Summary
Tissue transglutaminase (TG2) is a ubiquitously expressed member of the transglutaminase family of Ca2+-dependent cross-linking enzymes. Unlike other family members, TG2 is a multifunctional protein, which has several other well documented enzymatic and non-enzymatic functions. A significant body of evidence accumulated over the last decade reveals multiple and complex activities of this protein on the cell surface and in the extracellular matrix (ECM), including its role in the regulation of cell-ECM interactions and outside-in signaling by several types of transmembrane receptors. Moreover, recent findings indicate a dynamic regulation of the levels and functions of extracellular TG2 by several complementary mechanisms. This review summarizes and assesses recent research into the emerging functions and regulation of extracellular TG2.
Keywords: transglutaminase, cell-ECM interactions, integrin, syndecan, signaling, non-classical protein secretion, protein-phospholipid interaction, membrane trafficking, endosomal recycling, endocytosis
Introduction
There are 8 related transglutaminase genes in the human genome [1]. TG2 is a member of the transglutaminase family which displays the transamidating / cross-linking function [1,2]. In this series, the minireviews by Kiraly and coauthors [3] and by Walther and colleagues [4] explore the novel aspects of transamidating function of TG2, including its regulation by Ca2+ and its role in monoaminylation of cellular proteins under both physiological and pathological conditions. In addition to transamidation / protein cross-linking, TG2 also displays GTPase, disulfide isomerase, and even more surprising protein kinase activities, as well as additional non-enzymatic functions [1,2]. The majority of cellular TG2 pool is present in the cytoplasm, some is also found in the mitochondria and the nucleus, while no TG2 is detected in the ER or Golgi compartments [1,2]. The emerging nuclear functions of TG2 are discussed in this minireview series by Kuo and Kojima [5]. Depending on cell type, a significant fraction of TG2 (1–20%) is localized extracellularly, both on the plasma membrane and in the ECM [6]. Recent work from several laboratories showed that TG2 has important enzymatic and non-enzymatic functions at these locations where it cross-links various ECM proteins and modulates the interactions of cells with the ECM and soluble growth factors by non-covalent interactions with and regulation of integrins [6–8], syndecan-4 [9,10] and growth factor receptors [11,12]. Intriguingly, mounting data suggest that TG2 has some common, related, or overlapping function(s) inside and outside the cells, such as regulation of cell survival and apoptotic response [13–15]. Numerous studies implicate TG2 in a wide range of pathological conditions (reviewed in [2]). Therefore, elucidation of the emerging adhesive/signaling function of extracellular TG2 appears important for understanding the involvement of this protein in tissue fibrosis, regeneration and response to injury, inflammation, cardiovascular and neurodegenerative diseases, and cancer progression and metastasis [1,2,6]. Here our current understanding of non-ezymatic functions of extracellular TG2 and mechanisms of its dynamic regulation by cells is reviewed.
Extracellular TG2: enzymatic and non-enzymatic functions
Early observations in the 1990s showed the presence of extracellular TG2 in the ECM [16] and suggested its potential role in the cell-ECM interactions as overexpression of this protein in fibroblasts was found to strikingly promote cell-substrate adhesion [17]. Subsequent studies based on immuno-fluorescence and immunoelectron microscopy showed the presence of this protein outside the cells in a close association with the plasma membrane, and in the ECM [18,19]. A number of enzymatic TG2 substrates were identified among the ECM proteins, including fibronectin, fibrin(ogen), collagen, vitronectin, osteopontin, etc. [20]. However, somewhat surprisingly, recent work indicated that extracellular TG2 is mostly enzymatically inactive in vivo, but can be activated by mechanical injury or inflammatory stimuli [21]. The balance between inactive and active TG2 in the extracellular environment is likely maintained by reversible oxidation of the key cysteine triad (Cys230, Cys370 and Cys371) and reversible formation of intramolecular Cys370–Cys371 disulfide bond [22], which is antagonized by Ca2+ (see also [3] for schematic of the protein). In addition, NO-dependent modification of cysteines in TG2 leads to its S-nitrosylation, which inhibits enzymatic activity of the protein in vitro [23] and in vivo in blood vessels [24]. The latter mechanism may modulate vascular stiffness and its age-dependent dysregulation is likely to contribute to elevated TG2-mediated ECM cross-linking in the blood vessels, leading to decreased arterial compliance and hypertension [24]. Similarly, nitrosylation of tyrosine residues in TG2 was suggested to inhibit its cross-linking activity in fibroblasts [25]. On the other hand, an interaction of TG2 with negatively charged glycosaminoglycans may augment its transamidating activity in the ECM [26]. Moreover, mechanical force generated by actomyosin and applied to the ECM-bound TG2 may shift the protein conformation toward the "open" form, thus facilitating access of glutamine substrates to the enzyme's active center [27]. Despite some recent progress, much remains to be learned about regulation of the TG2 cross-linking function in vivo (see an accompanying review by Kiraly et al. [3]). Yet, over the past two decades it became increasingly evident that, in addition to enzymatic cross-linking of ECM proteins, extracellular TG2 has other functions that are separate and independent from its transamidating / protein cross-linking enzymatic activity [6–12,28]. These emerging non-enzymatic functions of extracellular TG2 are the focus of current review.
Adhesive / signaling functions of extracellular TG2
Integrin-TG2 interaction and its role in cell-ECM adhesion and signaling
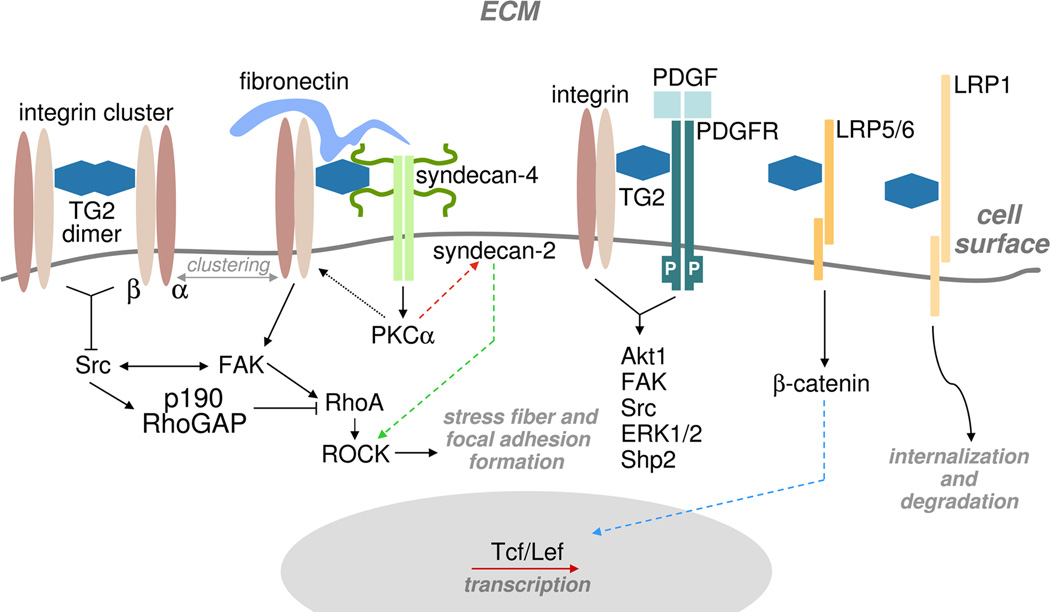
It has long been known that TG2 non-covalently interacts with the ubiquitous and abundant ECM protein, fibronectin, in vitro [29]. Subsequent work demonstrated the ability of cell surface TG2 to bind soluble fibronectin and to promote its deposition into ECM [28,30]. Importantly, the ability of TG2 to promote cell-ECM adhesion, cell migration, and assembly of fibronectin fibrillar matrices depends on this interaction [6–8,28,31]. TG2 binds with high affinity to the gelatin-binding region of fibronectin that consists of modules I6II1,2I7–9 and does not overlap with the major integrin-binding sites on the fibronectin molecule [6,32]. Cell surface TG2 was also found to collaborate with integrins in cell adhesion through direct non-covalent interaction with the β1, β3 and β5 integrin subunits and formation of stable ternary complexes with both integrins and fibronectin (see Fig. 1) [6–8,28,31]. Due to modest affinity for the integrin-fibronectin binding [33] and strong non-covalent association of TG2 with both these proteins [6,7], TG2 significantly enhances the interaction of cells with fibronectin serving as a bridge between integrins and fibronectin [6]. Precise mapping of the integrin-TG2 interaction appears difficult as the composite integrin-binding site on TG2 involves both the first and fourth domains of the protein, whereas TG2 binding site on integrins includes several membrane-proximal EGF-like repeats of the β subunit away from the ligand-binding site (Akimov SS & Belkin AM, unpublished results). Importantly, all TG2 on the surface of human erythroleukemia cells and THP-1 macrophages is bound to β1/β3/β5 integrins [7,31]. Reciprocally, in various cell types, a significant fraction of integrins (up to 40% of β1 integrins in macrophages) is associated with TG2 [7,8,31]. In addition, TG2 appears to control cell surface levels of certain integrins in cancer cells [14,34] and in macrophages [35], however molecular mechanisms of such regulation still remain unknown.
Fig. 1.
Several types of TG2-containing adhesive / signaling complexes present on the cell surface. Solid black lines indicate TG2-mediated activation of cytoplasmic targets by transmembrane signaling receptors. Dotted black line marks binding of activated PKCα to the integrin cytoplasmic tails, which causes their redistribution on the cell surface. Red dashed line outlines the activation of syndecan-2 by intracellular PKCα, and green dashed line - syndecan-2-mediated activation of ROCK, which induces stress fiber and focal adhesion formation. Blue dashed line marks nuclear translocation of β-catenin which leads to its complex formation with Tcf/Lef and activation of gene transcription. Curved black line indicates the principal pathway of surface TG2 internalization.
A significant impact of extracellular TG2 on integrin-mediated cell adhesion, spreading, migration, survival, differentiation, ECM contraction, and fibronectin matrix deposition was demonstrated for a wide range of normal and transformed cells [7,8,14,28,31,34–37]. Moreover, the interaction between integrin-bound TG2 and fibronectin on the cell surface is likely involved in various pathophysiological mechanisms. For instance, it is thought to facilitate the anchoring of ovarian cancer cells to the mesothelial lining of the peritoneal cavity and promote the subsequent metastasis during the progression of this type of cancer [34]. In addition, TG2 on the surface of astrocytes was proposed to mediate their adhesion and migration on fibronectin in multiple sclerosis lesions, thus contributing to tissue remodeling and glial scarring [38]. Therefore, targeting the TG2-fibronectin interaction (see a schematic in [3] for the fibronectin-binding site in TG2) might be a new promising venue for developing novel therapeutics that block the cell-ECM adhesion of tumor cells in ovarian cancer and activated astrocytes in multiple sclerosis.
In contrast, boosting the formation of integrin-TG2-fibronectin adhesive/signaling complexes might have important benefits for certain therapeutic applications. Transplantation therapy with autologous mesenchymal stem cell (MSC) for repair of myocardial injury has inherent limitations due to poor viability of these cells after the implantation. Cell-ECM adhesion is prerequisite for cell survival and also a key factor for differentiation of MSCs. As a novel pro-survival improvement strategy, genetically engineered MSCs that overexpress TG2 were used to enhance cell adhesion and survival after the implantation [37]. The MSCs overexpressing TG2 showed significant retention in the infarcted rat myocardium and developed into cardiac myocyte-like cells as judged by expression of cardiac-specific proteins [37]. Transplantation of these cells into the ischemic or infarcted rat myocardium further restored cardiac function as compared with MSC transplantation alone, suggesting that TG2 is important for integrin-mediated adhesion and pro-survival signaling of MSCs in the implanted tissues [37].
Another aspect of functional collaboration between integrins and TG2 in cell adhesion is reflected in the alteration of integrin clustering levels on the cell surface even in the absence of fibronectin (Fig. 1) [6,8]. Whereas no TG2-mediated changes in ligand-binding affinity of integrins were detected, TG2 was found to increase integrin avidity (clustering) [8]. In fibroblasts expressing TG2, a sizeable fraction of integrins was found within large protein complexes that were identified both biochemically and by immunofluorescence [8]. The molecular mechanisms of integrin clustering by surface TG2 remain unknown. Both the ability of TG2 to oligomerize [8,39] and the presence of other integrin-binding proteins, such as caveolin-1 and tetraspanins, within these complexes, may promote integrin aggregation. Furthermore, the reported co-distribution of TG2 and β1 integrins in lipid rafts and caveolae [40] is likely to enhance the linkage of cell-ECM adhesions to these cholesterol-enriched membrane microdomains, thereby regulating membrane protein trafficking and compartmentalization of cell signaling.
Importantly, the association of TG2 with integrins on the cell surface, and TG2-mediated integrin clustering potentiate outside-in signaling triggered by these transmembrane adhesion receptors [6–8]. The formation of complexes between these two proteins was shown to modulate the activities of focal adhesion kinase (FAK), src, p190RhoGAP, and up-regulate activation levels of RhoA GTPase and its downstream signaling target, ROCK, thus contributing to increased formation of focal adhesions, actin stress fibers and elevated actomyosin contractility in the cells expressing TG2 [8]. Likely, the activation of many other integrin-dependent signaling pathways is potentiated by TG2, suggesting that it serves as a general amplifier of outside-in integrin signaling (Fig. 1).
A convincing example of functional collaboration between extracellular TG2 and integrins came from the recent studies of TG2−/− macrophages which appear deficient in phagocytosis [35]. TG2 was found to be required for accumulation of β3 integrin at the engulfing portals of macrophages [35]. In these cells, the signaling via β3 integrin, which is required for the formation of phagocytic cup and effective uptake of apoptotic cells, was shown to depend on the interaction of this receptor with TG2 [35]. Moreover, TG2 enhances the activation of essential downstream signaling targets of β3 integrin, RhoG and Rac1, which is prerequisite for efficient phagocytosis [35]. Overexpression of β3 integrin in TG2−/− macrophages partially compensates for these signaling defects, further proving the functional collaboration between these proteins in phagocytosis [41]. The mechanistic basis for this partnership was defined when TG2 was found to interact with high affinity with the protein milk fat globulin EGF factor 8 (MGF-E8), which is involved in bridging the β3 integrin to apoptotic cells [35]. Hence, the TG2-mediated stabilization of β3 integrin / MGF-E8 complexes in macrophages is involved in phagocytic uptake of apoptotic cells.
Syndecan-4-TG2 complexes on the cell surface: a parallel adhesive / signaling platform
While early work indicated an interaction between TG2 and heparin in vitro, two recent studies with fibroblasts revealed heparan sulfate proteoglycan (HSPG) syndecan-4 as another important binding partner for extracellular TG2 (Fig. 1) [9,10]. Unlike syndecans-1, -2, and -3, syndecan-4 was previously shown to accumulate in focal adhesions where it interacts via heparan sulfate chains with the Hep-2 region of fibronectin and collaborates with integrins in cell adhesion to fibronectin and adhesion-dependent, RhoA-mediated development of focal adhesions, stress fibers, and actomyosin contractility [42]. The high affinity interaction of extracellular TG2 with syndecan-4 is thought to maintain the activation of protein kinase Cα (PKCα), which, in turn, directly binds to the β1 integrin cytoplasmic tails, and is important for controlling integrin levels and clustering throughout the cell surface [9,10,43–47]. Furthermore, latest work showed that the ability of activated PKCα to maintain the RGD-independent adhesion of fibroblasts [44] and osteoblasts [45] due to interaction of fibronectin-TG2 heterocomplexes with syndecan-4 is mediated by syndecan-2, which does not bind TG2, but acts as a downstream signaling effector in modulating the cytoskeletal organization through the ROCK pathway. These data also imply a major role for fibronectin-TG2-syndecan-4 complexes as a parallel adhesive/signaling platform that cells may utilize in the case of integrin function deficiency [46,47]. Meanwhile, the integrin- and syndecan-4-based adhesion systems are likely to physically interact, since these two receptors bind to separate and non-adjacent regions of fibronectin [42] and functionally collaborate by jointly regulating p190RhoGAP activity and localization during cell adhesion to this ECM protein [48]. Therefore, an emerging model indicates existence of quarternary adhesion/signaling complexes comprised of integrins, syndecan-4, their joint ECM ligand fibronectin, as well as TG2 (Fig. 1). In addition to fibronectin, TG2 appears to be involved in orchestrating the formation of such complexes due to high affinity interactions with all its other components [7,9,10,29]. Moreover, distinct and non-overlapping binding sites for integrins and heparan sulfate chains on the TG2 molecule [6,46] may even suggest a direct bridging function for extracellular TG2 in linking integrin and syndecan-4 adhesion receptors (Fig. 1). The interaction of integrin-bound TG2 with syndecan-4 on the cell surface might be required in response to extensive tissue damage and ECM degradation, which interferes with integrin-mediated adhesion and the associated outside-in signaling [9,10,49]. Thus, upregulation of TG2 expression during wound healing and tissue repair should enhance the adhesive/signaling function of extracellular TG2 and compensate for deficiency of integrin-dependent adhesion and assembly of fibronectin matrices [44,49]. In turn, this should lead to clustering its binding partners on the cell surface and enhanced adhesion, thereby preventing anoikis, inducing pro-survival signaling, and ultimately, increasing cell survival [9,10,44].
Cell surface TG2 as a new regulator of joint integrin / growth factor receptor signaling
An important paradigm in the field of cell adhesion entails both physical association and functional collaboration between integrins and receptor tyrosine kinases in the regulation of cell responses to the ECM and soluble growth factors (Fig. 1) [50]. In particular, the engagement of β1 and αvβ3 integrins with ECM ligands transiently activates platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF), epidermal growth factor (EGF), and fibroblast growth factor (FGF) receptor tyrosine kinases even in the absence of their soluble ligands and promotes and sustains growth factor-initiated signaling by these receptors [50]. Despite significance of this synergistic signaling, molecular mechanisms underlying the crosstalk between the two receptor systems remain largely unknown. A physical interaction between these two types of signaling receptors was proposed to be enhanced by their co-sequestering in cholesterol-enriched membrane microdomains [51]. Since integrins and growth factor receptors share many downstream signaling targets, integrin-ECM interaction may also increase availability of signal relay enzymes and adapter proteins to growth factor receptors by promoting their recruitment from cytosol to the plasma membrane [52]. A novel mechanistic insight into the crosstalk between integrin and PDGFR signaling pathways was provided recently when TG2 was found to interact with PDGFR in vitro and on the surface of fibroblasts and mediate its physical association with integrins (Fig. 1) [12]. In fibroblasts, TG2 enhances the PDGFR-integrin association by bridging these receptors on the cell surface [12]. The interaction between TG2 and PDGFR also reduces cellular levels of the receptor by accelerating its turnover [12]. Moreover, the association of PDGFR with TG2 causes receptor clustering, increases PDGF binding, promotes both adhesion-mediated and growth factor-induced PDGFR activation, and upregulates downstream signaling mediated by this receptor [12]. Importantly, TG2 appears to be required for efficient PDGF-dependent proliferation and migration of fibroblasts [12]. Further, other principal responses of mesenchyme-derived cells, such as survival and differentiation, might depend on TG2-mediated crosstalk between integrins and PDGFR. These findings show a novel function of extracellular TG2 in the regulation of the joint PDGFR/integrin signaling and PDGFR-dependent cell responses by coupling the adhesion-mediated and growth factor-dependent signaling pathways. They also suggest that this activity of TG2 might be involved in the pro-inflammatory function of this protein in normal wound healing and tissue fibrosis, vascular restenosis in response to vessel wall injury, and tumor metastasis, diverse pathophysiological processes that often involve overactivation or misregulation of PDGF/PDGFR-mediated signaling [53]. Moreover, the interaction of extracellular TG2 with a wide range of growth factor receptors might be a general phenomenon, as this protein was found to interact with VEGFR on the surface of endothelial cells and modulate VEGF-induced signaling in this cell type [11]. Additional work is needed to define the molecular motifs involved in the association of cell surface TG2 with growth factor receptors and determine whether TG2 interacts with other structurally related receptor tyrosine kinases, including EGFR and FGFR, and impacts their joint signaling with integrins.
Extracellular TG2 acts through LRP5/6 transmembrane receptors to induce β-catenin signaling
Recent study showed that, in addition to integrins and growth factor receptors, extracellular TG2 is capable of signaling through low density lipoprotein receptor-related proteins (LRP) 5 and 6 (Fig. 1) [54]. In search for TG2 binding partners on the surface of vascular smooth muscle cells, the transmembrane receptors LRP5/6 were identified as its major interactors [54]. Furthermore, the binding of TG2 to these receptors triggers the activation of β-catenin pathway by driving nuclear translocation of β-catenin, induction of the Tcf/Lef transcription factors, and decreasing p21 expression [54]. In turn, the TG2-mediated activation of the β-catenin pathway, which is inherently silent in vascular smooth muscle cells, was shown to promote calcification of these cells in culture [54]. Future studies should help to assess the in vivo contribution of extracellular TG2 to pathologic calcification in the vessel wall.
Other interacting partners of extracellular TG2
Several other proteins were shown to non-covalently interact with TG2 on the cell surface and in the ECM. GPR56, an atypical G protein-coupled receptor, which is down-regulated in highly metastatic melanoma cells, was found to interact with TG2 in the tumor stroma [55]. Thus, TG2 was proposed as a novel GPR56 ligand that may cooperate in the tumor suppressive role of this orphan receptor; however the precise mechanism involved in such regulation remains unknown.
An anti-tumor ECM protein angiocidin, produced by both endothelial and tumor cells, was reported to inhibit angiogenesis and interact with both collagen and the collagen-binding α2β1 integrin. More recently, angiocidin was found to co-localize with TG2 in the ECM of endothelial cells and interact non-covalently with TG2 via its C-terminal integrin- and collagen-binding domain [56]. Intriguingly, the angiocidin-TG2 interaction was found to prevent the deposition of fibronectin in the ECM of tumor and endothelial cells, suggesting that angiocidin-mediated disruption of the TG2-fibronectin interaction is involved in its tumor suppressive activity [56].
The C-terminal fragment of the α1 chain of collagen XVIII, endostatin, which binds to α5β1 and αvβ3 integrins, glypicans 1 and 4, and VEGFR2, is known as a potent anti-angiogenic protein localized on the surface of endothelial cells [57]. It suppresses integrin-mediated activation of FAK/c-Raf/MEK1/2-ERK1/2 signaling pathway and prevents binding of VEGF165 to endothelial cells, thereby inhibiting the VEGF-mediated activation of VEGFR [57]. Endostatin was reported to bind TG2 with high affinity in vitro via its C-terminal integrin-binding domain and co-localize with TG2 in the ECM [57]. This novel interaction was suggested to play a role in the regulation of angiogenesis and tumor growth [57].
Unlike the integrin-TG2-fibronectin adhesion complexes in which TG2 and integrin can simultaneously bind to separate non-overlapping sites on fibronectin [6,7], these proteins were reported to interact with the same sites in angocidin and endostatin [45,57]. Thus, on the surface of endothelial cells, TG2 is likely to bridge angiocidin and endostatin to integrin receptors and promote their clustering, thereby contributing to anti-angiogenic properties of these proteins. Further mechanistic analysis of these interactions is needed to prove this contention.
Finally, recent studies from our laboratory showed that TG2 forms complexes with matrix metalloproteinase MMP-2 [58] and the major endocytic receptor LRP1 (Fig. 1) [40] on the cell surface. The role of these ineteractions in the regulation of surface TG2 levels, and their consequences for cell-ECM adhesion and signaling is discussed below. Other interacting partners of TG2 are likely to be identified on the cell surface and in the ECM, further expanding the adhesive/signaling function of this protein, and helping to define its role in a wide range of pathophysiological processes.
Dynamic regulation of extracellular TG2
Given the significance of TG2 functions in the extracellular environment, a precise dynamic regulation of its levels and interactions on the cell surface and in the ECM appears important. Latest studies revealed that several membrane trafficking and proteolytic mechanisms are employed by cells to maintain and modulate the extracellular levels and functions of this protein.
Unconventional secretion of TG2
TG2 is constitutively externalized from undamaged cells and various cell types including fibroblasts, osteoblasts, endothelial cells, smooth muscle cells, and monocytes/macrophages, all contain it on their surface and in the ECM [1,2,6]. There are no secretory signal sequences and hydrophobic or transmembrane domains in TG2 [39,59], the protein is not localized in the ER / Golgi compartments and nothing is known regarding the factors that control its secretion [1,2,6]. While many growth factors and cytokines regulate TG2 cellular levels, biosynthesis and degradation, they all concurrently modulate the levels of TG2 outside the cell, suggesting general pathway(s) for trafficking of this protein to the cell surface. Meanwhile, a significant portion of the protein is present in the so-called “particulate fraction”, indicating its association with membranes in various cell types [1]. This association may depend on stable TG2 interactions with transmembrane proteins, such as integrins or adrenergic receptors [1,6]. Otherwise, in the absence of known posttranslational modifications of TG2 that mediate association with the lipid bilayer, a direct or indirect binding to lipids [60] may target this protein to the intracellular membranes. Although some reports proposed that fibronectin and heparan sulfate proteoglycans, two extracellular binding partners of TG2, and its own transamidating activity may affect its exportation [10,61,62], they are more likely to impact the retention of TG2 on the cell surface rather than its intracellular trafficking en route to the surface. Together, the available data suggest that TG2 is secreted by unconventional mechanisms. However, until recently, the pathway(s) of its externalization and mechanisms(s) of its translocation across lipid bilayers remained unknown.
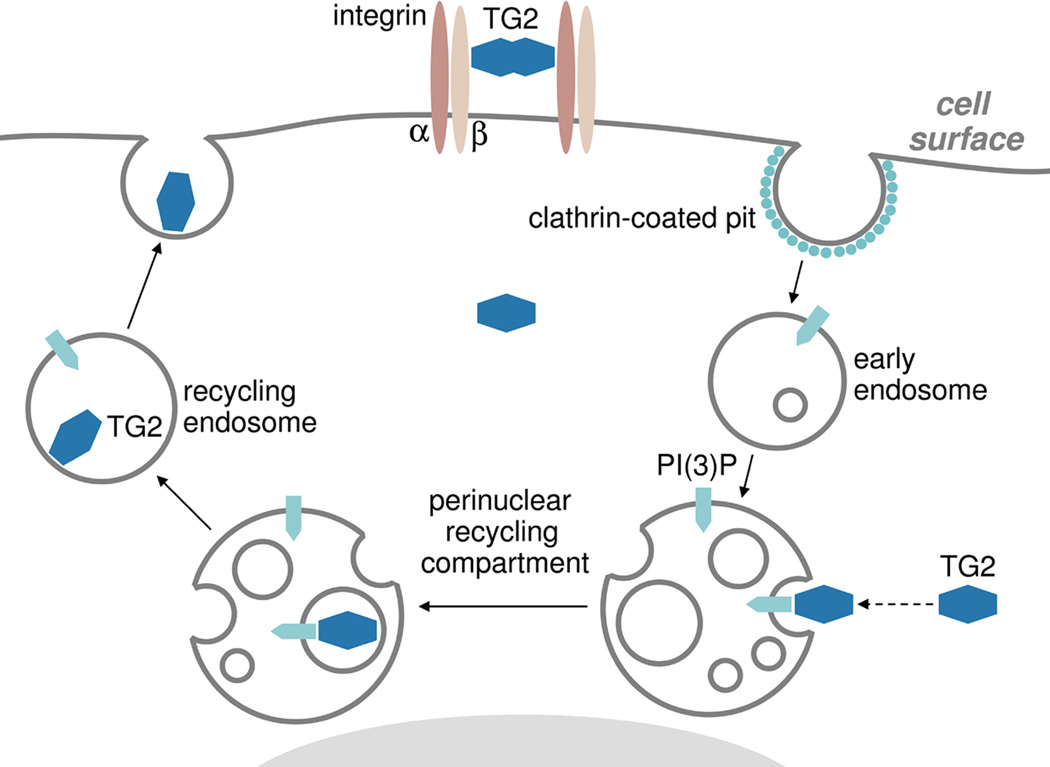
Recent work from our laboratory began to delineate the secretion pathway of cytoplasmic TG2 by focusing on its intracellular trafficking routes (Fig. 2) [63]. We found that in fibroblasts recycling endosomes are essential for TG2 externalization. Instead of being directed to the classical ER/Golgi-dependent secretion pathway, de novo synthesized cytoplasmic TG2 is targeted to perinuclear recycling endosomes, and is delivered inside these transport vesicles prior to exportation [63]. Inactivation of recycling endosomes, blocking endosome fusion with the plasma membrane, or downregulation of Rab11 GTPase that controls outbound trafficking of perinuclear recycling endosomes, all abrogate TG2 secretion [63]. The initial recruitment of cytoplasmic TG2 to the recycling endosomes and subsequent externalization depend on its binding to phosphoinositides on endosomal membranes, as mutation of the newly identified phospholipid-binding motif Lys590-Lys602 in TG2 (see a schematic in [3]) interferes with both processes [63]. The interaction of TG2 with intracellular transport vesicles likely represents a two step process with its initial tethering to endosomal phosphoinositides and subsequent tight binding to yet unidentified endosomal membrane protein(s). The search for such TG2 "receptor" on the recycling endosomes is currently underway. Together, these findings begin to unravel an unconventional mechanism of TG2 secretion which utilizes the long loop of endosomal recycling pathway and indicate involvement of endosomal trafficking in non-classical protein secretion. Unlike most routes of unconventional secretion, including the ones for FGF2 in fibroblasts or IL-1β in macrophages [64], the default TG2 exportation pathway is likely common for many cell types that express this protein [63]. While TG2 exportation operates via a constitutive secretion route, it is likely modulated by a wide range of factors, including intracellular Ca2+ and regulatory proteins that control various endosomal recycling pathways [63].
Fig. 2.
The unconventional pathway of cytoplasmic TG2 secretion involves phospholipid-dependent delivery into recycling endosomes. Solid lines mark the major endosomal recycling pathway which operates via the perinuclear recycling endosomal compartment. Dashed line indicates the PI(3)P-dependent recruitment of cytoplasmic TG2 to the membranes of the perinuclear recycling compartment.
The emerging relationship of the TG2 trafficking pathway to the general recycling routes of transmembrane receptors has important functional implications. Several features of TG2 secretion, including its dependence on Rab11A/B function and VAMP3- and SNAP23-mediated endosome-plasma membrane fusion coincide with those governing integrin recycling [63,65,66], arguing that TG2 is likely exported inside the same vesicles that contain integrins undergoing the recycling process. Whereas our earlier studies indicated that TG2 binds β1 integrin within 30–60 min after onset of biosynthesis [7], it remained unclear where these complexes are formed in the cells, since the lack of TG2 in the ER/Golgi left this issue unresolved. The targeting of cytoplasmic TG2 to perinuclear recycling endosomal compartment may provide a plausible explanation for these earlier findings. Both β1 and β3 integrins undergo internalization and recycling with the former utilizing the long, and the latter - the short endosomal recycling routes [65]. The presence of TG2 inside the recycling endosomes should facilitate its interaction with internalized integrins undergoing recycling process inside these vesicles and lead to externalization of the newly formed integrin-TG2 complexes via the recycling routes [63,65]. In turn, targeted delivery of these adhesive/signaling complexes to lamellipodia should strengthen adhesion to the ECM at the leading edge of migrating cell and contribute to directionality of cell migration. This hypothesis will be tested in our future work.
The balance between cell surface and matrix TG2: what is the translocation mechanism?
It has long been known that in addition to its localization on the plasma membrane, the protein is also present in the ECM away from the cell surface [16,20]. The mechanism(s) of TG2 translocation from the cell surface to the ECM remain unknown. Recent reports showed that TG2 nitrosylation increases relative surface levels of the protein, but reduces its deposition into the ECM [25]. Also, treatment of cells with reducing agents decreases the levels of surface TG2 and integrin-TG2 complexes (our unpublished data), suggesting that the integrin-TG2 interaction is further stabilized by the formation of disulfide bond(s). Thus, the oxidation state of TG2, which might be regulated by nitric oxide, reactive oxygen species, and disulfide modification / exchange [22–25], appears crucial for the retention of TG2 on the cell surface and its translocation to the ECM where it binds fibronectin. In addition, the ternary integrin-TG2-fibronectin complexes might be mechanically disrupted during cell movement and contraction. Since mechanical stretching alters the conformations of both integrin and fibronectin [33], an excessive tension applied to the cytoskeleton-ECM scaffold is likely to disrupt the integrin-TG2 complexes on the plasma membrane. More studies are needed to test these hypothetical mechanisms.
Internalization of TG2 from the cell surface
A novel principal mechanism of surface TG2 regulation was reported to operate via internalization and subsequent lysosomal degradation of the protein (Fig. 3) [40]. In fibroblasts, constitutive endocytosis of cell surface TG2 depends on plasma membrane cholesterol and requires the activity of dynamin-2 GTPase [40]. Internalization of TG2 from the cell surface involves both clathrin-coated pits and lipid rafts or caveolae, proceeds through early and late endosomes, and results in lysosomal accumulation and proteolysis of TG2 [40]. No recycling of the internalized TG2 occurs in fibroblastic cells [40]. The constitutive endocytosis of TG2 in fibroblasts is rather efficient with the half-life of the protein on the surface ~20 min [40]. Further, soluble fibronectin, the key ECM ligand of TG2, and PDGF, both promote its endocytosis from the cell surface [40]. In contrast, fibronectin in the ECM anchors TG2 on the plasma membrane and prevents its internalization [40]. Since all TG2 is associated with integrins on the cell surface, it appears plausible that it is internalized as a complex with integrins, although experimental evidence for such co-endocytosis is yet to be provided.
Fig. 3.
The constitutive LRP1-dependent internalization and lysosomal degradation of cell surface TG2. Solid lines mark the major endosomal recycling and lysosomal degradative pathways.
Importantly, TG2 was found to interact with the major endocytic receptor, LRP1, in vitro and on the cell surface, and internalization of TG2 from the surface requires the LRP1 function (Fig. 3) [40]. However, somewhat surprisingly, TG2 was found to bind LRP1 away from the ligand-binding repeats of this receptor, as physiological inhibitor of the LRP1-dependent endocytosis, the receptor-associated protein (RAP), failed to block the internalization of TG2 [40]. It remains to be determined whether the direct interaction between TG2 and LRP1 triggers its endocytosis, or extracellular fibronectin facilitates this process by bridging TG2 to LRP1 on the cell surface.
Notably, LRP1 deficiency or blockade of endolysosomal function both upregulate TG2 on the cell surface, thus leading to increased adhesion to the ECM [40]. These findings characterize a previously unknown pathway of TG2 internalization and degradation that might be crucial for regulation of adhesive and signaling capacity of cell surface TG2 [40]. They also add to the emerging theme in the field that highlights an intricate functional relationship between cell-ECM adhesion and endocytosis [64]. Future work will determine a contribution of this endocytic mechanism to the regulation of adhesive and signaling functions of cell surface TG2 under pathophysiologic conditions that include impairment of LRP1-mediated endocytosis and/or lysosomal function.
Finally, it would be important to precisely map the binding site(s) for TG2 on the LRP1 molecule. The molecular motif(s) for TG2 binding in LRP1 might be similar or identical to those in the homologous LRP5/6 receptors that bind and signal through extracellular TG2 [54]. A plausible scenario is emerging in which LRP1 competes with LRP5/6 for binding cell surface TG2, thereby adjusting the levels of TG2-mediated signaling to β-catenin via the latter receptors. Both pro-inflammatory function of TG2 in vascular smooth muscle [67] and atheroprotective role of LRP1 in the vessel wall [68] suggest that overactivation of signaling mediated by TG2 due to impairment of its endocytosis from the cell surface might be involved in the progression of vascular diseases.
Pericellular proteolysis controls the fate of extracellular TG2
Unlike integrins, which are extremely resistant to proteolysis, cell surface TG2 is highly sensitive to proteolytic degradation [6]. Cell invasion requires cooperation between adhesion receptors and matrix metalloproteinases (MMPs) [69]. Membrane type (MT)-MMPs have been thought to be primarily involved in the ECM degradation [69]. But, in addition to the ECM breakdown, MT-MMPs appear to be engaged in proteolysis of TG2 as a principal adhesion receptor on tumor cell surfaces [70]. The initial proteolysis of TG2 by MT1-MMP occurs, in the order of appearance, at His461Leu; Arg458Ala; and Pro375Val cleavage sites in vitro and in cells [70]. Notably, overexpression of MT1-MMP by glioma and fibrosarcoma cells leads to proteolytic degradation of TG2 at the leading edge of motile cancer cells [70]. In agreement, structurally related MT1-MMP, MT2-MMP, and MT3-MMP, but not evolutionary distant MT4-MMP, efficiently degrade purified TG2 in vitro [70]. Moreover, the proteolytic degradation of TG2 by MT1-MMP specifically suppresses cell adhesion and migration on fibronectin [70]. Reciprocally, fibronectin in vitro and in the ECM of cultured cells protects its surface receptor, TG2, from proteolysis by MT1-MMP, thereby supporting cell adhesion and locomotion [70]. Together, these observations suggest a novel regulatory function of membrane-anchored MMPs in cancer cell adhesion and locomotion [6,70]. Importantly, proteolysis of TG2 colocalized with MT-MMPs at discrete regions on the surface of migrating tumor cells might be controlled by composition of the surrounding ECM. Apparently, proteolysis by MT-MMPs of the TG2 component of the cell surface integrin-TG2 complexes alters the pattern of cell-ECM recognition and switches cells from the binding to fibronectin to a more efficient interaction with collagens or other ECM proteins [3,70]. Degradation of adhesion proteins, including surface TG2, by MT-MMPs, may allow cells to avoid invasion of deficient, degraded matrices and, on the contrary, to trigger their detachment and subsequent metastasis. These versatile adjustment mechanisms may regulate migration of cells within composite matrices including connective tissue and tissue barriers such as the basement membrane.
MT1-MMP, a prototypic member of the membrane-type MMP subfamily, is an invasion promoting protease and proteolytic activator of soluble metalloproteinase MMP-2 [69]. MMP-2, functioning in concert with MT1-MMP, was reported to hydrolyze cell-surface-associated TG2, thereby further promoting the effect initiated by the activator of MMP-2 [58]. In turn, TG2 is preferentially associated with the activation intermediate of MMP-2 on the cell surfaces [58]. This interaction regulates the rate of MMP-2 maturation and protects TG2 against proteolysis by MMP-2 [36,58]. Cell culture, in vitro experiments, and in silico modeling indicated that the catalytic domain of MMP-2 directly associates with the core enzymatic domain 2 of TG2 [58]. The follow-up cleavage of the domain 2 of TG2 by MMP-2 was found to eliminate both the adhesive and enzymatic activities of TG2 [6,58]. These findings illuminate the coordinated interplay involving the MT1-MMP/MMP-2 protease tandem in the regulation of surface TG2 levels and functions and explain the underlying biochemical mechanisms of the extensive TG2 proteolysis that exists at the normal tissue / tumor boundary [1,2,6,58,70]. They also suggest that neoplasms, which express functionally active MT1-MMP and, therefore, activate soluble MMP-2, contribute to the degradation of TG2 present on the surface of the neighboring host cells [6,58]. The loss of adhesive and enzymatic activities of TG2 at the interface between tumor and normal tissue may decrease the cell-ECM interactions and inhibit ECM cross-linking, causing multiple pathological alterations in host cell adhesion and locomotion.
The significance of pericellular proteolysis of surface TG2 extends beyond its role in regulation of cancer cell invasiveness. Thrombospondin is a multifunctional ECM protein that is involved in cell responses to injury, angiogenesis, and the assembly and stabilization of collagen fibrils in the ECM [71]. Dermal fibroblasts from thrombospondin-2-null mice were reported to display an attachment defect that results from increased MMP-2 levels in their conditioned media [72]. A search for molecular mechanisms responsible for this defect identified surface TG2 as a key proteolytic target of MMP-2 in thrombospondin-2-null fibroblasts [72]. Moreover, the thrombospondin-2-null mice have reduced TG2 levels and activity in the skin [72]. These observations suggest that thrombospondin-2 prevents the MMP-2-induced degradation of TG2 in dermal fibroblasts, thus supporting their adhesion and collagen fibril assembly [72]. Therefore, like in the case of cancer cells, the ECM composition and organization appears to control the levels and functions of TG2 on the surface of fibroblasts by modulation of its pericellular proteolysis. The ECM-dependent proteolytic regulation of surface TG2 is likely to represent a general mechanism that includes various types of cells, matrices, and pericellular proteases.
Conclusions
The developing paradigm in this field supports the complex non-enzymatic functions of extracellular TG2, which is based on non-covalent interactions of this protein with integrins, fibronectin, syndecan-4, growth factor receptors, and other cell surface or ECM proteins. The ability of surface TG2 to regulate cell-ECM adhesion and outside-in signaling by several transmembrane receptors appears important for regulation of various cell functions. Moreover, cells dynamically regulate surface TG2 levels and functions via intricate and complementary secretory, endocytic, and ECM-dependent proteolytic mechanisms. Finally, mounting evidence indicates that this novel TG2 function is involved in numerous disease states, including cancer, vascular inflammation, tissue fibrosis and autoimmunity. Therefore, targeting the molecular interactions of TG2 with its extracellular partners is emerging as a new promising therapeutic strategy.
Acknowledgements
Studies in the author's laboratory were supported by National Institutes of Health grant RO1-GM62895 and by Maryland Stem Cell Research Fund exploratory grant 2009-MSCRFE-0028.
Abbreviations
- ECM
extracellular matrix
- EGFR
epidermal growth factor receptor
- FAK
focal adhesion kinase
- FGFR
fibroblast growth factor receptor
- HSPG
heparan sulfate proteoglycan
- LRP
low-density lipoprotein receptor-related protein
- MGF-E8
milk fat globulin EGF factor 8
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- MT-MMP
membrane type MMP
- PDGFR
platelet derived growth factor receptor
- PKCα
protein kinase Cα
- Tcf/Lef
T-cell factor/lymphoid enhancer factor
- TG2
transglutaminase 2
- VEGFR
vascular endothelial growth factor receptor
References
- 1.Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. doi: 10.1038/nrm1014. [DOI] [PubMed] [Google Scholar]
- 2.Iismaa SE, Mearns BM, Lorand L, Graham RM. Transglutaminases and disease: lessons from genetically engineered mouse models and inherited disorders. Physiol Rev. 2009;89:991–1023. doi: 10.1152/physrev.00044.2008. [DOI] [PubMed] [Google Scholar]
- 3.Kiraly R, Demeny M, Fesus L. Protein transamidation by transglutaminase 2 in cells: a disputed Ca2+-dependent action of a multifunctional protein. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08345.x. submitted. [DOI] [PubMed] [Google Scholar]
- 4.Walther DJ, Stahlberg S, Vowinckel J. Novel roles for primeval chemicals: from monoamines in transglutaminase-mediated posttranslational protein modification to mono-aminylation deregulation diseases. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08347.x. submitted. [DOI] [PubMed] [Google Scholar]
- 5.Kuo F-T, Kojima S. new insights into functions and localization of nuclear transglutaminase 2. FEBS J. 2011 doi: 10.1111/j.1742-4658.2011.08409.x. submitted. [DOI] [PubMed] [Google Scholar]
- 6.Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM. The role of tissue transglutaminase in cell-matrix interactions. Front Biosci. 2006;11:1057–1076. doi: 10.2741/1863. [DOI] [PubMed] [Google Scholar]
- 7.Akimov SS, Krylov D, Fleischman LF, Belkin AM. Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol. 2000;148:825–838. doi: 10.1083/jcb.148.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janiak A, Zemskov EA, Belkin AM. Cell surface transglutaminase promotes RhoA activation via integrin clustering and suppression of the Src-p190RhoGAP signaling pathway. Mol Biol Cell. 2006;17:1606–1619. doi: 10.1091/mbc.E05-06-0549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Telci D, Wang Z, Li X, Verderio EA, Humphries MJ, Baccarini M, Basaga H, Griffin M. Fibronectin-tissue transglutaminase matrix rescues RGD-impaired adhesion through syndecan-4 and beta1 integrin co-signaling. J Biol Chem. 2008;283:20937–20947. doi: 10.1074/jbc.M801763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scarpellini A, Germack R, Lortat-Jacob H, Muramatsu T, Billett E, Johnson T, Verderio EAM. Heparan sulfate proteoglycans are receptors for the cell-surface trafficking and biological activity of transglutaminase-2. J Biol Chem. 2009;284:18411–18423. doi: 10.1074/jbc.M109.012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dardik R, Inbal A. Complex formation between tissue transglutaminase II (tTG) and vascular endothelial growth factor receptor 2 (VEGFR-2): proposed mechanism for modulation of endothelial cell response to VEGF. Exp Cell Res. 2006;312:2973–2982. doi: 10.1016/j.yexcr.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 12.Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem. 2009;284:16693–16703. doi: 10.1074/jbc.M109.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mann AP, Verma A, Sethi G, Manavathi B, Wang H, Fok JY, Kunnumakkara AB, Kumar R, Aggarwal BB, Mehta K. Overexpression of tissue transglutaminase leads to constitutive activation of nuclear factor-kappaB in cancer cells: delineation of a novel pathway. Cancer Res. 2006;66:8788–8795. doi: 10.1158/0008-5472.CAN-06-1457. [DOI] [PubMed] [Google Scholar]
- 14.Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K. Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene. 2007;26:2459–2470. doi: 10.1038/sj.onc.1210035. [DOI] [PubMed] [Google Scholar]
- 15.Radisky DC, Stallings-Mann M, Hirai Y, Bissell MJ. Single proteins might have dual but related functions in intracellular and extracellular microenvironments. Nat Rev Mol Cell Biol. 2009;10:228–235. doi: 10.1038/nrm2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Upchurch HF, Conway E, Maxwell MD. Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix-bound enzyme. J Cell Physiol. 1991;149:375–382. doi: 10.1002/jcp.1041490304. [DOI] [PubMed] [Google Scholar]
- 17.Gentile V, Thomazy V, Piacentini M, Fesus L, Davies PJ. Expression of tissue transglutaminase in Balb-C 3T3 fibroblasts: effects on cell morphology abd adhesion. J Cell Biol. 1992;119:463–474. doi: 10.1083/jcb.119.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aeschlimann D, Wetterwald A, Fleisch H, Paulsson M. Expression of tissue trans-glutaminase in skeletal tissues correlates with events of terminal differentiation of chondrocytes. J Cell Biol. 1993;120:461–470. doi: 10.1083/jcb.120.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaudry CA, Verderio E, Jones RA, Smith C, Griffin M. Tissue transglutaminase is an important player at the surface of human endothelial cells: evidence for its externalization and its colocalization with the beta(1) integrin. Exp Cell Res. 1999;252:104–113. doi: 10.1006/excr.1999.4633. [DOI] [PubMed] [Google Scholar]
- 20.Aeschlimann D, Thomazy V. Protein crosslinking in assembly and remodelling of extra-cellular matrices: the role of transglutaminases. Connective Tissue Res. 2000;41:1–26. doi: 10.3109/03008200009005638. [DOI] [PubMed] [Google Scholar]
- 21.Siegel M, Strnad P, Watts RE, Choi K, Jabri B, Omary MB, Khosla C. Extracellular transglutaminase 2 is catalytically inactive, but is transiently activated upon tissue injury. PLoS One. 2008;3:e1861. doi: 10.1371/journal.pone.0001861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM. Redox regulation of transglutaminase 2 activity. J Biol Chem. 2010;285:25402–25309. doi: 10.1074/jbc.M109.097162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lai TS, Hausladen A, Slaughter TF, Eu JP, Stamler JS, Greenberg CS. Calcium regulates S-nitrosylation, denitrosylation, and activity of tissue transglutaminase. Biochemistry. 2001;40:4904–4910. doi: 10.1021/bi002321t. [DOI] [PubMed] [Google Scholar]
- 24.Santhanam L, Tuday EC, Webb AK, Dowzicky P, Kim JH, Oh YJ, Sikka G, Kuo M, Halushka MK, Macgregor AM, Dunn J, Gutbrod S, Yin D, Shoukas A, Nyhan D, Flavahan NA, Belkin AM, Berkowitz DE. Decreased S-nitrosylation of tissue transglutaminase contributes to age-related increases in vascular stiffness. Circ Res. 2010;107:117–125. doi: 10.1161/CIRCRESAHA.109.215228. [DOI] [PubMed] [Google Scholar]
- 25.Telci D, Collighan RJ, Basaga H, Griffin M. Increased TG2 expression can result in induction of transforming growth factor beta1, causing increased synthesis and deposition of matrix proteins, which can be regulated by nitric oxide. J Biol Chem. 2009;284:29547–29558. doi: 10.1074/jbc.M109.041806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sane DC, Moser TL, Parker CJ, Seiffert D, Loskutoff DJ, Greenberg CS. Highly sulfated glycosaminoglycans augment the cross-linking of vitronectin by guinea pig liver transglutaminase. Functional studies of the cross-linked vitronectin multimers. J Biol Chem. 1990;265:3543–3548. [PubMed] [Google Scholar]
- 27.Pinkas DM, Strop P, Brunger AT, Khosla C. Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol. 2007;5:e327. doi: 10.1371/journal.pbio.0050327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Akimov SS, Belkin AM. Cell-surface transglutaminase promotes fibronectin assembly via interaction with the gelatin-binding domain of fibronectin: a role in TGFbeta-dependent matrix deposition. J Cell Sci. 2001;114:2989–3000. doi: 10.1242/jcs.114.16.2989. [DOI] [PubMed] [Google Scholar]
- 29.Turner PM, Lorand L. Complexation of fibronectin with tissue transglutaminase. Biochemistry. 1989;28:628–635. doi: 10.1021/bi00428a032. [DOI] [PubMed] [Google Scholar]
- 30.Martinez J, Chalupowicz DG, Roush RK, Sheth A, Barsigian C. Transglutaminase-mediated processing of fibronectin by endothelial cell monolayers. Biochemistry. 1994;33:2538–2545. doi: 10.1021/bi00175a024. [DOI] [PubMed] [Google Scholar]
- 31.Akimov SS, Belkin AM. Cell surface tissue transglutaminase is involved in adhesion and migration of monocytic cells on fibronectin. Blood. 2001;98:1567–1576. doi: 10.1182/blood.v98.5.1567. [DOI] [PubMed] [Google Scholar]
- 32.Radek JT, Jeong JM, Murthy SN, Ingham KC, Lorand L. Affinity of human erythrocyte transglutaminase for a 42-kDa gelatin-binding fragment of human plasma fibronectin. Proc Natl Acad Sci USA. 1984;90:3152–3156. doi: 10.1073/pnas.90.8.3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leiss M, Beckmann K, Giros A, Costell M, Fassler R. The role of integrin binding sites in fibronectin matrix assembly in vivo. Curr Opin Cell Biol. 2010;20:502–507. doi: 10.1016/j.ceb.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 34.Satpathy M, Cao L, Pincheira R, Emerson R, Bigsby R, Nakshatri H, Matei D. Enhanced peritoneal ovarian tumor dissemination by tissue transglutaminase. Cancer Res. 2007;67:7194–7202. doi: 10.1158/0008-5472.CAN-07-0307. [DOI] [PubMed] [Google Scholar]
- 35.Tóth B, Garabuczi E, Sarang Z, Vereb G, Vámosi G, Aeschlimann D, Blaskó B, Bécsi B, Erdõdi F, Lacy-Hulbert A, Zhang A, Falasca L, Birge RB, Balajthy Z, Melino G, Fésüs L, Szondy Z. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–2092. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 36.Stephens P, Grenard P, Aeschlimann P, Langley M, Blain E, Errington R, Kipling D, Thomas D, Aeschlimann D. Crosslinking and G-protein functions of transglutaminase 2 contribute differentially to fibroblast wound healing responses. J Cell Sci. 2004;117:3389–3403. doi: 10.1242/jcs.01188. [DOI] [PubMed] [Google Scholar]
- 37.Song H, Chang W, Lim S, Seo HS, Shim CY, Park S, Yoo KJ, Kim BS, Min BH, Lee H, Jang Y, Chung N, Hwang KC. Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells. 2007;25:1431–1438. doi: 10.1634/stemcells.2006-0467. [DOI] [PubMed] [Google Scholar]
- 38.van Strien ME, Drukarch B, Bol JG, van der Valk P, van Horssen J, Gerritsen WH, Breve JJ, van Dam AM. Appearance of tissue transglutaminase in astrocytes in multiple sclerosis lesions: a role in cell adhesion and migration? Brain Pathol. 2010;21:44–54. doi: 10.1111/j.1750-3639.2010.00428.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Cerione RA, Clardy J. Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci USA. 2002;99:2743–2747. doi: 10.1073/pnas.042454899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zemskov EA, Mikhailenko I, Strickland DK, Belkin AM. Cell surface transglutaminase undergoes internalization and lysosomal degradation: an essential role for LRP1. J Cell Sci. 2007;120:3188–3199. doi: 10.1242/jcs.010397. [DOI] [PubMed] [Google Scholar]
- 41.Tóth B, Sarang Z, Vereb G, Zhang A, Tanaka S, Melino G, Fésüs L, Szondy Z. Over-expression of integrin beta3 can partially overcome the defect of integrin beta3 signaling in transglutaminase 2 null macrophages. Immunol Lett. 2009;126:22–28. doi: 10.1016/j.imlet.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 42.Xian X, Gopal S, Couchman JR. Syndecans as receptors and organizers of the extracellular matrix. Cell Tissue Res. 2009;339:31–46. doi: 10.1007/s00441-009-0829-3. [DOI] [PubMed] [Google Scholar]
- 43.Parsons M, Keppler MD, Kline A, Messent A, Humphries MJ, Gilchrist R, Hart IR, Quittau-Prevostel C, Hughes WE, Parker PJ, Ng T. Site-directed perturbation of protein kinase C-integrin interaction blocks carcinoma cell chemotaxis. Mol Cell Biol. 2002;22:5897–5911. doi: 10.1128/MCB.22.16.5897-5911.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Z, Collighan RJ, Gross SR, Danen EH, Orend G, Telci D, Griffin M. RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 and {alpha}5{beta}1 integrin co-signaling. J Biol Chem. 2010;285:40212–40229. doi: 10.1074/jbc.M110.123703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Z, Telci D, Griffin M. Importance of syndecan-4 and syndecan -2 in osteoblast cell adhesion and survival mediated by a tissue transglutaminase-fibronectin complex. Exp Cell Res. 2011;317:367–381. doi: 10.1016/j.yexcr.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 46.Verderio E, Scarpellini A, Johnson TS. Novel interactions of TG2 with heparan sulfate proteoglycans: reflection on physiological implications. Amino Acids. 2009;36:671–677. doi: 10.1007/s00726-008-0134-6. [DOI] [PubMed] [Google Scholar]
- 47.Verderio E, Scarpellini A. Significance of syndecan-4-transglutaminase-2 interaction. Sci World J. 2010;10:1073–1077. doi: 10.1100/tsw.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bass MD, Morgan MR, Roach KA, Settleman J, Goryachev AB, Humphries MJ. p190RhoGAP is the convergence point of adhesion signals from α5β1 integrin and syndecan-4. J Cell Biol. 2008;181:1013–1026. doi: 10.1083/jcb.200711129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Telci D, Griffin M. Tissue transglutaminase (TG2) - a wound response enzyme. Front Biosci. 2006;11:867–882. doi: 10.2741/1843. [DOI] [PubMed] [Google Scholar]
- 50.Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nature Cell Biol. 2002;4:E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- 51.Baron W, Decker L, Colognato H, ffrench-Constant C. Regulation of integrin growth factor interactions in oligodendrocytes by lipid raft microdomains. Curr Biol. 2003;13:151–155. doi: 10.1016/s0960-9822(02)01437-9. [DOI] [PubMed] [Google Scholar]
- 52.DeMali KA, Balciunaite E, Kazlauskas A. Integrins enhance platelet-derived growth factor (PDGF)-dependent responses by altering the signal relay enzymes that are recruited to the PDGF beta receptor. J Biol Chem. 1999;274:19551–19558. doi: 10.1074/jbc.274.28.19551. [DOI] [PubMed] [Google Scholar]
- 53.Heldin C-H, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- 54.Faverman L, Mikhaylova L, Malmquist J, Nurminskaya M. Extracellular transglutaminase 2 activates beta-catenin signaling in calcifying vascular smooth muscle cells. FEBS Lett. 2008;582:1552–1557. doi: 10.1016/j.febslet.2008.03.053. [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Begum S, Hearn JD, Hynes RO. GPR56, an atypical G protein-coupled receptor, binds tissue transglutaminase, TG2, and inhibits melanoma tumor growth and metastasis. Proc Natl Acad Sci USA. 2006;103:9023–9028. doi: 10.1073/pnas.0602681103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.L'Heureux DZ, Rothman VL, Tuszynski GP. The interaction of angiocidin with tissue transglutaminase. Exp Mol Pathol. 2010;88:15–25. doi: 10.1016/j.yexmp.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Faye C, Inforzato A, Bignon M, Hartmann DJ, Muller L, Ballut L, Olsen BR, Day AJ, Ricard-Blum S. Transglutaminase-2: a new endostatin partner in the extracellular matrix of endothelial cells. Biochem J. 2010;427:467–475. doi: 10.1042/BJ20091594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Belkin AM, Zemskov EA, Hang J, Akimov SS, Sikora S, Strongin AY. Cell-surface-associated tissue transglutaminase is a target of MMP-2 proteolysis. Biochemistry. 2004;43:11760–11769. doi: 10.1021/bi049266z. [DOI] [PubMed] [Google Scholar]
- 59.Gentile V, Saydak M, Chiocca EA, Akande O, Birckbichler PJ, Lee KN, Stein JP, Davies PJ. Isolation and characterization of cDNA clones to mouse macrophage and human endothelial cell tissue transglutaminases. J Biol Chem. 1991;266:478–483. [PubMed] [Google Scholar]
- 60.Harsfalvi J, Arato G, Fesus L. Lipids associated with tissue transglutaminase. Biochim Biophys Acta. 1987;923:42–45. doi: 10.1016/0304-4165(87)90123-1. [DOI] [PubMed] [Google Scholar]
- 61.Gaudry CA, Verderio E, Aeschlimann D, Cox A, Smith C, Griffin M. Cell surface localization of tissue transglutaminase is dependent on a fibronectin-binding site in its N-terminal beta-sandwich domain. J Biol Chem. 1999;274:30707–30714. doi: 10.1074/jbc.274.43.30707. [DOI] [PubMed] [Google Scholar]
- 62.Balklava Z, Verderio E, Collighan R, Gross S, Adams J, Griffin M. Analysis of tissue transglutaminase function in the migration of Swiss 3T3 fibroblasts: the active-state conformation of the enzyme does not affect cell motility but is important for its secretion. J Biol Chem. 2002;277:16567–16575. doi: 10.1074/jbc.M109836200. [DOI] [PubMed] [Google Scholar]
- 63.Zemskov EA, Mikhailenko I, Hsia RC, Zaritskaya L, Belkin AM. Unconventional secretion of tissue transglutaminase involves phospholipid-dependent delivery into recycling endosomes. PLoS One. 2011;6:e19414. doi: 10.1371/journal.pone.0019414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nickel W, Rabouille C. Mechanisms of regulated unconventional protein secretion. Nat Rev Mol Cell Biol. 2009;10:148–155. doi: 10.1038/nrm2617. [DOI] [PubMed] [Google Scholar]
- 65.Caswell PT, Vadrevu S, Norman JC. Integrins: masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 66.Skalski M, Yi Q, Kean MJ, Myers DW, Williams KC, Burtnik A, Coppolino MG. Lamellipodium extension and membrane ruffling require different SNARE-mediated trafficking pathways. BMC Cell Biology. 2010;11:62–78. doi: 10.1186/1471-2121-11-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bakker EN, Pistea A, VanBavel E. Transglutaminases in vascular biology: relevance for vascular remodeling and atherosclerosis. J Vasc Res. 2008;45:271–278. doi: 10.1159/000113599. [DOI] [PubMed] [Google Scholar]
- 68.Lillis AP, van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kessenbrock K, Plaks V, Werb Z. Matrix metalloproteinases: regulators of the tumor microenvironment. Cell. 2010;141:52–67. doi: 10.1016/j.cell.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Belkin AM, Akimov SS, Zaritskaya LS, Ratnikov BI, Deryugina EI, Strongin AY. Matrix-dependent proteolysis of surface transglutaminase by membrane-type metalloproteinase regulates cancer cell adhesion and locomotion. J Biol Chem. 2001;276:18415–18422. doi: 10.1074/jbc.M010135200. [DOI] [PubMed] [Google Scholar]
- 71.Adams JC, Lawler J. The thrombospondins. Int J Biochem Cell Biol. 2004;36:961–968. doi: 10.1016/j.biocel.2004.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Agah A, Kyriakides TR, Bornstein P. Proteolysis of cell-surface tissue transglutaminase by matrix metalloproteinase-2 contributes to the adhesive defect and matrix abnormalities in thrombospondin-2-null fibroblasts and mice. Am J Pathol. 2005;167:81–88. doi: 10.1016/S0002-9440(10)62955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]