Abstract
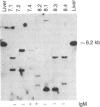
An in vitro system for the study of B-lymphocyte differentiation from precursor elements has been established. Two separate sets of culture conditions are utilized to control B-lymphocyte precursor growth versus differentiation. The first, modeled after the bone marrow culture system of Dexter and colleagues [Dexter, T. M. & Testa, N. G. (1976) in Methods in Cell Biology, ed. Prescott, D. M. (Academic, New York), Vol. 14, pp. 387-395], allows precursor replication whereas the second set of culture conditions is permissive for differentiation to more mature members of the B-lymphocyte lineage. The shift from one set of conditions to the other allows us to follow the kinetics of this differentiation from precursor cells to pre-B and B lymphocytes over a 5-week period. The resulting population of B-lineage cells synthesizes heterogeneous immunoglobulin molecules as analyzed by two-dimensional gel electrophoresis and has a heterogeneous array of immunoglobulin gene rearrangements, suggesting an origin from very early uncommitted precursors. This in vitro system will allow the study of genes that regulate B-lymphocyte differentiation and will aid in the isolation of the cellular intermediates in the B-cell developmental pathway.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Miller R. G., Phillips R. A. The identification in adult bone marrow of pluripotent and restricted stem cells of the myeloid and lymphoid systems. J Exp Med. 1977 Jun 1;145(6):1567–1579. doi: 10.1084/jem.145.6.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F. W., Yancopoulos G. D., Blackwell T. K., Wood C., Thomas E., Boss M., Coffman R., Rosenberg N., Tonegawa S., Baltimore D. Ordered rearrangement of immunoglobulin heavy chain variable region segments. EMBO J. 1984 Jun;3(6):1209–1219. doi: 10.1002/j.1460-2075.1984.tb01955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alt F., Rosenberg N., Lewis S., Thomas E., Baltimore D. Organization and reorganization of immunoglobulin genes in A-MULV-transformed cells: rearrangement of heavy but not light chain genes. Cell. 1981 Dec;27(2 Pt 1):381–390. doi: 10.1016/0092-8674(81)90421-9. [DOI] [PubMed] [Google Scholar]
- Aspinall R., Owen J. J. An investigation into the B lymphopoietic capacity of long-term bone marrow cultures. Immunology. 1983 Jan;48(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Bazill G. W., Haynes M., Garland J., Dexter T. M. Characterization and partial purification of a haemopoietic cell growth factor in WEHI-3 cell conditioned medium. Biochem J. 1983 Mar 15;210(3):747–759. doi: 10.1042/bj2100747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger D., Anderson S., Dexter T. M. Effect of src infection on long-term marrow cultures: increased self-renewal of hemopoietic progenitor cells without leukemia. Cell. 1984 Mar;36(3):763–773. doi: 10.1016/0092-8674(84)90356-8. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bradley T. R., Metcalf D. The growth of mouse bone marrow cells in vitro. Aust J Exp Biol Med Sci. 1966 Jun;44(3):287–299. doi: 10.1038/icb.1966.28. [DOI] [PubMed] [Google Scholar]
- Cohen D. I., Hedrick S. M., Nielsen E. A., D'Eustachio P., Ruddle F., Steinberg A. D., Paul W. E., Davis M. M. Isolation of a cDNA clone corresponding to an X-linked gene family (XLR) closely linked to the murine immunodeficiency disorder xid. 1985 Mar 28-Apr 3Nature. 314(6009):369–372. doi: 10.1038/314369a0. [DOI] [PubMed] [Google Scholar]
- Denis K. A., Treiman L. J., St Claire J. I., Witte O. N. Long-term cultures of murine fetal liver retain very early B lymphoid phenotype. J Exp Med. 1984 Oct 1;160(4):1087–1101. doi: 10.1084/jem.160.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dexter T. M., Allen T. D., Lajtha L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J Cell Physiol. 1977 Jun;91(3):335–344. doi: 10.1002/jcp.1040910303. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E. Loss of immunoreactivity in long-term bone marrow culture. Nature. 1978 Sep 14;275(5676):135–136. doi: 10.1038/275135a0. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Spooncer E., Toksoz D., Lajtha L. G. The role of cells and their products in the regulation of in vitro stem cell proliferation and granulocyte development. J Supramol Struct. 1980;13(4):513–524. doi: 10.1002/jss.400130410. [DOI] [PubMed] [Google Scholar]
- Dexter T. M., Testa N. G. Differentiation and proliferation of hemopoietic cells in culture. Methods Cell Biol. 1976;14:387–405. doi: 10.1016/s0091-679x(08)60498-7. [DOI] [PubMed] [Google Scholar]
- Dorshkind K., Phillips R. A. Characterization of early B lymphocyte precursors present in long-term bone marrow cultures. J Immunol. 1983 Nov;131(5):2240–2245. [PubMed] [Google Scholar]
- Dorshkind K., Phillips R. A. Maturational state of lymphoid cells in long-term bone marrow cultures. J Immunol. 1982 Dec;129(6):2444–2450. [PubMed] [Google Scholar]
- Early P., Huang H., Davis M., Calame K., Hood L. An immunoglobulin heavy chain variable region gene is generated from three segments of DNA: VH, D and JH. Cell. 1980 Apr;19(4):981–992. doi: 10.1016/0092-8674(80)90089-6. [DOI] [PubMed] [Google Scholar]
- Friedman R. L. Expression of human adenosine deaminase using a transmissable murine retrovirus vector system. Proc Natl Acad Sci U S A. 1985 Feb;82(3):703–707. doi: 10.1073/pnas.82.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S., Good R. A. Markers of human lymphocyte subpopulations in primary immunodeficiency and lymphoproliferative disorders. Semin Hematol. 1980 Jan;17(1):1–29. [PubMed] [Google Scholar]
- Hofman F. M., Billing R. J., Parker J. W., Taylor C. R. Cytoplasmic as opposed to surface Ia antigens expressed on human peripheral blood lymphocytes and monocytes. Clin Exp Immunol. 1982 Aug;49(2):355–363. [PMC free article] [PubMed] [Google Scholar]
- Jones-Villeneuve E., Phillips R. A. Potentials for lymphoid differentiation by cells from long term cultures of bone marrow. Exp Hematol. 1980 Jan;8(1):65–76. [PubMed] [Google Scholar]
- Kincade P. W., Paige C. J., Parkhouse R. M., Lee G. Characterization of murine colony-forming B cells. I. Distribution, resistance to anti-immunoglobulin antibodies, and expression of Ia antigens. J Immunol. 1978 Apr;120(4):1289–1294. [PubMed] [Google Scholar]
- Kurland J. I., Ziegler S. F., Witte O. N. Long-term cultured B lymphocytes and their precursors reconstitute the B-lymphocyte lineage in vivo. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7554–7558. doi: 10.1073/pnas.81.23.7554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P. The genetics of antibody diversity. Sci Am. 1982 May;246(5):102–115. doi: 10.1038/scientificamerican0582-102. [DOI] [PubMed] [Google Scholar]
- Nottenburg C., Weissman I. L. Cmu gene rearrangement of mouse immunoglobulin genes in normal B cells occurs on both the expressed and nonexpressed chromosomes. Proc Natl Acad Sci U S A. 1981 Jan;78(1):484–488. doi: 10.1073/pnas.78.1.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Peterson R. C., Cheung L. C., Mattaliano R. J., Chang L. M., Bollum F. J. Molecular cloning of human terminal deoxynucleotidyltransferase. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4363–4367. doi: 10.1073/pnas.81.14.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg N., Baltimore D. A quantitative assay for transformation of bone marrow cells by Abelson murine leukemia virus. J Exp Med. 1976 Jun 1;143(6):1453–1463. doi: 10.1084/jem.143.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J. W., Schrader S. In vitro studies on lymphocyte differentiation. I. Long term in vitro culture of cells giving rise to functional lymphocytes in irradiated mice. J Exp Med. 1978 Sep 1;148(3):823–828. doi: 10.1084/jem.148.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- TILL J. E., McCULLOCH E. A. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961 Feb;14:213–222. [PubMed] [Google Scholar]
- Till J. E., McCulloch E. A. Hemopoietic stem cell differentiation. Biochim Biophys Acta. 1980 Nov 26;605(4):431–459. doi: 10.1016/0304-419x(80)90009-8. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983 Apr 14;302(5909):575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Wall R., Kuehl M. Biosynthesis and regulation of immunoglobulins. Annu Rev Immunol. 1983;1:393–422. doi: 10.1146/annurev.iy.01.040183.002141. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Robertson D., Witte O. N. Murine B cell lymphopoiesis in long term culture. J Immunol Methods. 1984 Mar 16;67(2):353–369. doi: 10.1016/0022-1759(84)90475-7. [DOI] [PubMed] [Google Scholar]
- Whitlock C. A., Witte O. N. Long-term culture of B lymphocytes and their precursors from murine bone marrow. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3608–3612. doi: 10.1073/pnas.79.11.3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
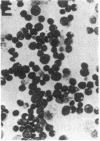
- Whitlock C. A., Ziegler S. F., Treiman L. J., Stafford J. I., Witte O. N. Differentiation of cloned populations of immature B cells after transformation with Abelson murine leukemia virus. Cell. 1983 Mar;32(3):903–911. doi: 10.1016/0092-8674(83)90075-2. [DOI] [PubMed] [Google Scholar]