Veterinary Medical Science. 2000;62(6):583–587. doi: 10.1292/jvms.62.583. [DOI] [PubMed] [Google Scholar]
- 41.Omer FM, De Souza JB, Riley EM. Differential induction of TGF-β regulates proinflammatory cytokine production and determines the outcome of lethal and nonlethal Plasmodium yoelii infections. Journal of Immunology. 2003;171(10):5430–5436. doi: 10.4049/jimmunol.171.10.5430. [DOI] [PubMed] [Google Scholar]
- 42.Couper KN, Blount DG, Wilson MS, et al. IL-10 from CD4+CD25−Foxp3−CD127− adaptive regulatory T cells modulates parasite clearance and pathology during malaria infection. PLoS Pathogens. 2008;4(2) doi: 10.1371/journal.ppat.1000004. Article ID e1000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jankovic D, Kullberg MC, Feng CG, et al. Conventional T-bet+Foxp3− Th1 cells are the major source of host-protective regulatory IL-10 during intracellular protozoan infection. Journal of Experimental Medicine. 2007;204(2):273–283. doi: 10.1084/jem.20062175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson CF, Oukka M, Kuchroo VJ, Sacks D. CD4+CD25-Foxp3− Th1 cells are the source of IL-10-mediated immune suppression in chronic cutaneous leishmaniasis. Journal of Experimental Medicine. 2007;204(2):285–297. doi: 10.1084/jem.20061886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lutz HU, Pfister M, Hornig R. Tissue homeostatic role of naturally occurring anti-band 3 antibodies. Cellular and Molecular Biology. 1996;42(7):995–1005. [PubMed] [Google Scholar]
- 46.Clark IA, Chaudhri G. Tumour necrosis factor may contribute to the anaemia of malaria by causing dyserythropoiesis and erythrophagocytosis. British Journal of Haematology. 1988;70(1):99–103. doi: 10.1111/j.1365-2141.1988.tb02440.x. [DOI] [PubMed] [Google Scholar]
- 47.Niikura M, Kamiya S, Kita K, Kobayashi F. Coinfection with nonlethal murine malaria parasites suppresses pathogenesis caused by Plasmodium berghei NK65. Journal of Immunology. 2008;180(10):6877–6884. doi: 10.4049/jimmunol.180.10.6877. [DOI] [PubMed] [Google Scholar]
- 48.Findlay EG, Greig R, Stumhofer JS, et al. Essential role for IL-27 receptor signaling in prevention of Th1-mediated immunopathology during malaria infection. Journal of Immunology. 2010;185(4):2482–2492. doi: 10.4049/jimmunol.0904019. [DOI] [PubMed] [Google Scholar]
- 49.Sanni LA, Jarra W, Li C, Langhorne J. Cerebral edema and cerebral hemorrhages in interleukin-10-deficient mice infected with Plasmodium chabaudi. Infection and Immunity. 2004;72(5):3054–3058. doi: 10.1128/IAI.72.5.3054-3058.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Niikura M, Kamiya S, Nakane A, Kita K, Kobayashi F. IL-10 plays a crucial role for the protection of experimental cerebral malaria by co-infection with non-lethal malaria parasites. International Journal for Parasitology. 2010;40(1):101–108. doi: 10.1016/j.ijpara.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 51.Adachi K, Tsutsui H, Kashiwamura SI, et al. Plasmodium berghei infection in mice induces liver injury by an IL-12- and toll-like receptor/myeloid differentiation factor 88-dependent mechanism. Journal of Immunology. 2001;167(10):5928–5934. doi: 10.4049/jimmunol.167.10.5928. [DOI] [PubMed] [Google Scholar]
- 52.Yoeli M. Cerebral malaria: the quest for suitable experimental models in parasitic diseases of man. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1976;70(1):24–35. doi: 10.1016/0035-9203(76)90003-1. [DOI] [PubMed] [Google Scholar]
- 53.Lou J, Lucas R, Grau GE. Pathogenesis of cerebral malaria: recent experimental data and possible applications for humans. Clinical Microbiology Reviews. 2001;14(4):810–820. doi: 10.1128/CMR.14.4.810-820.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hunt NH, Grau GE. Cytokines: accelerators and brakes in the pathogenesis of cerebral malaria. Trends in Immunology. 2003;24(9):491–499. doi: 10.1016/s1471-4906(03)00229-1. [DOI] [PubMed] [Google Scholar]
- 55.Eckwalanga M, Marussig M, Tavares MD, et al. Murine AIDS protects mice against experimental cerebral malaria: down- regulation by interleukin 10 of a T-helper type 1 CD4+ cell-mediated pathology. Proceedings of the National Academy of Sciences of the United States of America. 1994;91(17):8097–8101. doi: 10.1073/pnas.91.17.8097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kossodo S, Monso C, Juillard P, Velu T, Goldman M, Grau GE. Interleukin-10 modulates susceptibility in experimental cerebral malaria. Immunology. 1997;91(4):536–540. doi: 10.1046/j.1365-2567.1997.00290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mitchell AJ, Hansen AM, Hee L, et al. Early cytokine production is associated with protection from murine cerebral malaria. Infection and Immunity. 2005;73(9):5645–5653. doi: 10.1128/IAI.73.9.5645-5653.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Randall LM, Amante FH, McSweeney KA, et al. Common strategies to prevent and modulate experimental cerebral malaria in mouse strains with different susceptibilities. Infection and Immunity. 2008;76(7):3312–3320. doi: 10.1128/IAI.01475-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Specht S, Ruiz DF, Dubben B, Deininger S, Hoerauf A. Filaria-induced IL-10 suppresses murine cerebral malaria. Microbes and Infection. 2010;12(8-9):635–642. doi: 10.1016/j.micinf.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Voza T, Vigário AM, Belnoue E, et al. Species-specific inhibition of cerebral malaria in mice coinfected with Plasmodium spp. Infection and Immunity. 2005;73(8):4777–4786. doi: 10.1128/IAI.73.8.4777-4786.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Haque A, Best SE, Amante FH, et al. Cd4+ natural regulatory t cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathogens. 2010;6(12) doi: 10.1371/journal.ppat.1001221. Article ID e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hansen DS, Bernard NJ, Nie CQ, Scholeld L. NK cells stimulate recruitment of CXCR3+ T cells to the brain during Plasmodium berghei-mediated cerebral malaria. Journal of Immunology. 2007;178(9):5779–5788. doi: 10.4049/jimmunol.178.9.5779. [DOI] [PubMed] [Google Scholar]
- 63.Campanella GSV, Tager AM, El Khoury JK, et al. Chemokine receptor CXCR3 and its ligands CXCL9 and CXCL10 are required for the development of murine cerebral malaria. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(12):4814–4819. doi: 10.1073/pnas.0801544105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hisaeda H, Tetsutani K, Imai T, et al. Malaria parasites require TLR9 signaling for immune evasion by activating regulatory T cells. Journal of Immunology. 2008;180(4):2496–2503. doi: 10.4049/jimmunol.180.4.2496. [DOI] [PubMed] [Google Scholar]
- 65.Kawai T, Akira S. The role of pattern-recognition receptors in innate immunity: update on toll-like receptors. Nature Immunology. 2010;11(5):373–384. doi: 10.1038/ni.1863. [DOI] [PubMed] [Google Scholar]
- 66.Cramer JP, Lepenies B, Kamena F, et al. MyD88/IL-18-dependent pathways rather than TLRs control early parasitaemia in non-lethal Plasmodium yoelii infection. Microbes and Infection. 2008;10(12-13):1259–1265. doi: 10.1016/j.micinf.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 67.Millington OR, Di Lorenzo C, Phillips RS, Garside P, Brewer JM. Suppression of adaptive immunity to heterologous antigens during Plasmodium infection through hemozoin-induced failure of dendritic cell function. Journal of Biology. 2006;5, article no. 5 doi: 10.1186/jbiol34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yeo SJ, Yoon JG, Hong SC, Yi AK. CpG DNA induces self and cross-hyporesponsiveness of RAW264.7 cells in response to CpG DNA and lipopolysaccharide: alterations in IL-1 receptor-associated kinase expression. Journal of Immunology. 2003;170(2):1052–1061. doi: 10.4049/jimmunol.170.2.1052. [DOI] [PubMed] [Google Scholar]
- 69.Coban C, Ishii KJ, Kawai T, et al. Toll-like receptor 9 mediates innate immune activation by the malaria pigment hemozoin. Journal of Experimental Medicine. 2005;201(1):19–25. doi: 10.1084/jem.20041836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu X, Gowda NM, Kumar S, Gowda DC. Protein-DNA complex is the exclusive malaria parasite component that activates dendritic cells and triggers innate immune responses. Journal of Immunology. 2010;184(8):4338–4348. doi: 10.4049/jimmunol.0903824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Coban C, Uematsu S, Arisue N, et al. Pathological role of Toll-like receptor signaling in cerebral malaria. International Immunology. 2007;19(1):67–79. doi: 10.1093/intimm/dxl123. [DOI] [PubMed] [Google Scholar]
- 72.Griffith JW, O’Connor C, Bernard K, Town T, Goldstein DR, Bucala R. Toll-like receptor modulation of murine cerebral malaria is dependent on the genetic background of the host. Journal of Infectious Diseases. 2007;196(10):1553–1564. doi: 10.1086/522865. [DOI] [PubMed] [Google Scholar]
- 73.Franklin BS, Ishizaka ST, Lamphier M, et al. Therapeutical targeting of nucleic acid-sensing Toll-like receptors prevents experimental cerebral malaria. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(9):3689–3694. doi: 10.1073/pnas.1015406108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Togbe D, Schofield L, Grau GE, et al. Murine cerebral malaria development is independent of toll-like receptor signaling. American Journal of Pathology. 2007;170(5):1640–1648. doi: 10.2353/ajpath.2007.060889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lepenies B, Cramer JP, Burchard GD, Wagner H, Kirschning CJ, Jacobs T. Induction of experimental cerebral malaria is independent of TLR2/4/9. Medical Microbiology and Immunology. 2008;197(1):39–44. doi: 10.1007/s00430-007-0057-y. [DOI] [PubMed] [Google Scholar]


