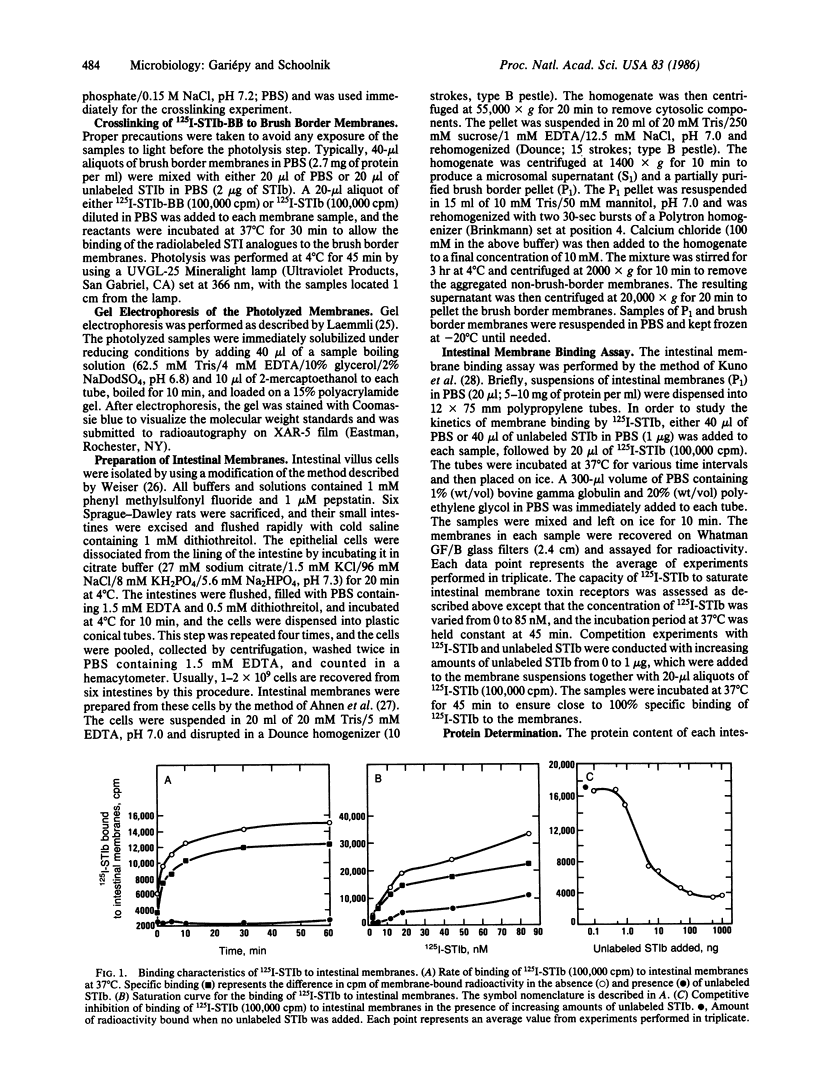
Abstract
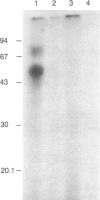
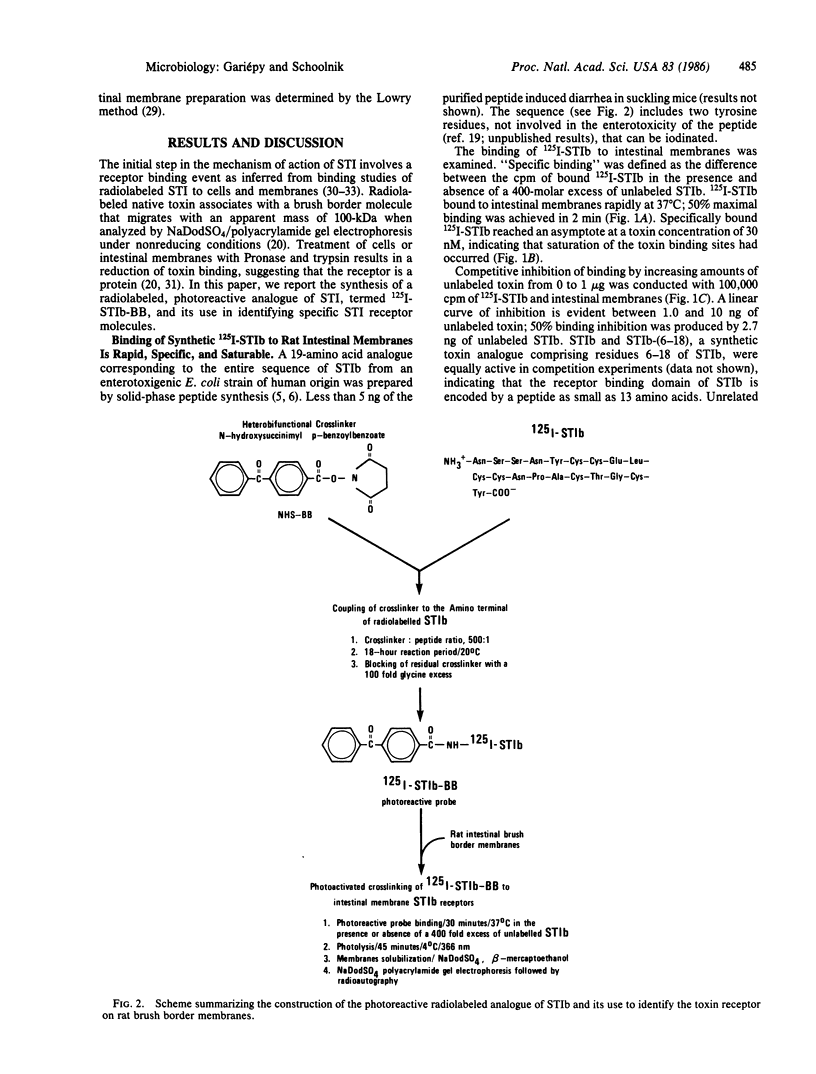
The Escherichia coli heat-stable enterotoxin, STIb was prepared by solid-phase peptide synthesis and purified to homogeneity by high-pressure liquid chromatography. This analogue was iodinated and shown to bind specifically to rat intestinal membranes. The radiolabeled peptide was derivatized at the amino terminus with the photoreactive heterobifunctional crosslinking agent N-hydroxysuccinimidyl p-benzoylbenzoate. This photoreactive probe also exhibited binding specificity. It was mixed with rat intestinal brush border membranes and photolyzed in the presence or absence of excess unlabeled STIb. Polyacrylamide gel electrophoresis performed in the presence of sodium dodecyl sulfate and 2-mercaptoethanol indicated that the peptide probe was crosslinked specifically to two molecular species of 57 and 75 kDa. One or both of these molecules appear to constitute the enterotoxin receptor or to be in close proximity to it.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahnen D. J., Santiago N. A., Cezard J. P., Gray G. M. Intestinal aminooligopeptidase. In vivo synthesis on intracellular membranes of rat jejunum. J Biol Chem. 1982 Oct 25;257(20):12129–12135. [PubMed] [Google Scholar]
- Aimoto S., Takao T., Shimonishi Y., Hara S., Takeda T., Takeda Y., Miwatani T. Amino-acid sequence of a heat-stable enterotoxin produced by human enterotoxigenic Escherichia coli. Eur J Biochem. 1982 Dec 15;129(2):257–263. doi: 10.1111/j.1432-1033.1982.tb07047.x. [DOI] [PubMed] [Google Scholar]
- Burgess M. N., Bywater R. J., Cowley C. M., Mullan N. A., Newsome P. M. Biological evaluation of a methanol-soluble, heat-stable Escherichia coli enterotoxin in infant mice, pigs, rabbits, and calves. Infect Immun. 1978 Aug;21(2):526–531. doi: 10.1128/iai.21.2.526-531.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Dreyfus L. A., Jaso-Friedmann L., Robertson D. C. Characterization of the mechanism of action of Escherichia coli heat-stable enterotoxin. Infect Immun. 1984 May;44(2):493–501. doi: 10.1128/iai.44.2.493-501.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus L. A., Robertson D. C. Solubilization and partial characterization of the intestinal receptor for Escherichia coli heat-stable enterotoxin. Infect Immun. 1984 Nov;46(2):537–543. doi: 10.1128/iai.46.2.537-543.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field M., Graf L. H., Jr, Laird W. J., Smith P. L. Heat-stable enterotoxin of Escherichia coli: in vitro effects on guanylate cyclase activity, cyclic GMP concentration, and ion transport in small intestine. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2800–2804. doi: 10.1073/pnas.75.6.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz J. C., Jaso-Friedman L., Robertson D. C. Binding of Escherichia coli heat-stable enterotoxin to rat intestinal cells and brush border membranes. Infect Immun. 1984 Feb;43(2):622–630. doi: 10.1128/iai.43.2.622-630.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gariepy J., O'Hanley P., Waldman S. A., Murad F., Schoolnik G. K. A common antigenic determinant found in two functionally unrelated toxins. J Exp Med. 1984 Oct 1;160(4):1253–1258. doi: 10.1084/jem.160.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Drake K. W. Effect of purified Escherichia coli heat-stable enterotoxin on intestinal cyclic nucleotide metabolism and fluid secretion. Infect Immun. 1979 Apr;24(1):19–23. doi: 10.1128/iai.24.1.19-23.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannella R. A., Luttrell M., Thompson M. Binding of Escherichia coli heat-stable enterotoxin to receptors on rat intestinal cells. Am J Physiol. 1983 Oct;245(4):G492–G498. doi: 10.1152/ajpgi.1983.245.4.G492. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Richardson S. H. Adenosine diphosphate-ribosylation of adenylate cyclase catalyzed by heat-labile enterotoxin of Escherichia coli: comparison with cholera toxin. J Infect Dis. 1980 Jan;141(1):64–70. doi: 10.1093/infdis/141.1.64. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Hughes J. M., Chang B., Robertson D. C., Murad F. Activation of intestinal guanylate cyclase by heat-stable enterotoxin of Escherichia coli: studies of tissue specificity, potential receptors, and intermediates. J Infect Dis. 1980 Aug;142(2):220–228. doi: 10.1093/infdis/142.2.220. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Limitations of the infant mouse test for Escherichia coli heat stable enterotoxin. Can J Comp Med. 1979 Oct;43(4):371–379. [PMC free article] [PubMed] [Google Scholar]
- Hughes J. M., Murad F., Chang B., Guerrant R. L. Role of cyclic GMP in the action of heat-stable enterotoxin of Escherichia coli. Nature. 1978 Feb 23;271(5647):755–756. doi: 10.1038/271755a0. [DOI] [PubMed] [Google Scholar]
- Kennedy D. J., Greenberg R. N., Dunn J. A., Abernathy R., Ryerse J. S., Guerrant R. L. Effects of Escherichia coli heat-stable enterotoxin STb on intestines of mice, rats, rabbits, and piglets. Infect Immun. 1984 Dec;46(3):639–643. doi: 10.1128/iai.46.3.639-643.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuno T., Saijoh K., Tanaka C. Solubilization of D2 dopamine receptor coupled to guanine nucleotide regulatory protein from bovine striatum. J Neurochem. 1983 Sep;41(3):841–847. doi: 10.1111/j.1471-4159.1983.tb04817.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lallier R., Bernard F., Gendreau M., Lazure C., Seidah N. G., Chrétien M., St-Pierre S. A. Isolation and purification of Escherichia coli heat-stable enterotoxin of porcine origin. Anal Biochem. 1982 Dec;127(2):267–275. doi: 10.1016/0003-2697(82)90171-3. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Moseley S. L., Moon H. W., Whipp S. C., Gyles C. L., So M. Characterization of the gene encoding heat-stable toxin II and preliminary molecular epidemiological studies of enterotoxigenic Escherichia coli heat-stable toxin II producers. Infect Immun. 1983 Oct;42(1):264–268. doi: 10.1128/iai.42.1.264-268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. J., Schultz G. S., Levy R. S. Rapid purification of radioiodinated peptides with Sep-Pak reversed phase cartridges and HPLC. Int J Pept Protein Res. 1984 Aug;24(2):112–122. doi: 10.1111/j.1399-3011.1984.tb00935.x. [DOI] [PubMed] [Google Scholar]
- Moseley S. L., Hardy J. W., Hug M. I., Echeverria P., Falkow S. Isolation and nucleotide sequence determination of a gene encoding a heat-stable enterotoxin of Escherichia coli. Infect Immun. 1983 Mar;39(3):1167–1174. doi: 10.1128/iai.39.3.1167-1174.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picken R. N., Mazaitis A. J., Maas W. K., Rey M., Heyneker H. Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect Immun. 1983 Oct;42(1):269–275. doi: 10.1128/iai.42.1.269-275.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M. C., Guandalini S., Smith P. L., Field M. Mode of action of heat-stable Escherichia coli enterotoxin. Tissue and subcellular specificities and role of cyclic GMP. Biochim Biophys Acta. 1980 Sep 17;632(1):35–46. doi: 10.1016/0304-4165(80)90247-0. [DOI] [PubMed] [Google Scholar]
- Rönnberg B., Wadström T., Jörnvall H. Structure of a heat-stable enterotoxin produced by a human strain of Escherichia coli. Differences from the toxin of another human strain suggest the presence of compensated amino acid exchanges. FEBS Lett. 1983 May 8;155(2):183–186. doi: 10.1016/0014-5793(82)80598-x. [DOI] [PubMed] [Google Scholar]
- Shapiro A. L., Viñuela E., Maizel J. V., Jr Molecular weight estimation of polypeptide chains by electrophoresis in SDS-polyacrylamide gels. Biochem Biophys Res Commun. 1967 Sep 7;28(5):815–820. doi: 10.1016/0006-291x(67)90391-9. [DOI] [PubMed] [Google Scholar]
- So M., McCarthy B. J. Nucleotide sequence of the bacterial transposon Tn1681 encoding a heat-stable (ST) toxin and its identification in enterotoxigenic Escherichia coli strains. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4011–4015. doi: 10.1073/pnas.77.7.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Knoop F. C. Effect of heat-stable enterotoxin of Escherichia coli on cultured mammalian cells. J Infect Dis. 1983 Mar;147(3):450–459. doi: 10.1093/infdis/147.3.450. [DOI] [PubMed] [Google Scholar]
- Thompson M. R., Giannella R. A. Revised amino acid sequence for a heat-stable enterotoxin produced by an Escherichia coli strain (18D) that is pathogenic for humans. Infect Immun. 1985 Mar;47(3):834–836. doi: 10.1128/iai.47.3.834-836.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman S. A., O'Hanley P., Falkow S., Schoolnik G., Murad F. A simple, sensitive, and specific assay for the heat-stable enterotoxin of Escherichia coli. J Infect Dis. 1984 Jan;149(1):83–89. doi: 10.1093/infdis/149.1.83. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Weiser M. M. Intestinal epithelial cell surface membrane glycoprotein synthesis. I. An indicator of cellular differentiation. J Biol Chem. 1973 Apr 10;248(7):2536–2541. [PubMed] [Google Scholar]
- Yoshimura S., Ikemura H., Watanabe H., Aimoto S., Shimonishi Y., Hara S., Takeda T., Miwatani T., Takeda Y. Essential structure for full enterotoxigenic activity of heat-stable enterotoxin produced by enterotoxigenic Escherichia coli. FEBS Lett. 1985 Feb 11;181(1):138–142. doi: 10.1016/0014-5793(85)81129-7. [DOI] [PubMed] [Google Scholar]