Abstract
Background
Neuroadaptations within the nucleus accumbens (NAc) have been implicated in molecular mechanisms underlying the development and/or maintenance of alcohol abuse disorders. We recently reported that the activation of mammalian target of rapamycin complex 1 (mTORC1) signaling pathway in the NAc of rodents, following exposure to alcohol, contributes to alcohol drinking behaviors. The kinase AKT, is the main upstream activator of the mTORC1 pathway. We therefore hypothesized that the activation of AKT in the NAc in response to alcohol exposure plays an important role in mechanisms that underlie excessive alcohol consumption.
Methods
Western blot analysis was used to assess the phosphorylation levels of enzymes. Acute exposure of mice to alcohol was achieved by the administration of 2 g/kg alcohol intraperitoneally, (i.p.). Two-bottle choice and operant self-administration procedures were used to assess drinking behaviors in rats.
Results
We found that acute systemic administration of alcohol and recurring cycles of excessive voluntary consumption of alcohol and withdrawal result in the activation of AKT signaling in the NAc of rodents. Importantly, we show that inhibition of AKT, or its upstream activator, phosphatidylinositol-3-kinase (PI3K), within the NAc of rats attenuates binge drinking as well as alcohol but not sucrose operant self-administration.
Conclusions
Our results suggest that the activation of the AKT pathway in the NAc in response to alcohol exposure is an important contributor to the molecular mechanisms underlying alcohol-drinking behaviors. AKT signaling pathway inhibitors are therefore potential candidates for drug development for the treatment of alcohol use and abuse disorders.
Keywords: alcohol, addiction, AKT, GSK-3, PI3K, nucleus accumbens
Introduction
Alcohol addiction is a psychiatric disorder in which symptoms persist despite negative consequences (1). Although alcohol use and abuse disorders are major health and socioeconomic problems, only a limited number of medications are available to treat adverse phenotypes such as excessive drinking, craving and relapse (1). Therefore, unraveling the molecular and neuronal processes responsible for the development and persistence of these pathological behaviors may lead to the development of new strategies to treat the disease.
The use of animal models allows the exploration of processes that underlie some key characteristics of adverse behaviors related to alcohol use and abuse disorders such as the consumption of an excessive amount of alcohol (1–2). For example, a progressive escalation of alcohol intake can be obtained in rats which undergo cycles of voluntary alcohol intake and withdrawal in a 24-hr-intermittent 2-bottle choice access procedure (3–4). This paradigm also leads to a stable and high level of voluntary consumption (3–4) that results in a blood alcohol concentration (BAC) of 80.9 ± 7 mg%, 30 min after the beginning of an alcohol drinking session (3). This BAC corresponds to human binge drinking as defined by the National Institute on Alcohol Abuse and Alcoholism (5), and therefore allows the investigation of the neuronal processes underlying excessive drinking of alcohol.
The NAc, a key component of the reward circuit, is a major substrate of all drugs of abuse (2, 6), and, as such, plays a key role in the expression of behavioral phenotypes associated with alcohol exposure (1). Accordingly, using rodent models of excessive alcohol consumption, we recently established that the mTORC1-mediated signaling pathway in the NAc of rodents is activated in response to binge drinking of alcohol, and that the activation persists even 24 hrs after alcohol withdrawal (7). We further showed that the inhibition of the mTORC1 pathway results in the attenuation of alcohol-related behaviors such as locomotor sensitization, conditioned place preference and excessive drinking (7).
mTORC1 is a downstream target of the phosphatidylinositol-3-kinase (PI3K) pathway (8–9). Specifically, activation of PI3K results in the production of phosphatidylinositol (3,4,5)-trisphosphate (PIP3) at the plasma membrane leading to the recruitment of AKT and its kinase, the phosphoinositide-dependent protein kinase 1 (PDK1), to the membrane. Upon co-localization, PDK1 phosphorylates AKT at the threonine 308 (10). Activation of AKT also requires its phosphorylation on the serine 473 residue by the PDK2/mammalian target of rapamycin complex 2 (mTORC2) (10–13). AKT, in turn, phosphorylates and inhibits tuberin, a negative regulator of the Ras-homolog enriched in brain (Rheb) and mTORC1, leading to the activation of the mTORC1 kinase (8–9, 14). In addition to this dominant PI3K/AKT signaling cascade (8), the activation of the Ras/Raf/ERK1/2 (extracellular-signal-regulated kinase 1 and 2) pathway can also lead to the activation of mTORC1 through the direct phosphorylation and inhibition of tuberin by ERK1/2 (8, 14–15).
Here, we set out to examine whether AKT and/or ERK1/2 are activated in the NAc in response to alcohol exposure and, if so, to test for the possible contribution of the pathway(s) to the expression and/or maintenance of alcohol drinking behaviors.
Material and methods
Animals
Nine-week old male C57BL/6J mice were obtained from Jackson Laboratories and male Long-Evans rats (250–300 g) were purchased from Harlan. Mice and rats were housed in a temperature and humidity-controlled room under a 12 hr light:dark cycle (lights on at 07:00 am) with food and water available ad libitum. All animal procedures in this report were approved by the Gallo Center Institutional Animal Care and Use Committee and were conducted in agreement with the Guide for the Care and Use of Laboratory Animals, National Research Council, 1996.
Materials
See Supplement.
Preparation of solutions
See Supplement.
Systemic administration of alcohol
See Supplement.
Western Blot Analyses
Rats were anesthetized by isoflurane and were killed by decapitation. Mice were killed by cervical dislocation. The NAc was immediately collected and homogenized in a RadioImmuno Precipitation Assay (RIPA) buffer containing 25 mM Tris-HCl pH 7.6, 150 mM NaCl, EDTA 1mM, 1% (vol/vol) NP-40, 0.5% (weight/vol) sodium deoxycholate and 0.1% (weight/vol) SDS, protease and phosphatase inhibitors. Forty µg of samples were resolved on a 4–12% SDS-PAGE, and transferred to a nitrocellulose membrane. Membranes were blocked for 1 hr with 5% (weight/vol) non-fat milk in Tris Buffer Saline/0.1% (vol/vol) Tween 20 (TBS-T) and then incubated overnight at 4°C in the same blocking solution including the appropriate antibody. After extensive washing with TBS-T, bound primary antibodies were detected with HRP-conjugated secondary antibody and visualized by Enhanced Chemiluminescence (ECL). After measuring the level of phospho-proteins, membranes were then stripped for 30 min at 50°C in buffer containing 100 mM 2-β-mercaptoethanol, 2% (weight/vol) SDS, 62.5 mM Tris-HCl pH 6.7, followed by extensive washing in TBS-T before re-blocking and re-probing with the appropriate total antibody. The optical density of the relevant immunoreactive band was quantified using NIH Image 1.63 program. The values of the phospho-protein signal were normalized to the signal of the total protein in the same sample. Results were expressed as a percentage of the control.
Intermittent-access 20% alcohol 2-bottle choice drinking paradigm
Animals were given 24-hr concurrent access to one bottle of 20% vol/vol alcohol in tap water and one bottle of water, starting at 12:00 p.m. on Monday, Wednesday, and Friday, with 24 or 48-hr alcohol-deprivation periods between the alcohol-drinking sessions. The placement (left or right) of each solution was alternated between each session to control for side preference. The water and alcohol bottles were weighed after 30 min and 24 h of access. After 6 weeks of alcohol exposure, animals were implanted bilaterally with guide cannulae in the NAc. Following 4 days of recovery, intermittent-access 20% alcohol 2-bottle choice drinking procedure was resumed and microinfusions of wortmannin and triciribine were conducted. For more details, see Supplement.
Surgery
Rats were anesthetized with isoflurane (Baxter Health Care Corporation) and then bilaterally implanted with 26-gauge stainless-steel guide cannulas (Plastics One, Roanoke, VA) aimed at the NAc (AP +1.6 mm, ML ±1 mm from Bregma and DV −5.9 mm from the skull surface). For more details, see Supplement.
Operant self-administration of alcohol
Rats were trained to self-administer a 20% alcohol solution in operant self-administration chambers (Med Associates) under a fixed ratio 3 (FR3) schedule of reinforcement, wherein 3 lever presses resulted in the delivery of 0.1 ml of alcohol. Surgery and microinfusions of the inhibitors started after 6 weeks of alcohol self-administration upon acquisition of a stable baseline of responding. In the first experiment, all subjects received vehicle or wortmannin in a counterbalanced manner, with one microinfusion per week. One week later, the same animals and procedure were used to test the effect of the triciribine. For more details, see Supplement.
Intra-NAc infusions of wortmannin and triciribine
Rats were infused with vehicle or wortmannin using doses (0.1 or 0.4 µg/side) based on previous studies (16–17), and triciribine (0.05 or 0.5 µg /side) (18). The half-life of wortmannin is shorter (one hour) (19–20) than the half-life of triciribine (several hours) (21). Therefore wortmannin and triciribine were infused into the NAc of rats, 1 hr or 3 hrs respectively, before the 24-hr alcohol-drinking session or the 30-min operant self-administration. A total of 1 µl/side of each inhibitor or vehicle was infused over 2.5 min into the NAc of gently restrained rats via injection cannulae extending 1 mm beyond the guide cannula tip. Injection cannulae were left in place for an additional 1 min. After infusion, stylets were replaced in the guide cannulae and the animal was put back in the home cage. All subjects received each dose of inhibitors in a counterbalanced manner, with one microinjection per week.
Operant self-administration of sucrose
Rats were trained to self-administer a solution of 1.5 % of sucrose under an FR3 schedule 5 days per week during 30-min sessions. Experiments started when the rats reached a stable level of presses. In a first experiment, all subjects received vehicle or wortmannin in a counterbalanced manner, with one microinfusion per week. Two weeks later, the same animals were used to test the effect of triciribine.
Histology
After completion of the experiments, rats implanted with cannulae were sacrificed by i.p. injection of pentobarbital and perfused transcardically with 4% paraformaldehyde. Coronal sections of the forebrain were stained with thionin to allow visualization of probe tracks in the NAc (Figure S1 in the Supplement). Only subjects with injection cannulae located in the NAc were included in the study.
Data analysis
Western blot data were analyzed with a one-tailed unpaired t-test. Rat 2-bottle choice and operant self-administration experiments were conducted in a within-subject design. Data were analyzed with one-way or two-way ANOVA with repeated measures. Significant main effects and interactions of the ANOVAs were further investigated with the Student-Newman-Keuls test or the method of contrasts (one-tailed paired t-test). Statistical significance was set at p < 0.05. Data are presented as mean ± SEM.
Results
Systemic administration of alcohol results in the activation of AKT pathway in the NAc of mice
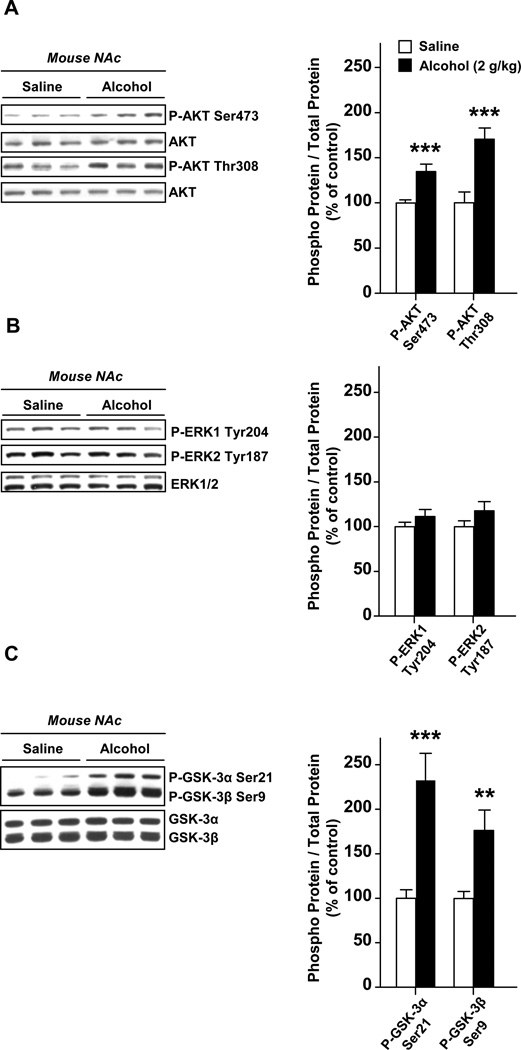
First, we aimed to determine whether AKT is activated in the NAc of mice treated with alcohol. Animals were systemically administered (i.p) with a non-hypnotic dose (2 g/kg) of alcohol and the phosphorylation of AKT was assessed 15 min later. As shown in Fig. 1A, we found that acute treatment of mice with alcohol results in the activation of AKT in the NAc as reflected by the increase in the phosphorylation level of amino acids threonine 308 and the serine 473. Another signaling cascade upstream mTORC1 is the ERK1/2 pathway; however, we did not detect any alteration in the phosphorylation level of ERK1/2 following alcohol administration (Fig. 1B), suggesting that this pathway is not activated in the NAc in response to acute alcohol administration.
Figure 1. Systemic administration of alcohol to mice results in a rapid activation of the AKT pathway in the NAc.
Mice were systemically administered (i.p.) with 2 g/kg of alcohol or saline solution, and the NAc was removed 15 min later. The level of AKT phosphorylation on threonine 308 and serine 473 (A), of ERK1/2 on tyrosine 204/187 (B), as well as the levels of GSK3α and GSK3β phosphorylation on serine 21 and serine 9 respectively (C), were determined by western blot analyses. Optical density quantification of the phosphorylation is expressed as the ratio of the phosphorylated protein to the total protein. n=9 per group. Data are presented as mean ± SEM and expressed as percentage of control. **p< 0.01, ***p<0.001, one-tailed unpaired t-test.
GSK-3 (Glycogen synthase kinase-3) is a serine and threonine kinase, which is a well-described downstream target of AKT (22–23). Therefore, we tested whether the activation of AKT in the NAc in response to alcohol results in the phosphorylation of the two GSK-3 isoforms, GSK-3α and GSK-3β. We found that acute administration of alcohol to mice results in the induction of the phosphorylation of GSK-3α and GSK-3β on serine 21 and serine 9 residues, respectively (Fig. 1C). Together, these data indicate that alcohol treatment induces a rapid activation of the AKT but not ERK1/2 pathway in the NAc.
AKT is activated in the NAc of rats with a history of excessive alcohol consumption
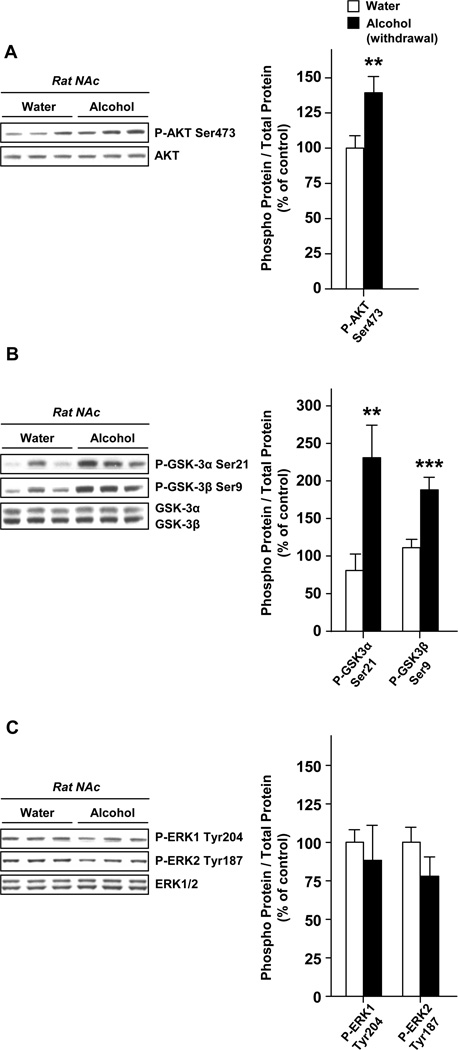
Next, we aimed to determine whether alterations of AKT signaling induced by alcohol in the NAc contribute to neuroadaptations that underlie alcohol consumption. To do so we first examined whether the AKT signaling within the NAc was activated in response to cycles of excessive alcohol consumption and withdrawal periods by measuring the phosphorylation levels of AKT, as well as its substrates GSK-3α and GSK-3β, 24 hrs after the last drinking session. We observed an elevation of the phosphorylation of AKT (Fig. 2A) and both of the GSK-3 isoforms (Fig. 2B). However, we did not observe any elevation in ERK1/2 phosphorylation suggesting that ERK1/2 activity was not increased in the NAc in response to alcohol exposure (Fig. 3C). Hence, excessive alcohol intake results in a sustained activation of the AKT but not ERK1/2 pathway in the NAc.
Figure 2. Excessive alcohol consumption results in the activation of AKT but not ERK1/2 signaling in the NAc of rats.
Rats experienced 2 months of intermittent-access 20% alcohol 2-bottle choice drinking sessions. Control animals underwent the same paradigm but had access to water only. The NAc were removed after the last 24 hrs of alcohol deprivation session (24 hr of withdrawal). The levels of AKT (A), GSK-3α, GSK-3β (B) and ERK1/2 (C) phosphorylation were determined by western blot analyses. n=16 (A), n=8–10 (B), n=6 (C) per group. Data are presented as mean ± SEM and expressed as percentage of control. **p<0.01, ***p<0.001, one-tailed unpaired t-test.
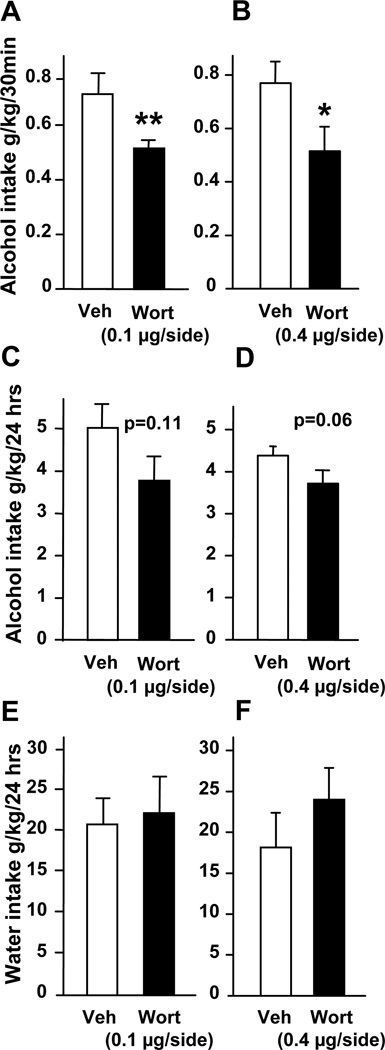
Figure 3. Intra-NAc infusion of wortmannin reduces binge drinking of alcohol in rats.
Vehicle (Veh) or wortmannin (Wort, 0.1 or 0.4 µg/side) were infused into the NAc 1 hr before the beginning of the 24-hr alcohol-drinking session in rats trained to consume a high amount of a 20% solution of alcohol in a 2-bottle choice paradigm. (A, B) Alcohol intake was measured 30 min after the beginning of the session. Alcohol (C, D) and water (E, F) intakes were also measured at the end of the 24-hr drinking session. Consumption is expressed in grams per kilogram (g/kg) of body weight. n=8–9 per group. Data are presented as mean ± SEM. One-way ANOVA with repeated measures showed significant effects of treatment for A [F(1,7) = 13.84, P = 0.007] and B [F(1,8) = 8.38, P = 0.02] but not for C [F(1,7) = 3.28, P = 0.11] and D [F(1,8) = 4.71, P = 0.062], *p< 0.05 and **p<0.01.
Inhibition of the AKT pathway within the NAc of rats attenuates binge drinking of alcohol
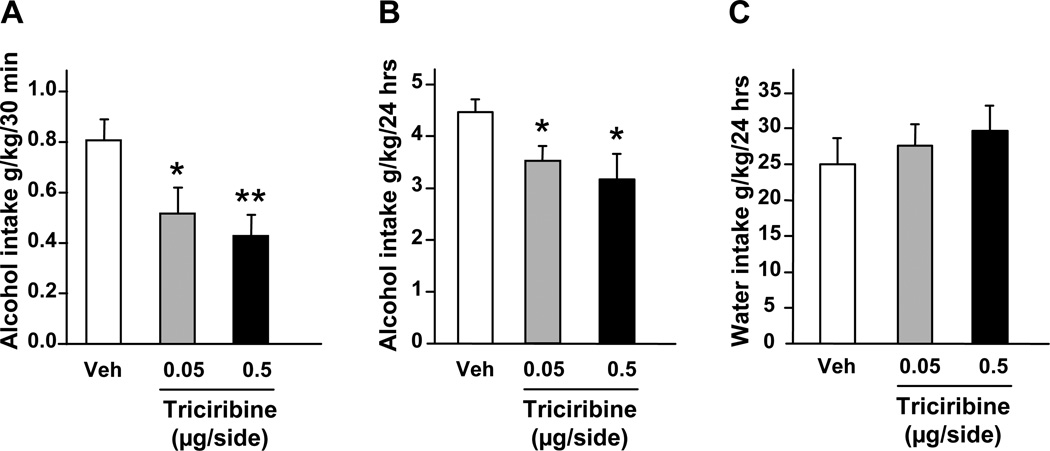
To test for the possible functional consequences of alcohol-mediated activation of AKT signaling in the NAc, we used the specific PI3K inhibitor, wortmannin (24). We first confirmed that intra-NAc infusion of wortmannin results in a selective inhibition of AKT (Figure S2 in the Supplement). Next, we established that the inhibition of PI3K by wortmannin in the NAc attenuates alcohol-mediated phosphorylation of AKT. As shown in Fig. S3 (see Supplement), the increase in AKT phosphorylation was observed in the NAc after acute systemic administration of alcohol in vehicle treated but not wortmannin treated mice. In addition to wortmannin, triciribine was used to directly inhibit the activity of AKT (18, 25). Wortmannin and triciribine were infused into the NAc of rats 1 and 3 hrs respectively (Figure S1A in the Supplement), before the beginning of a drinking session, and alcohol and water consumptions were monitored (see also methods). We found that intra-NAc infusion of both inhibitors attenuated binge drinking of alcohol as revealed by a decrease in alcohol intake during the first 30 min of the drinking session (Figs. 3A–B and 4A). We further observed that intra-NAc administration of triciribine (Fig. 4B) but not wortmannin (Fig. 3C–D), also significantly decreased alcohol intake over a period of 24 hr access. Importantly, intra-NAc inhibition of the AKT pathway by wortmannin (Fig. 3E–F) and triciribine (Fig. 4C) did not affect water intake. Together, these data indicate that the AKT pathway within the NAc contributes to the molecular mechanisms underlying the expression and/or maintenance of excessive alcohol consumption.
Figure 4. Intra-NAc infusion of triciribine reduces binge drinking of alcohol in rats.
Vehicle (Veh) or triciribine (0.05 or 0.5 µg/side) were infused into the NAc 3 hrs before the beginning of the 24-hr alcohol-drinking session in rats trained to consume a high amount of a 20% solution of alcohol in a 2-bottle choice paradigm. (A) Alcohol intake was measured 30 min after the beginning of the session. Alcohol (B) and water (C) intakes were also measured at the end of the 24-hr drinking session. Alcohol and water intakes are expressed in grams per kilogram (g/kg) of body weight. n=9 per group. Data are presented as mean ± SEM. One-way ANOVA with repeated measures showed significant effects of treatment for (A) [F(2,16) = 6.62, P = 0.008] and (B) [F(2,16) = 5.71, P = 0.013]; *p<0.05 and **p<0.01 compared to vehicle (Newman-Keuls post-hoc test).
Inhibition of AKT pathway within the NAc of rats attenuates operant self-administration of alcohol
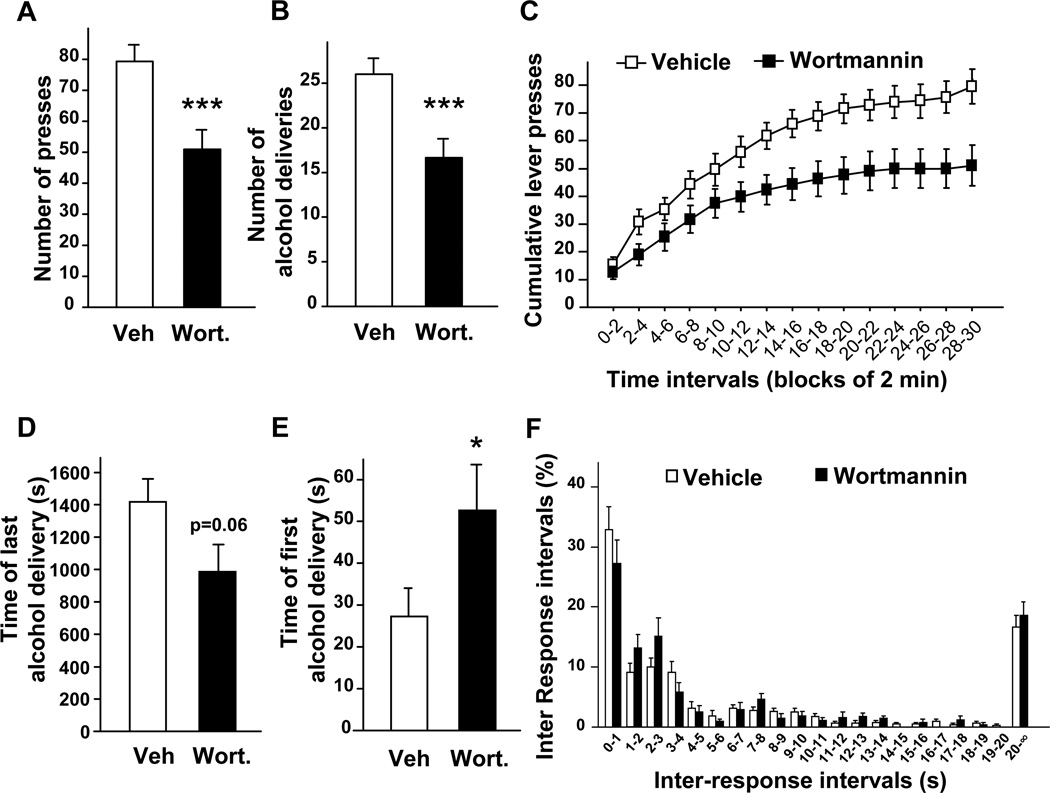
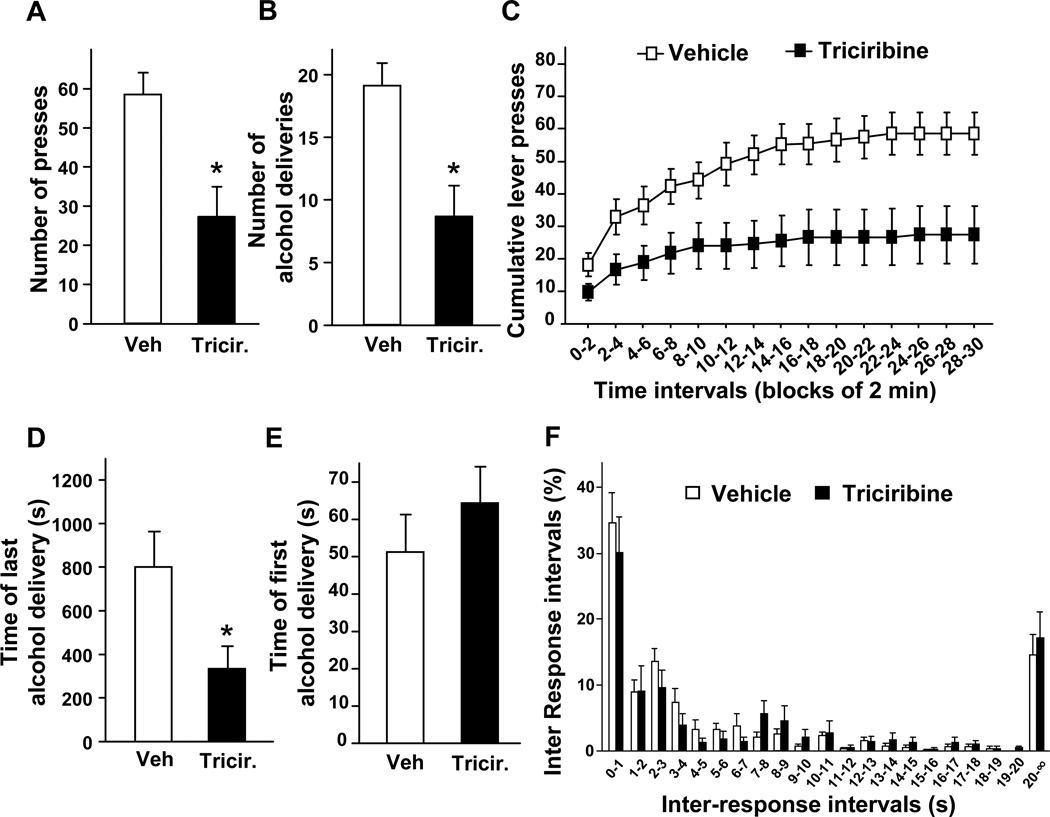
Next, we tested the contribution of the AKT pathway to the motivation of rats to drink alcohol. To do so, we used an operant conditioning paradigm in which rats with a history of excessive voluntary alcohol consumption were trained to self-administer alcohol in an operant procedure on an FR3 schedule. Once animals reached a stable responding for the alcohol lever over a 30-min self-administration session, wortmannin and triciribine were infused into the NAc (Fig. S1B), 1 hr and 3 hrs respectively, before the beginning of a session. Consistent with the results described above, we found that inhibition of the AKT pathway within the NAc reduced operant responding for alcohol (Figs. 5A and 6A). Consequently, the decrease in the number of lever-presses also resulted in a reduction of the number of alcohol deliveries during the 30-min session (Fig. 5B and 6B), without altering the responding for the inactive lever (1.90 ± 0.46 press for vehicle versus 2.22 ± 0.49 presses for wortmannin and 1.45 ± 0.51 press for vehicle versus 1.18 ± 0.57 press for triciribine). Furthermore, analysis of cumulative active lever-press responding within the test session (Figs. 5C and 6C), and the time of the last alcohol delivery (Figs. 5D and 6D), suggest that the decrease in operant responding for alcohol induced by wortmannin and triciribine results from an early termination of the drinking episode. We also observed that intra-NAc infusion of wortmannin (Fig. 5E), but not triciribine (Fig. 6E) delays the time of the first alcohol delivery. The distribution of inter-response intervals was similar for wortmannin, triciribine and their corresponding controls (no effect of treatment [Fwortmannin (1,160) = 0.41, P = 0.54], [Ftriciribine (1,160) = 0.31, P = 0.59] and no interaction between treatment and time intervals [Fwortmannin (20,160) = 1.33, P = 0.17], [Ftriciribine (20,160) = 0.77, P = 0.75]) (Figs. 5F and 6F). Importantly, we did not find any changes in the number of fast responses (p > 0.05 for inter-response interval < 1.0 s) (Figs. 5F and 6F). These last two observations indicate that the inhibitory effects of intra-NAc infusion of wortmannin and triciribine on operant self-administration of alcohol is unlikely to be due to an alteration of rat locomotor activity. Together, these data suggest that the inhibition of AKT pathway in the NAc of rats attenuates alcohol intake by decreasing the motivation of the animals to consume alcohol.
Figure 5. Intra-NAc infusion of wortmannin reduces operant self-administration of alcohol in rats.
(A–F) Rats with a history of high levels of alcohol consumption were trained to self-administer a solution of 20% alcohol in an operant procedure on a fixed ratio 3 (FR3) schedule. One hr before the beginning of a 30-min session, wortmannin (0.1 µg/side) was infused into the NAc of rats. (A) Number of active-lever presses during the 30-min operant alcohol self-administration session. (B) Number of alcohol deliveries (0.1 ml of 20% alcohol per delivery) obtained during the 30-min session. (C) Cumulative mean presses in bins of 2 min, indicative of the rate of presses for alcohol during the session. Two-way ANOVA with repeated measures showed a significant main effect of time [F(14,112) = 98.52, P < 0.001] and treatment [F(1,112) = 7.32, P = 0.027] and an interaction between time and treatment [F(14,112) = 2.87, P = 0.001]. Subsequent analysis using the method of contrasts detected a significant difference between vehicle and wortmannin for all time intervals except for the first interval 0–2 min (all other p’s < 0.05). (D) Time of the last alcohol delivery. (E) Time of the first alcohol delivery. (F) Distribution of inter-response intervals. n=9 per group. Data are represented as mean ± SEM. One-way ANOVA with repeated measures showed main effects of treatment for (A) [F(1,8) = 29.21, P < 0.001]; (B) [F(1,8) = 27.03, P < 0.001] and (E) [F(1,8) = 10.45, P = 0.012] but not for (D) [F(1,8) = 4.65, P = 0.063], *p<0.05 and ***p<0.001.
Figure 6. Intra-NAc infusion of triciribine reduces operant self-administration of alcohol in rats.
(A–F) Rats with a history of high levels of alcohol consumption were trained to self-administer a solution of 20% alcohol. Three hrs before the beginning of a 30-min session, triciribine (0.5 µg/side) was infused into the NAc of rats. (A) Number of active-lever presses during the 30-min operant alcohol self-administration session. (B) Number of alcohol deliveries (0.1 ml of alcohol 20% per delivery) obtained during the 30-min session. (C) Cumulative mean presses in bins of 2 min, indicative of the rate of presses for alcohol during the session. Two-way ANOVA with repeated measures showed a significant main effect of time [F(14,112) = 27.44, P < 0.001] and treatment [F(1,112) = 5.93, P = 0.041] and an interaction between time and treatment [F(14,112) = 3.34, P < 0.001]. Subsequent analysis using the method of contrasts detected a significant difference between vehicle and triciribine for all time intervals (all p’s < 0.05). (D) Time of the last alcohol delivery. (E) Time of the first alcohol delivery. (F) Distribution of inter-response intervals. n=9 per group. Data are represented as mean ± SEM. One-way ANOVA with repeated measures showed main effects of treatment for (A) [F(1,8) = 10.37, P = 0.012]; (B) [F(1,8) = 11.22, P = 0.01] and (D) [F(1,8) = 8.57, P = 0.019], *p<0.05.
Inhibition of the AKT pathway within the NAc of rats does not affect self-administration of sucrose
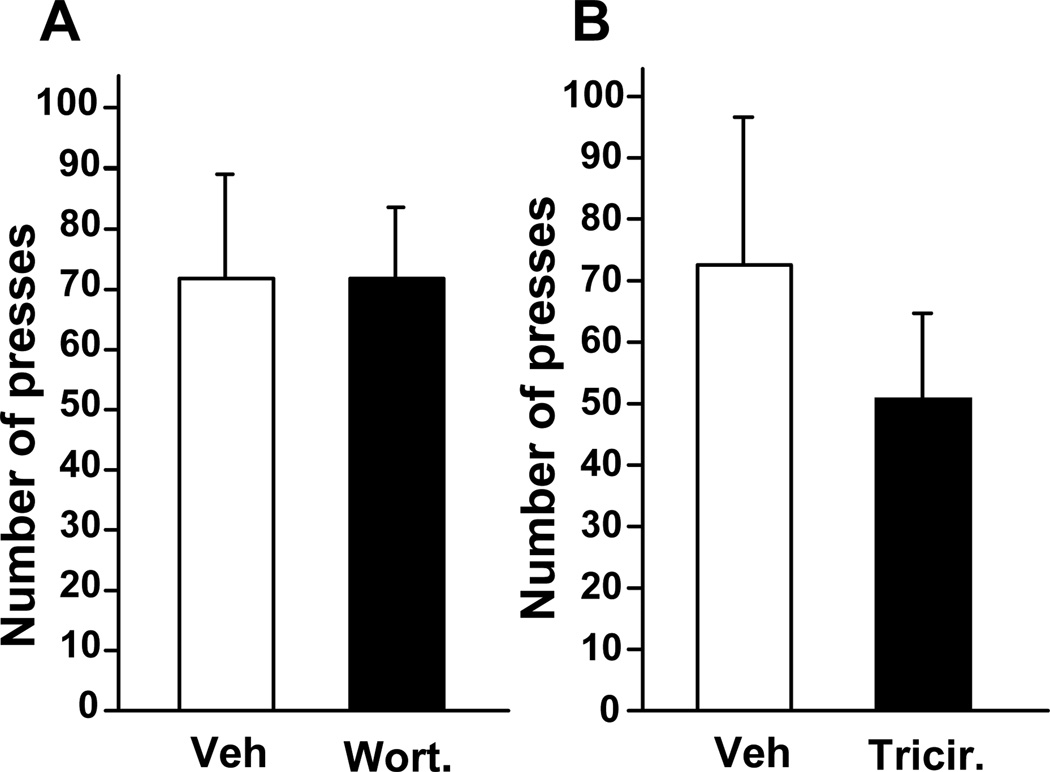
Finally, we tested whether the reduction in operant self-administration by wortmannin and triciribine in the NAc is specific for alcohol. To do so, we tested the capacity of wortmannin and triciribine to modulate the self-administration of the non-drug reinforcer, sucrose. Rats were therefore trained to self-administer a solution of sucrose under an FR3 schedule. Upon reaching stable responding, wortmannin and triciribine were infused into the NAc (Figure S1C in the Supplement), 1 hr or 3 hrs, respectively, before the sucrose operant self-administration session. As shown Fig. 7, the PI3K and AKT inhibitors did not alter lever-press responding for sucrose. These data suggest that the effect of both inhibitors on alcohol self-administration is not due to a general reduction in motivation to consume rewarding substances. These results also suggest that the attenuation of alcohol self-administration is not due to a non-specific alteration of rats’ behavior such as locomotor activity or memory.
Figure 7. Intra-NAc infusion of wortmannin and triciribine does not alter operant self-administration of sucrose in rats.
Rats were trained to self-administer a solution of 1.5% sucrose in an operant procedure. One hr or 3 hrs before the beginning of a 30-min session, 0.1 µg/side of wortmannin (A) or 0.5 µg/side of triciribine (B) respectively were infused into the NAc of rats and the total number of presses on the sucrose lever was recorded. n=8 per group. Data are represented as mean ± SEM.
Discussion
In the present study we show that AKT is activated in the NAc of rodents in response to acute systemic administration of alcohol as well as a result of recurring cycles of excessive alcohol consumption and withdrawal. The consequences of alcohol-mediated activation of AKT are the phosphorylation (and thus inhibition) of GSK-3 kinase, and the activation of the mTORC1 pathway (7). Importantly, our results imply that the AKT-mediated signaling within the NAc contributes to mechanisms underlying excessive alcohol-drinking behaviors (diagram, Figure S4 in the Supplement).
We did not detect any increase in the phosphorylation and thus activation state of ERK1/2 in the NAc of rodents following alcohol exposure. This observation is in agreement with previous studies that reported a small decrease or no change in ERK1/2 phosphorylation following acute systemic administration of alcohol or intermittent exposure to alcohol in a vapor chamber (26–27). In contrast, Ibba et al. recently reported an activation of ERK1/2 pathway in the NAc following administration of alcohol by gavage (28). The differences between the results by Ibba et al. and ours and others, could be due to the mode of alcohol administration. In addition, the fact that gavage induces a significant stress response should be taken into consideration.
We observed that systemic administration of alcohol to mice results in the phosphorylation of AKT on threonine 308 and serine 473 in the NAc. These results are in line with Bjork et al, who reported that AKT is phosphorylated on threonine 308 in mouse striatum after systemic administration of alcohol (29). The observation that alcohol administration leads to the phosphorylation of AKT at both threonine 308 and serine 473 is of interest since the phosphorylation of AKT on threonine 308 and serine 473 is thought to be regulated by two distinct kinases, PDK1 and mTORC2, respectively (12–13, 23). Therefore, our data suggest that alcohol exposure may also result in the activation of mTORC2 in the NAc leading to AKT phosphorylation on serine 473. We recently reported that the mTORC1 signaling pathway is activated in the NAc after alcohol exposure and plays a key role in the molecular mechanisms that underlie alcohol-related behaviors (7). Whereas mTORC1 activation in the brain leads to the translation of synaptic proteins (8, 30), the activation of mTORC2 results in the phosphorylation of substrates such as AKT, serum- and glucocorticoid-induced protein kinase (SGK) and protein kinase C (PKC) (31), which in turn, regulate diverse biological responses (31–32). Interestingly, the role of PKC isoforms in mechanisms underlying alcohol’s action in the CNS is well established (33). Therefore, these data and ours raise the possibility that mTORC2 may also contribute to mechanisms that underlie alcohol-related behaviors by regulating AKT activity through its phosphorylation on serine 473 as well as via other kinases such as SGK and PKC, and this possibility merits further investigation.
The serine and threonine kinase GSK-3 is a substrate of AKT. The two highly homologous isoforms GSK-3α and GSK-3β are encoded by two different genes (34), and the phosphorylation of the isoforms by AKT on serine 21 and 9, respectively, leads to their inhibition (12, 23). The GSK-3β isoform is enriched in the brain (35) where it has been reported to regulate cytoskeleton dynamics (36), as well as the activity of several transcription factors such as the cAMP response element binding protein (CREB) (37), and the function of ionotropic glutamate receptors (12, 35, 38). GSK-3β has also been shown to play a critical role in neuronal development (37) and synaptic plasticity (35). We found that a consequence of alcohol-mediated increase in AKT activity in the NAc is the phosphorylation of both GSK-3α and GSK-3β on serine 21 and serine 9 respectively within the NAc. Specifically, we found that systemic administration of alcohol in mice and voluntary consumption of high amounts of alcohol followed by periods of withdrawal in rats result in increased levels of phosphorylated GSK-3α and GSK-3β in the NAc. These data suggest that AKT-induced GSK-3 inhibition is potentially another mechanism whereby AKT regulates alcohol-drinking behaviors.
In contrast to the inhibitory actions of alcohol on the activity of GSK-3 in the NAc, cocaine-induced GSK-3 activation in the NAc has been implicated in the mechanisms that underlie locomotor sensitization (39). This is yet another example of clear differences in the molecular pathways that underlie the actions of alcohol and stimulants. For instance, whereas cocaine and amphetamine activate ERK1/2 pathway within the NAc (40–42) we and others (26–27), found no increase of ERK1/2 activity in the NAc following alcohol exposure.
Importantly, we observed that repeated cycles of consumption and withdrawal result in an increase in the phosphorylation and thus activation of AKT, and that the blockade of the AKT pathway within the NAc decreases excessive voluntary consumption and self-administration of alcohol. Specifically, we show that intra-NAc infusion of the PI3K inhibitor wortmannin attenuates binge drinking in rats indicating that PI3K activity regulates excessive alcohol intake. It is possible that the mGluR5/Homer2 system contributes to alcohol-mediated activation of PI3K as suggested by Cozzoli et al. (16). We further found that inhibition of AKT by triciribine has the same consequence on alcohol consumption suggesting that the effect of PI3K blockade on binge drinking is due to the subsequent inhibition of AKT. The differences in the inhibition profiles of the two inhibitors on voluntary consumption and self-administration of alcohol could be due to their pharmacokinetic properties (e.g., wortmannin has a shorter half-life compared to triciribine) (19–21), or to the fact that AKT is positioned at a focal point of the PI3K/AKT cascade. Importantly, we also observed that intra-NAc infusion of both wortmannin and triciribine does not reduce operant self-administration of sucrose. This result implies that blockade of the AKT pathway within the NAc does not result in a general reduction of the motivation to obtain a reward, but rather in a selective inhibition of alcohol self-administration. This finding is in agreement with our recent study where we showed that the inhibition of mTORC1, a signaling cascade which is known to be activated by AKT (8–9, 14), decreases the level of motivation of rats to self-administer alcohol but not sucrose (7).
Regarding the neuronal mechanism underlying AKT contribution to excessive alcohol drinking, it is noteworthy that PI3K/AKT pathway has been reported to control synaptic strength in several forebrain regions (17, 43–44). Importantly, alcohol-increased neuronal excitability within the NAc has been associated with increased alcohol consumption (45). Therefore, local inhibition of AKT pathway within the NAc using wortmannin and triciribine may abate neuronal activity that drives alcohol directed behaviors such as excessive consumption.
In conclusion, in the present work we provide biochemical and behavioral data to support the conclusion that the AKT signaling pathway within the NAc contributes to the mechanisms that underlie excessive drinking of alcohol, a hallmark of alcohol addiction (1). Importantly, we found that the inhibition of the AKT pathway within the NAc does not alter the motivational state of rats trained to self-administer a non-drug reward such as sucrose, which is a critical issue from a therapeutic development perspective (46). Our findings therefore suggest that inhibitors of the AKT pathway, which are actively being developed for the treatment of several kinds of cancers (10, 47–48), are potential drug candidates that could be developed for the treatment of alcohol use and abuse disorders.
Supplementary Material
Acknowledgments
This work was supported by NIH-NIAAA P50 AA017072 (D.R.), and the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (D.R.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions:
J.N., S.B.H., Q.Y., and S.C. performed research; J.N., S.B.H., S.C. and D.R. designed research; J.N., S.B.H. and D.R. analyzed data and wrote the manuscript.
Financial disclosure: All authors reported no biomedical financial interests or potential conflict of interest.
References
- 1.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol Rev. 2009;89:649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 2.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simms JA, Steensland P, Medina B, Abernathy KE, Chandler LJ, Wise R, et al. Intermittent access to 20% ethanol induces high ethanol consumption in Long-Evans and Wistar rats. Alcohol Clin Exp Res. 2008;32:1816–1823. doi: 10.1111/j.1530-0277.2008.00753.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.NIAAA. Council approves definition of binge drinking. NIAAA Newsletter 3. 2004 [Google Scholar]
- 6.Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- 7.Neasta J, Ben Hamida S, Yowell Q, Carnicella S, Ron D. Role for mammalian target of rapamycin complex 1 signaling in neuroadaptations underlying alcohol-related disorders. Proc Natl Acad Sci U S A. 2010;107:20093–20098. doi: 10.1073/pnas.1005554107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Costa-Mattioli M, Sossin WS, Klann E, Sonenberg N. Translational control of long-lasting synaptic plasticity and memory. Neuron. 2009;61:10–26. doi: 10.1016/j.neuron.2008.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes Dev. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- 10.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 12.Beaulieu JM, Gainetdinov RR, Caron MG. Akt/GSK3 signaling in the action of psychotropic drugs. Annu Rev Pharmacol Toxicol. 2009;49:327–347. doi: 10.1146/annurev.pharmtox.011008.145634. [DOI] [PubMed] [Google Scholar]
- 13.Yang WL, Wu CY, Wu J, Lin HK. Regulation of Akt signaling activation by ubiquitination. Cell Cycle. 2010;9:487–497. doi: 10.4161/cc.9.3.10508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–318. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 15.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 16.Cozzoli DK, Goulding SP, Zhang PW, Xiao B, Hu JH, Ary AW, et al. Binge drinking upregulates accumbens mGluR5-Homer2-PI3K signaling: functional implications for alcoholism. J Neurosci. 2009;29:8655–8668. doi: 10.1523/JNEUROSCI.5900-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin CH, Yeh SH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- 18.Berndt N, Yang H, Trinczek B, Betzi S, Zhang Z, Wu B, et al. The Akt activation inhibitor TCN-P inhibits Akt phosphorylation by binding to the PH domain of Akt and blocking its recruitment to the plasma membrane. Cell Death Differ. 2010;17:1795–1804. doi: 10.1038/cdd.2010.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holleran JL, Egorin MJ, Zuhowski EG, Parise RA, Musser SM, Pan SS. Use of high-performance liquid chromatography to characterize the rapid decomposition of wortmannin in tissue culture media. Anal Biochem. 2003;323:19–25. doi: 10.1016/j.ab.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 20.Yuan H, Luo J, Weissleder R, Cantley L, Josephson L. Wortmannin-C20 conjugates generate wortmannin. J Med Chem. 2006;49:740–747. doi: 10.1021/jm050699p. [DOI] [PubMed] [Google Scholar]
- 21.Powis G, Basseches PJ, Kroschel DM, Richardson RL, O'Connell MJ, Kvols LK. Disposition of tricyclic nucleoside-5'-monophosphate in blood and plasma of patients during phase I and II clinical trials. Cancer Treat Rep. 1986;70:359–362. [PubMed] [Google Scholar]
- 22.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci. 2007;28:166–172. doi: 10.1016/j.tips.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 23.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bain J, Plater L, Elliott M, Shpiro N, Hastie CJ, McLauchlan H, et al. The selectivity of protein kinase inhibitors: a further update. Biochem J. 2007;408:297–315. doi: 10.1042/BJ20070797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang L, Dan HC, Sun M, Liu Q, Sun XM, Feldman RI, et al. Akt/protein kinase B signaling inhibitor-2, a selective small molecule inhibitor of Akt signaling with antitumor activity in cancer cells overexpressing Akt. Cancer Res. 2004;64:4394–4399. doi: 10.1158/0008-5472.CAN-04-0343. [DOI] [PubMed] [Google Scholar]
- 26.Neznanova O, Bjork K, Rimondini R, Hansson AC, Hyytia P, Heilig M, et al. Acute ethanol challenge inhibits glycogen synthase kinase-3beta in the rat prefrontal cortex. Int J Neuropsychopharmacol. 2009;12:275–280. doi: 10.1017/S1461145708009620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanna PP, Simpson C, Lutjens R, Koob G. ERK regulation in chronic ethanol exposure and withdrawal. Brain Res. 2002;948:186–191. doi: 10.1016/s0006-8993(02)03191-8. [DOI] [PubMed] [Google Scholar]
- 28.Ibba F, Vinci S, Spiga S, Peana AT, Assaretti AR, Spina L, et al. Ethanol-induced extracellular signal regulated kinase: role of dopamine D1 receptors. Alcohol Clin Exp Res. 2009;33:858–867. doi: 10.1111/j.1530-0277.2009.00907.x. [DOI] [PubMed] [Google Scholar]
- 29.Bjork K, Terasmaa A, Sun H, Thorsell A, Sommer WH, Heilig M. Ethanol-induced activation of AKT and DARPP-32 in the mouse striatum mediated by opioid receptors. Addict Biol. 2010;15:299–303. doi: 10.1111/j.1369-1600.2010.00212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoeffer CA, Klann E. mTOR signaling: at the crossroads of plasticity, memory and disease. Trends Neurosci. 2010;33:67–75. doi: 10.1016/j.tins.2009.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–3744. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dowling RJ, Topisirovic I, Fonseca BD, Sonenberg N. Dissecting the role of mTOR: Lessons from mTOR inhibitors. Biochim Biophys Acta. 2010;1804:433–439. doi: 10.1016/j.bbapap.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 33.Newton PM, Messing RO. Intracellular signaling pathways that regulate behavioral responses to ethanol. Pharmacol Ther. 2006;109:227–237. doi: 10.1016/j.pharmthera.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 34.Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:2431–2438. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peineau S, Bradley C, Taghibiglou C, Doherty A, Bortolotto ZA, Wang YT, et al. The role of GSK-3 in synaptic plasticity. Br J Pharmacol. 2008;153 Suppl 1:S428–S437. doi: 10.1038/bjp.2008.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou FQ, Snider WD. CELL BIOLOGY: GSK-3beta and Microtubule Assembly in Axons. Science. 2005;308:211–214. doi: 10.1126/science.1110301. [DOI] [PubMed] [Google Scholar]
- 37.Hur EM, Zhou FQ. GSK3 signalling in neural development. Nat Rev Neurosci. 2010;11:539–551. doi: 10.1038/nrn2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peineau S, Taghibiglou C, Bradley C, Wong TP, Liu L, Lu J, et al. LTP inhibits LTD in the hippocampus via regulation of GSK3beta. Neuron. 2007;53:703–717. doi: 10.1016/j.neuron.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 39.Xu CM, Wang J, Wu P, Zhu WL, Li QQ, Xue YX, et al. Glycogen synthase kinase 3beta in the nucleus accumbens core mediates cocaine-induced behavioral sensitization. J Neurochem. 2009;111:1357–1368. doi: 10.1111/j.1471-4159.2009.06414.x. [DOI] [PubMed] [Google Scholar]
- 40.Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 41.Valjent E, Corvol JC, Pages C, Besson MJ, Maldonado R, Caboche J. Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties. J Neurosci. 2000;20:8701–8709. doi: 10.1523/JNEUROSCI.20-23-08701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valjent E, Pascoli V, Svenningsson P, Paul S, Enslen H, Corvol JC, et al. Regulation of a protein phosphatase cascade allows convergent dopamine and glutamate signals to activate ERK in the striatum. Proc Natl Acad Sci U S A. 2005;102:491–496. doi: 10.1073/pnas.0408305102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang Q, Liu L, Pei L, Ju W, Ahmadian G, Lu J, et al. Control of synaptic strength, a novel function of Akt. Neuron. 2003;38:915–928. doi: 10.1016/s0896-6273(03)00356-8. [DOI] [PubMed] [Google Scholar]
- 44.Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, et al. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stuber GD, Hopf FW, Tye KM, Chen BT, Bonci A. Neuroplastic alterations in the limbic system following cocaine or alcohol exposure. Curr Top Behav Neurosci. 2010;3:3–27. doi: 10.1007/7854_2009_23. [DOI] [PubMed] [Google Scholar]
- 46.Mello NK, Negus SS. Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology. 1996;14:375–424. doi: 10.1016/0893-133X(95)00274-H. [DOI] [PubMed] [Google Scholar]
- 47.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 48.Yap TA, Garrett MD, Walton MI, Raynaud F, de Bono JS, Workman P. Targeting the PI3K-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol. 2008;8:393–412. doi: 10.1016/j.coph.2008.08.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.