Abstract
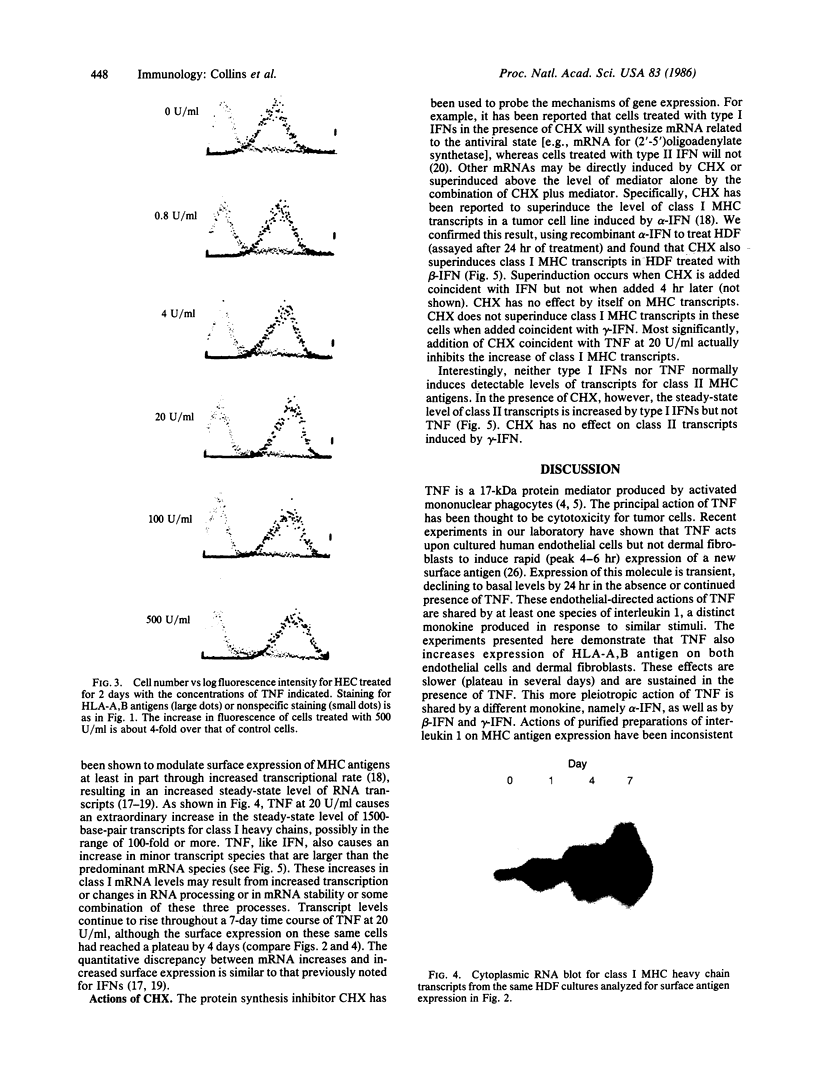
Recombinant human tumor necrosis factor (TNF), purified to greater than 99% homogeneity, increases surface expression of class I major histocompatibility complex (MHC) antigens to a maximum of 9-fold on cultured human endothelial cells (HEC) and human dermal fibroblasts (HDF). The increase is concentration dependent (peak 20-100 units/ml) and time dependent (nearly maximal by 4 days); expression remains elevated in the continued presence of TNF and requires greater than 7 days to return to basal levels upon TNF withdrawal. The increase in surface expression appears to result from increases in steady-state mRNA levels for the class I antigens, although the increase in mRNA is proportionately greater than for surface expression. No surface expression of or mRNA for class II MHC antigens is detectable in either control or TNF-treated HEC or HDF. These effects are similar to those produced by leukocyte or fibroblast (type I) interferons (IFNs). The protein synthesis inhibitor cycloheximide (CHX), when added coincidentally with type I IFNs, leads to superinduction of mRNA for class I MHC antigens and, unexpectedly, leads to the appearance of mRNA for class II MHC antigens. CHX has no effect by itself upon mRNA levels for class I or class II MHC antigens, nor does it modulate the increases in mRNA produced by immune (type II) IFN. Most interesting, CHX blocks the increase in mRNA for class I MHC antigens induced by TNF. Thus TNF appears to act on MHC gene expression through a newly synthesized protein intermediate. Our results provide direct evidence that TNF can modulate gene expression in normal (untransformed) cell types and contribute to understanding the complex nature of MHC gene regulation. Finally, they suggest that TNF may act in vivo as an immunoregulatory molecule.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Aggarwal B. B., Kohr W. J., Hass P. E., Moffat B., Spencer S. A., Henzel W. J., Bringman T. S., Nedwin G. E., Goeddel D. V., Harkins R. N. Human tumor necrosis factor. Production, purification, and characterization. J Biol Chem. 1985 Feb 25;260(4):2345–2354. [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Beutler B., Mahoney J., Le Trang N., Pekala P., Cerami A. Purification of cachectin, a lipoprotein lipase-suppressing hormone secreted by endotoxin-induced RAW 264.7 cells. J Exp Med. 1985 May 1;161(5):984–995. doi: 10.1084/jem.161.5.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Wheeler M. E., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 acts on cultured human vascular endothelium to increase the adhesion of polymorphonuclear leukocytes, monocytes, and related leukocyte cell lines. J Clin Invest. 1985 Nov;76(5):2003–2011. doi: 10.1172/JCI112200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski J. F., Welsh R. M. Interferon enhances the susceptibility of virus-infected fibroblasts to cytotoxic T cells. J Exp Med. 1985 Jan 1;161(1):257–262. doi: 10.1084/jem.161.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins T., Korman A. J., Wake C. T., Boss J. M., Kappes D. J., Fiers W., Ault K. A., Gimbrone M. A., Jr, Strominger J. L., Pober J. S. Immune interferon activates multiple class II major histocompatibility complex genes and the associated invariant chain gene in human endothelial cells and dermal fibroblasts. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4917–4921. doi: 10.1073/pnas.81.15.4917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didinsky J. B., Rheinwald J. G. Failure of hydrocortisone or growth factors to influence the senescence of fibroblasts in a new culture system for assessing replicative lifespan. J Cell Physiol. 1981 Oct;109(1):171–179. doi: 10.1002/jcp.1041090119. [DOI] [PubMed] [Google Scholar]
- Fiers W., Remaut E., Devos R., Cheroutre H., Contreras R., Gheysen D., Degrave W., Stanssens P., Tavernier J., Taya Y. The human fibroblast and human immune interferon genes and their expression in homologous and heterologous cells. Philos Trans R Soc Lond B Biol Sci. 1982 Sep 24;299(1094):29–38. doi: 10.1098/rstb.1982.0103. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Manly S. P., McMahon M., Kerr I. M., Stark G. R. Transcriptional and posttranscriptional regulation of interferon-induced gene expression in human cells. Cell. 1984 Oct;38(3):745–755. doi: 10.1016/0092-8674(84)90270-8. [DOI] [PubMed] [Google Scholar]
- Friedman R. L., Stark G. R. alpha-Interferon-induced transcription of HLA and metallothionein genes containing homologous upstream sequences. Nature. 1985 Apr 18;314(6012):637–639. doi: 10.1038/314637a0. [DOI] [PubMed] [Google Scholar]
- Gamble J. R., Harlan J. M., Klebanoff S. J., Vadas M. A. Stimulation of the adherence of neutrophils to umbilical vein endothelium by human recombinant tumor necrosis factor. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8667–8671. doi: 10.1073/pnas.82.24.8667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr Culture of vascular endothelium. Prog Hemost Thromb. 1976;3:1–28. [PubMed] [Google Scholar]
- Herman A., Parham P., Weissman S. M., Engelhard V. H. Recognition by xenogeneic cytotoxic T lymphocytes of cells expressing HLA-A2 or HLA-B7 after DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5056–5060. doi: 10.1073/pnas.80.16.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman A., Parham P., Weissman S. M., Engelhard V. H. Recognition by xenogeneic cytotoxic T lymphocytes of cells expressing HLA-A2 or HLA-B7 after DNA-mediated gene transfer. Proc Natl Acad Sci U S A. 1983 Aug;80(16):5056–5060. doi: 10.1073/pnas.80.16.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling P. D., Warren M. K., Vogel S. N. Antagonistic effect of interferon-beta on the interferon-gamma-induced expression of Ia antigen in murine macrophages. J Immunol. 1985 Sep;135(3):1857–1863. [PubMed] [Google Scholar]
- Muchmore A. V., Megson M., Decker J. M., Knudsen P., Mann D. L., Broder S. Inhibitory activity of antibodies to human Ia-like determinants: comparison of intact and pepsin-digested antibodies. J Immunol. 1983 Aug;131(2):725–730. [PubMed] [Google Scholar]
- Pober J. S., Gimbrone M. A., Jr, Cotran R. S., Reiss C. S., Burakoff S. J., Fiers W., Ault K. A. Ia expression by vascular endothelium is inducible by activated T cells and by human gamma interferon. J Exp Med. 1983 Apr 1;157(4):1339–1353. doi: 10.1084/jem.157.4.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scahill S. J., Devos R., Van der Heyden J., Fiers W. Expression and characterization of the product of a human immune interferon cDNA gene in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4654–4658. doi: 10.1073/pnas.80.15.4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton S. C., Mueller S. N., Levine E. M. Human endothelial cells: use of heparin in cloning and long-term serial cultivation. Science. 1983 Nov 11;222(4624):623–625. doi: 10.1126/science.6635659. [DOI] [PubMed] [Google Scholar]
- Torti F. M., Dieckmann B., Beutler B., Cerami A., Ringold G. M. A macrophage factor inhibits adipocyte gene expression: an in vitro model of cachexia. Science. 1985 Aug 30;229(4716):867–869. doi: 10.1126/science.3839597. [DOI] [PubMed] [Google Scholar]
- Wallach D., Fellous M., Revel M. Preferential effect of gamma interferon on the synthesis of HLA antigens and their mRNAs in human cells. Nature. 1982 Oct 28;299(5886):833–836. doi: 10.1038/299833a0. [DOI] [PubMed] [Google Scholar]