Abstract
Adenovirus (Ad)-based gene therapy represents a potentially viable strategy for treating colorectal cancer. The infectivity of serotype 5 adenovirus (Ad.5), routinely used as a transgene delivery vector, is dependent on Coxsackie-adenovirus receptors (CAR). CAR expression is downregulated in many cancers thus preventing optimum therapeutic efficiency of Ad.5-based therapies. To overcome the low CAR problem, a serotype chimerism approach was used to generate a recombinant Ad (Ad.5/3) that is capable of infecting cancer cells via Ad.3 receptors in a CAR-independent manner. We evaluated the improved transgene delivery and efficacy of Ad.5/3 recombinant virus expressing melanoma differentiation associated gene-7/interleukin-24 (mda-7/IL-24), an effective wide-spectrum cancer-selective therapeutic. In low CAR human colorectal cancer cells RKO, wild-type Ad.5 virus expressing mda-7/IL-24 (Ad.5-mda-7) failed to infect efficiently resulting in lack of expression of MDA-7/IL-24 or induction of apoptosis. However, a recombinant Ad.5/3 virus expressing mda-7/IL-24 (Ad.5/3-mda-7) efficiently infected RKO cells resulting in higher MDA-7/IL-24 expression and inhibition of cell growth both in vitro and in nude mice xenograft models. Addition of the novel Bcl-2 family pharmacological inhibitor Apogossypol derivative BI-97C1 (Sabutoclax) significantly augmented the efficacy of Ad.5/3-mda-7. A combination regimen of suboptimal doses of Ad.5/3-mda-7 and BI-97C1 profoundly enhanced cytotoxicity in RKO cells both in vitro and in vivo. Considering the fact that Ad.5-mda-7 has demonstrated significant objective responses in a Phase I clinical trial for advanced solid tumors, Ad.5/3-mda-7 alone or in combination with BI-97C1 would be predicted to exert significantly improved therapeutic efficacy in colorectal cancer patients.
Keywords: Viral gene therapy, Mcl-1 inhibition, apoptosis induction, anti-tumor activity
Introduction
According to the American Cancer Society (www.cancer.gov), colorectal cancer is the third most common cancer and third leading cause of cancer-related deaths in the US. During 2010, 142,570 new cases of colorectal cancer were diagnosed and it was expected to cause about 51,370 deaths. Over 20% of patients present with metastatic (stage IV) colorectal cancer at the time of diagnosis, and up to 25% of this group will have isolated liver metastasis that is potentially resectable (1). The National Cancer Institute (www.cancer.gov) reported that the lifetime risk of being diagnosed with cancer of the colon or rectum is about 1 in 19 in males and about 1 in 20 in females in the US. While 20%-25% of colorectal cancer cases occur among individuals with a family history of colorectal cancer or a predisposing illness, about 75% of cases occur in people without these risk factors (2). In these contexts, there is need to develop novel, effective therapeutic approaches for treating colorectal cancer and genetic therapies represent promising therapeutic options for this neoplasm.
Adenoviruses represent a new therapeutic modality that can be designed to deliver therapeutic genes and to specifically replicate in and kill cancer cells. Clinical studies proved that these viruses can safely be administered locally, regionally and systemically (3) and that these agents have efficacy in gastrointestinal cancer (4). Recombinant forms of the type 5 adenovirus (Ad.5) are the most frequently used serotype of Ad for gene therapy. However, to be able to infect the cells, Ad.5 requires the Coxsackie Adenovirus Receptor (CAR) (5-6). In many tumor types, such as malignant glioma, and ovarian, renal, bladder, prostate, colorectal cancers, and particularly when examined in primary tumor specimens, the expression of CAR is reduced or absent in tumor cells compared to surrounding non-tumor tissue (5, 7-10). Established cell lines express higher levels of CAR compared to that observed in primary tumors or in patients. Reduced CAR expression limits the efficiency of transduction of cancer cells by Ad.5 (11-12). This finding may in part explain why gene therapy approaches using Ad.5 have not been as robust or successful as the studies performed in vitro using established cell lines. An approach to circumventing the low efficiency of Ad.5 infection of tumor cells involves ‘tropism modification’ in which the virus capsid proteins that normally associate with CAR are modified, permitting CAR-independent infectivity of tumor cells. The infective type 3 Ad sequence within the Ad type 5 virus knob (Ad.5/3 recombinant virus) allows viral infectivity regardless of the CAR expression status of tumor cells thereby permitting efficient viral infectivity of low and high CAR expressing tumor cells (13-14).
Melanoma differentiation associated gene-7/interleukin-24(mda-7/IL-24) was cloned in our laboratory by subtraction hybridization technique by inducing human melanoma cells to terminal differentiation upon treatment with fibroblast interferon and mezerein (15-16). This novel tumor suppressor cytokine is a member of the interleukin-10 (IL-10) gene family (17-22). Ectopic expression of mda-7/IL-24, using Ad.5 (Ad.5-mda-7) inhibits growth and induces cell death in many types of cancer cell lines in vitro, without harming comparable normal human cells, including epithelial cells, fibroblasts, melanocytes or astrocytes (21-26). Considering its robust cancer-specific apoptosis-inducing ability (by inducing ER stress) and tumor growth-suppressing properties in nude mice xenograft models, Ad.mda-7 (INGN 241) was evaluated in a phase I clinical trial in patients with advanced cancers. The results from this trial demonstrated safety and clinical efficacy (27). In addition to its direct apoptosis inducing properties, Ad.mda-7 also shows antiangiogenic, immunostimulatory, and potent “bystander” antitumor activities (28-29). These exciting results provide direct support for using mda-7/IL-24 in developing an effective gene-based therapy for cancer. Recently, we reported that in prostate and ovarian cancers and in malignant gliomas, mda-7/IL-24 induced an ER stress response and subsequent apoptosis by suppressing expression of the anti-apoptotic Bcl-2 family members thus demonstrating the crucial role of Bcl-2 family proteins in regulating mda-7/IL-24 function (30-32). In this context antagonists of anti-apoptotic Bcl-2 family members might sensitize cancer cells to mda-7/IL-24-mediated cytotoxicity. The novel Apogossypol derivative BI-97C1 (Sabutoclax) suppresses the pro-survival Bcl-2 family members within a clinically achievable dose range (33). We recently demonstrated that BI-97C1 (Sabutoclax) sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity (34).
The present study explored the efficacy of a tropism-modified Ad-based cancer gene therapy approach for eradicating low CAR colorectal cancer cells. We show that in low CAR human colorectal cancer cells (RKO), a recombinant Ad.5/3 virus delivering mda-7/IL-24 (Ad.5/3-mda-7) is more efficient than Ad.5 delivering mda-7/IL-24 (Ad.5-mda-7) in expressing MDA-7/IL-24 protein, inducing cancer-specific apoptosis and inhibiting in vivo tumor growth in a nude mouse xenograft model. Further, our in vitro and in vivo data shows BI-97C1 (Sabutoclax) profoundly sensitizes mda-7/IL-24 mediated toxicity in colorectal cancer. Thus, Ad.5/3-mda-7, alone and/or in combination with BI-97C1 (Sabutoclax), might represent an improved and more effective therapeutic approach for colorectal and other cancers.
Materials and Methods
Cell lines, culture conditions, and viability assays
Human HCT116 colorectal carcinoma cell lines were obtained from the American Type Culture Collection (Rockville, MD, USA). Dr. Jeffrey Schlom kindly provided human RKO colorectal carcinoma cell line from the National Center Institute (NIH, Bethesda, MD, USA). The cells were cultured in DMEM High Glucose (GIBCO), supplemented with 10% FBS and 1% penicillin and streptomycin (Life technologies Inc.) at 37°C in a 5% CO2 95% air humidified incubator. Cell viability was determined by standard 3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyltetrazolium bromide (MTT) assays (35).
Generation of Ad.5.mda-7 and Ad.5/3-mda-7
Recombinant serotype 5 and serotype 5 /serotype 3 chimeric adenoviruses expressing mda-7/IL-24 (Ad.5.mda-7 and Ad.5/3-mda-7) and control empty adenovirus (Ad.5.vec and Ad.5/3.vec) were generated as described (25, 36).
Trypan blue dye exclusion and Annexin V binding assays
Cells were harvested by treatment with 0.05% trypsin/0.53 mM EDTA. Cells were centrifuged at 200 × g for 5 min to remove trypsin/EDTA. After the cell pellets were resuspended in 100 μl of PBS, 100 μl of 0.4% trypan blue solution were added and mixed. Trypan blue cell suspension was left at room temperature for 10 min before the cells were counted with a hemocytometer. Cell death was defined as the percentage of the stained dead cells in the whole-cell population (35). Annexin V binding assay was performed as previously described (37).
Detection of Coxsackie-adenovirus receptors (CARs) on the cell surface, and preparation of whole-cell lysates and Western blotting analyses
Quantification of surface expression of CAR was performed as described previously (36, 37). Preparation of whole-cell lysates and Western blotting analyses were performed as described (38). The primary antibodies used were anti-MDA-7/IL-24 (Gen Hunter Corporation), anti-EF1α (1:1,000; mouse monoclonal;, Millipore), anti-Mcl-1 (1:500; mouse monoclonal; Santa Cruz), anti-BiP/GRP78 (1:500; rabbit monoclonal; Santa Cruz), anti-GRP94 (1:1000; rabbit monoclonal; Sigma), anti-Bcl-2 (1:1000; mouse monoclonal; BD Biosciences) and anti-PARP (1:1000; rabbit monoclonal; Cell Signaling).
Human colorectal cancer xenografts in athymic nude mice and treatment with Ad.5/3-mda-7 and BI-97C1 (Sabutoclax)
RKO cells (2 × 106) were injected s.c. in 100 μL in male athymic nude mice (NCRnu/nu, 4 weeks old, ∼20 g body weight) (38-39). After establishment of visible tumors of ∼100-mm3, requiring ∼8-10 days, different adenoviruses were injected intratumorally. The injections were given 3× the first week and then 2× a week thereafter for a total of nine injections. BI-97C1 (Sabutoclax) was injected intraperitoneally (i.p.) at a sub-toxic level (33) at 5 mg/kg 3× a week throughout the study (total 12 injections). Compound was dissolved in 500 μL of solvent (ethanol/Cremophor EL/saline = 10:10:80). A minimum of five animals was used per experimental point. Tumor volume was measured with a caliper and calculated using the formula: π/6 × larger diameter × (smaller diameter)2. At the end of the experiment, the animals were sacrificed and the tumors were harvested and preserved in formalin before embedding in paraffin for immunohistochemical analysis.
Immunohistochemical staining
Immunohistochemical staining was performed as described (36) with anti-MDA-7/IL-24 (1:200) and monoclonal anti-CD31 (1:200) (Dako Corporation, Carpenteria, California, USA).
Statistical analysis
Statistical analysis was done using the Student t-test. P < 0.05 was considered significant.
Results
Enhanced infectivity of tropism-modified adenovirus (Ad.5/3) in RKO low CAR colorectal cancer cells
Experiments were designed to compare the infectivity of Ad.5/3 chimeric viruses (expressing luciferase or mda-7/IL-24) and Ad.5 viruses (expressing luciferase or mda-7/IL-24) in low and high CAR colorectal cancer cells. The Kolmogorov-Smirnov two-sample test was used to analyze flow cytometric histograms to determine the cumulative distribution function of fluorescence intensity for each sample and the difference between the cumulative distributions, with the D value indicating the greatest difference between the two curves. RKO cells have a reduced level of CAR (D value 0.03), whereas HCT116 cells have a high level of CAR (D value 0.82) (Fig. 1A). Luciferase (Luc) activity was evaluated following infection with Ad.5/3-Luc and Ad.5-Luc in RKO and HCT116 cells (Fig. 1B). The infection of RKO cells with Ad.5/3-Luc showed a dramatic increase in luciferase activity when compared to Ad.5-Luc. On the other hand the relative luciferase activity of Ad.5/3-Luc compared to that of Ad.5-Luc in HCT116 was much lower than that observed in RKO cells. These findings indicate that transduction efficiency in a low CAR tumor cell background was significantly enhanced by the Ad.5/3 modification. Next we compared Ad.5/3 and Ad.5 expressing mda-7/IL-24 (Ad.5/3-mda-7 and Ad.5-mda-7, respectively) by evaluating cell viability using standard MTT assay (Fig. 1C). Ad.5-vec (empty Ad.5 virus) and Ad.5/3-vec (empty Ad.5/3 virus) were used as controls. In RKO cells, Ad.5/3-mda-7 infected group had a significant reduction in cell viability whereas Ad.5-mda-7 treatment did not affect the cell viability. In HCT116 cells, both Ad.5-mda-7 and Ad.5/3-mda-7 inhibited growth with comparable efficiency (Fig. 1C).
Figure 1. Enhanced infectivity of tropism-modified adenovirus (Ad.5/3) in RKO low CAR colorectal cancer cells.
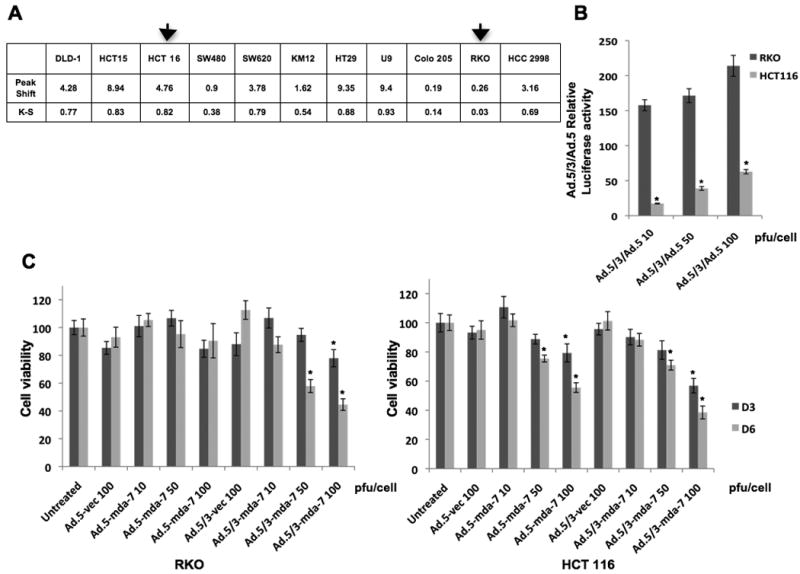
A) Coxsackie-adenovirus receptors (CARs) expression on the surface of different colorectal cancer cell lines, the K-S value of RKO is only 3%, whereas it reaches 83% for HCT116. B) HCT116 and RKO cells were infected with Ad.5-Luc and Ad.5/3-Luc at the indicated doses; and luciferase activity was measured after 48 hr. Data represent luciferase activity of Ad.5/3-Luc divided by that of Ad.5-Luc. C) HCT116 and RKO cells were infected with the indicated doses of virus and cell viability was quantified using MTT assay after 3 and 6 days of infection.
Ad.5/3-mda-7 induces MDA-7 expression and enhances killing in low CAR expressing RKO cells
We compared the ability of Ad.5/3-mda-7 and Ad.5-mda-7 (50 or 100 pfu/cell) in expressing mda-7/IL-24 in RKO and HCT116 cells (Fig. 2A and B). In RKO cells, Ad.5/3-mda-7 generated MDA-7/IL-24 at both high and low titer whereas Ad.5-mda-7 infected groups did not generate MDA-7/IL-24 protein (Fig. 2A). HCT116 cancer cells generated comparable amounts of MDA-7/IL-24 protein upon infection with both Ad.5/3-mda-7 and Ad.5-mda-7 (Fig. 2B). As a corollary, in RKO cells Ad.5/3-mda-7, and not Ad.5-mda-7 efficiently induced apoptosis, while both Ads were equally effective in inducing apoptosis in HCT116 cells as determined by PARP cleavage analysis (Fig. 2A and B). Similar profiles of induction of cell death were also observed using the trypan blue-dye exclusion test (Fig. 2C and D). Ad.5/3-mda-7, but not Ad.5-mda-7, induced cell killing (Fig. 2C) activity in RKO cells, which correlated with the expression level of MDA-7/IL-24 protein. As anticipated, both Ad.5-mda-7 and Ad.5/3-mda-7 induced cell death in HCT116 cells with comparable efficiency (Fig. 2D).
Figure 2. Ad.5/3-mda-7 induces MDA-7/IL-24 expression and enhances killing in low CAR expressing RKO cancer cells.
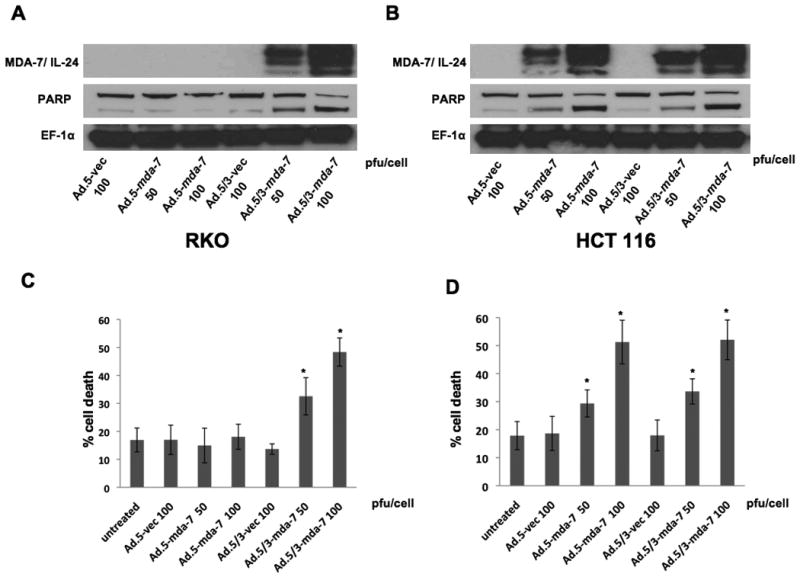
A and B. HCT116 and RKO cells were infected with the indicated doses of Ad.5-mda-7 or Ad.5/3-mda-7 and respective controls. After 48 hr of infection total proteins were isolated. The expression of MDA-7/IL-24, activation of PARP (as a marker for apoptosis) and expression of EF1α (as a loading control) were analyzed by Western blotting analyses in RKO (A) and HCT 116 (B) cells. C and D. Percentage of dead cells detected by Trypan blue dye exclusion staining for RKO (C) and HCT 116 (D) cells. Results are mean ± S.D. (n=3).
Annexin V staining followed by flow cytometry further quantified induction of apoptosis. In RKO cells, infection of Ad.5/3-mda-7 at 100 pfu/cell resulted in ∼70% apoptotic cells, while no apoptosis was observed with Ad.5-mda-7 (Fig. 3A and B). As a corollary, infection of RKO cells with Ad.5/3-mda-7, and not with Ad.5-mda-7, induced an ER stress response as evidenced by increased expression of ER stress markers BiP/GRP78 and GRP94 and downregulation of anti-apoptotic protein Bcl-2 (Fig. 3C) These findings confirm that Ad.5/3 chimerism maintains the bona fide downstream effects exerted by mda-7/IL-24.
Figure 3. Ad.5/3-mda-7 induces killing, apoptosis and ER stress in RKO low CAR colorectal cancer cells.
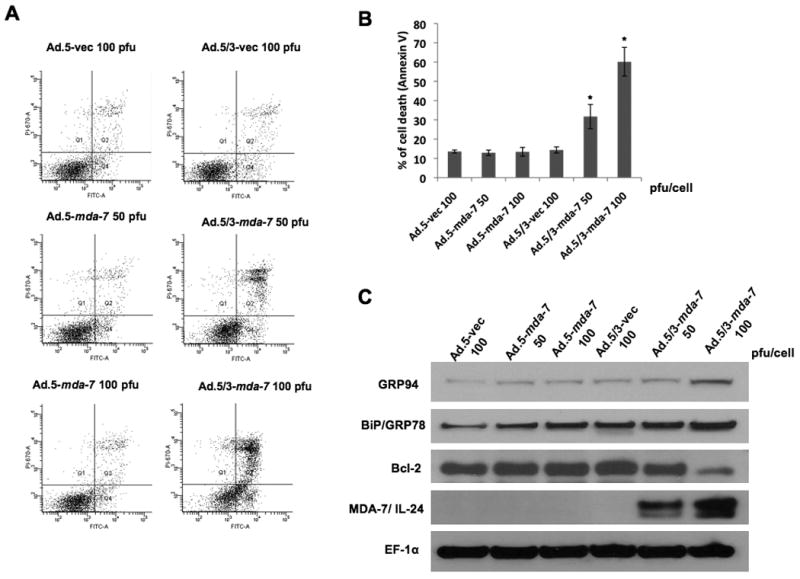
RKO Cells were treated with the indicated Ad for two days. A) Histogram of different groups assayed by flow cytometry following Annexin V-FITC/PI staining. B) Graph showing percentage of Annexin V- positive cells. Results are the mean ± S.D. (n=3). C) Changes in GRP94, BiP/GRP78, Bcl-2, MDA-7/IL-24 and EF1α were evaluated by Western blotting analysis.
Ad.5/3-mda-7 exerts anti-tumor effects in RKO xenografts in nude mice
To test if the in vitro superiority of Ad.5/3-mda-7 over Ad.5-mda-7 in low CAR colorectal cancer cells translates into the in vivo situation, we established subcutaneous xenografts of RKO cells in athymic nude mice. After palpable tumors of ∼100 mm3 developed, the animals received 9 intratumoral injections over a 4-week period with 4 × 108 pfu of Ad.5-vec, Ad.5/3-vec, Ad.5-mda-7 or Ad.5/3-mda-7 (Fig. 4). RKO cells formed large, aggressive and actively growing tumors in Ad.5-vec, Ad.5/3-vec and Ad.5-mda-7 treated animals, while Ad.5/3-mda-7 significantly inhibited the growth of the RKO tumors, which was evident at weeks 4 after initiating the therapeutic treatment protocol (Fig. 4A and B). The reduction in tumor growth by Ad.5/3-mda-7 was associated with increased expression of MDA-7/IL-24 and reduced expression of CD31, a marker for microvessels, in the tumor sections (Fig. 5A and B). H & E sections of the tumors also showed decreased cell density in Ad.5/3-mda-7-treated group compared to the other groups (Fig. 5C). These findings indicate that in low CAR colorectal cancer cells, Ad.5/3-mda-7 can efficiently generate MDA-7/IL-24 protein leading to inhibition of cell proliferation and angiogenesis.
Figure 4. Anti-tumor effect of Ad.5-mda-7 and Ad.5/3-mda-7 in RKO xenografts in nude mice.
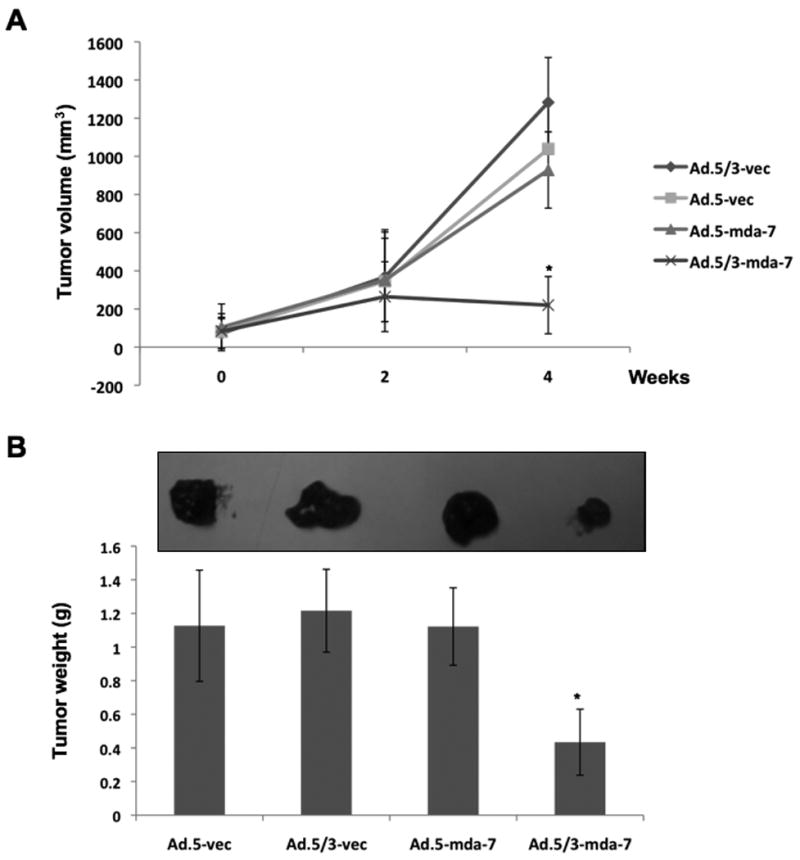
Subcutaneous tumor xenografts from RKO cells were established in athymic nude mice; tumors were injected with the indicated Ads over a 4-week period (total of nine injections). A) Measurements of RKO xenograft tumor volumes; points, average (with a minimum of five mice in each group); bars, ± S.D. B) measurement of tumor weights at the end of the study. Bars, ± S.D. C) Tumor images at the end of the study.
Figure 5. Ad.5/3-mda-7 shows enhanced MDA-7/IL-24 production and inhibition of proliferation and angiogenesis in RKO low CAR colorectal cancer cells.
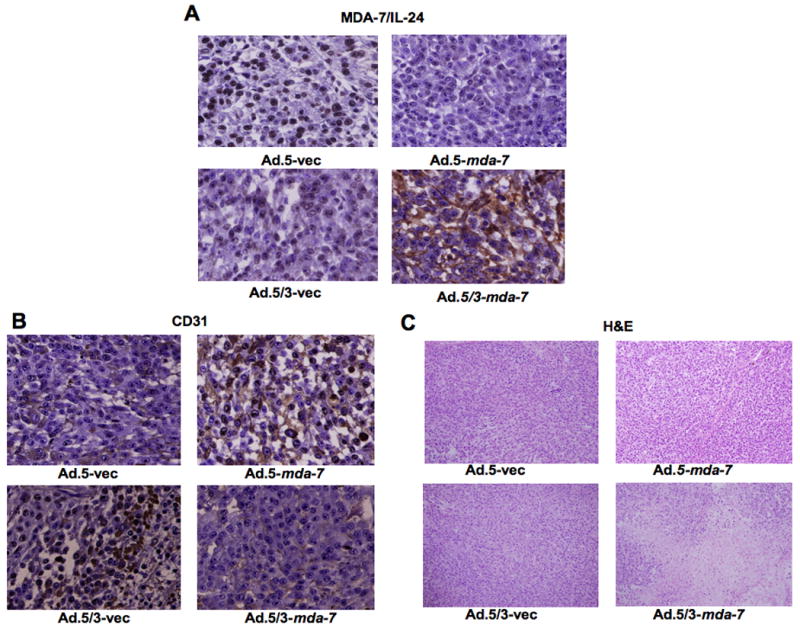
Subcutaneous tumor xenografts from RKO cells were established in athymic nude mice; the tumors were injected with the indicated Ads over a 4-week period (total of nine injections). At the end of the experiment the tumors were harvested and formalin-fixed, paraffin-embedded, sectioned and were subjected to further analysis A) Immunohistochemical analysis for MDA-7/IL-24 expression. B) Immunohistochemical analysis for CD31 expression. C) Monitoring of H&E stained sections for cellular density.
Suboptimal doses of Ad.5/3-mda-7 and BI-97C1 (Sabutoclax) promote synergistic anti-tumor effect in vitro and in vivo in RKO colorectal cancer cells
We determined if suboptimal doses of the combination regimen of Ad.5/3-mda-7 and BI-97C1 (Sabutoclax) are capable of inhibiting growth of RKO cells. We first evaluated the dose and time kinetics of BI-97C1 (Sabutoclax) alone on RKO cells viability in vitro. A significant inhibition of cell viability was observed with 3 μM BI-97C1 on day 3 and day 6 (Fig. 6A). A combination of suboptimal doses of BI-97C1 (2μM) and Ad.5/3-mda-7 (50 pfu/cell) resulted in significant inhibition of viability of RKO cells compared to either agent alone (Fig. 6B). The combinatorial treatment resulted in downregulation of prosurvival proteins Bcl-2 and Mcl-1 and induction of apoptosis as revealed by PARP cleavage (Fig. 6C).
Figure 6. Suboptimal doses of tropism-modified adenoviruses (Ad.5/3) and BI-97C1 (Sabutoclax) synergize resulting in decreased viability of RKO low CAR colorectal cancer cells.
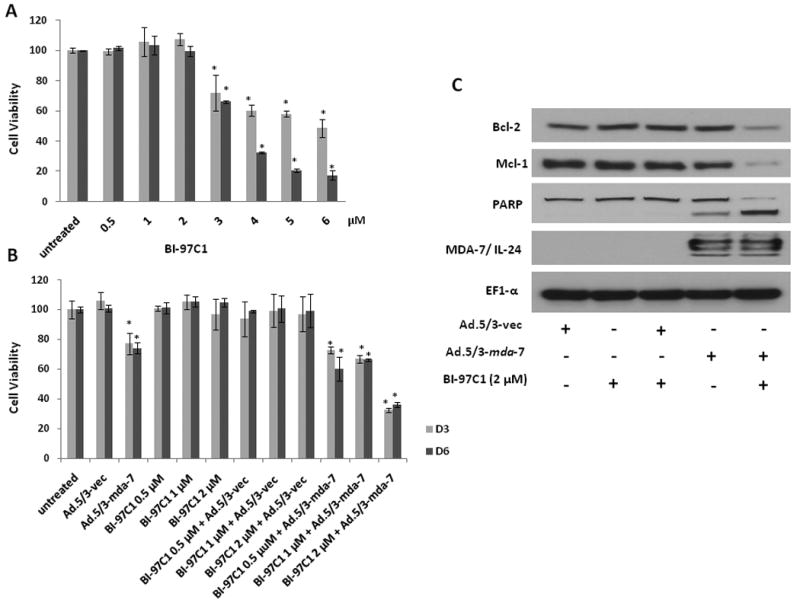
A) RKO cells were treated with the indicated concentrations of BI-97C1 (Sabutoclax). After 3 and 6 days, cell viability was evaluated by MTT assays. Bars, ± S.D. (n = 3). B) RKO cells were infected with 50 pfu/cell of Ad.5/3-vec or Ad.5/3-mda-7 for 24 hr after which cells were treated with indicated concentrations BI-79C1 (Sabutoclax) and MTT assay was performed at 3 and 6 days. C) Analysis of expression of the indicated proteins by Western blotting.
To evaluate the effect of combinatorial treatment in vivo, nude mice bearing subcutaneous RKO xenografts received nine intratumoral injections of a sub-optimal dose of 1 × 108 pfu per injection of Ad.5/3-vec or Ad.5/3-mda-7 and 12 intraperitoneal injections of 3 mg/kg of BI-97C1 (Sabutoclax) over a 4-week period (33-34). The treatment was started when tumor size was ∼100 mm3. Mice were divided into four groups; Ad.5/3-vec; Ad.5/3-mda-7; Ad.5/3-vec plus BI-97C1 (Sabutoclax); and Ad.5/3-mda-7 plus BI-97C1 (Sabutoclax). Tumor growth and weight were markedly inhibited by the combination treatment of Ad.5/3-mda-7 and BI-97C1 (Sabutoclax) compared to the other groups at the end of the treatment period (Fig. 7). These findings showed that BI-97C1 (Sabutoclax) is efficient in sensitizing low CAR colorectal cancer cells to Ad.5/3-mda-7 treatment. BI-97C1 (Sabutoclax) was non-toxic to athymic nude mice (NCRnu/nu) when injected intraperitonially (up to 10 mg/kg) (34).
Figure 7. The combination of Ad.5/3-mda-7 and BI-97C1 (Sabutoclax) additively inhibit the growth of low-CAR colorectal cancer xenografts in vivo.
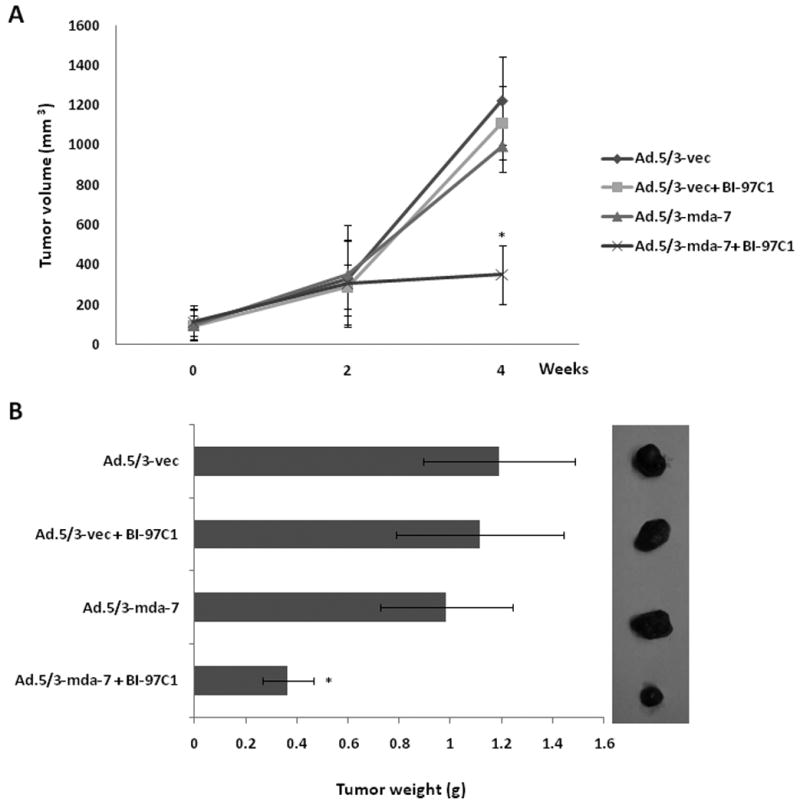
Subcutaneous tumor xenografts from RKO cells were established in athymic nude mice; the tumors were injected with the indicated Ads with and without BI-97C1 (Sabutoclax) over a 4-week period (total of nine injections each). A) Measurements of RKO xenograft tumor volumes; points, average (five mice in each group); bars, ± S.D. B) Images of the tumors at the end of the study. C) Measurement of tumor weight at the end of the study; columns, mean; bars, ± S.D.
Discussion
HCT116 cells have high CAR and therefore are efficiently infected with Ad.5. On the other hand RKO cells have low CAR, which makes it relatively resistant to Ad.5 infection. Our analysis reveals a variable level of CAR expression in different colorectal cancer cell lines (Fig. 1A) indicating that for Ad delivery to be effective a strategy facilitating efficient infection of all types of colorectal cancer cells needs to be utilized. To overcome the low infectivity limitation of Ad.5, multiple groups are trying to solve the relative inefficiency of Ad.5 infection by modifying specific sequences within the virus capsid proteins that directly associate with CAR; i.e., targeting strategies are being developed to enhance viral infectivity via CAR-independent pathways. Several laboratories have modified the infective viral capsid “knob” to bind to surface integrin proteins enhancing transformation (RGD modification) (6, 11-14). Additionally, insertion into the knob of multiple lysine residues (pK7) increases viral interaction with cells by electrostatic effects; and including portions of type 3 Ad in the viral capsid knob has also enhanced infectivity via CAR-independent pathways (6, 11-14). In the present study, the potent tumor suppressor gene mda-7/IL-24 driven by a CMV promoter has been delivered by a CAR-independent Ad containing a chimeric fiber with the knob domain of human Ad.3 in the Ad.5 capsid (Ad.5/3), which showed more efficient transduction of prostate, renal cancer and malignant glioma cells having low CAR compared with wild-type Ad.5 (36, 40-42). This concept is supported by our observation that Ad.5/3-Luc showed superior luciferase transgene expression when compared to Ad.5-Luc in low CAR colorectal cancer cells. Similarly, Ad.5/3-Luc also showed enhanced infectivity in ovarian cancer cells OV-4 and SKOV3 which display moderate or low levels of CAR (43), as well as in renal cancer cells (44), melanoma cells (45) and prostate cancer (36). We presently extend these observations by demonstrating that Ad.5/3 can effectively deliver the potent anti-cancer therapeutic, mda-7/IL-24, in low CAR colorectal cancer cells; and it has similar transduction efficiency as the wild-type Ad.5 in high CAR colorectal cancer cells. More importantly the 5/3 modification did not interfere with the downstream killing pathway of mda-7/IL-24 as evidenced by induction of ER stress response markers such as BiP/GRP78 and GRP94; downregulation of pro-apoptotic Bcl-2 family members such as Bcl-2 and induction of apoptosis upon Ad.5/3-mda-7 infection of RKO cells. Therefore, Ad.5/3 might be an effective tool for therapeutic delivery of mda-7/IL-24 into genetically diverse colorectal tumors in vivo.
mda-7/IL-24 induces apoptosis by downregulating the proapoptotic Bcl-2 family proteins (33, 37) and ectopic expression of anti-apoptotic Bcl-2 family proteins, including Mcl-1, interferes with the anti-tumor activity of mda-7/IL-24. Apogossypol derivatives, such as BI-97C1 (Sabutoclax), function as broad-spectrum Bcl-2 antagonist by binding to the BH3 domain and inhibiting activity (33). BI-97C1 (Sabutoclax) that also targets Mcl-1 profoundly sensitizes prostate cancer cells to mda-7/IL-24-mediated cell toxicity (34). Similarly, a combination of suboptimal doses of BI-97C1 (Sabutoclax) and Ad.5/3-mda-7 profoundly inhibited cell viability and promoted cell death in colorectal cancer cells and also inhibited Colo tumor xenografts in vivo. In our studies, BI-97C1 (Sabutoclax) was found to be non-toxic even at high doses and although clinical trials with BI-97C1 (Sabutoclax) remain to be initiated it is assumed that a suboptimal dose of BI-97C1 might not be toxic and will be well tolerated in human subjects as well. Based on the safety profile of the individual agents, and the fact that mda-7/IL-24 is now in the clinic and has been shown to be safe and efficacious, this novel combinatorial approach may be translatable into the clinic for the treatment of advanced colorectal cancers in the near future.
Acknowledgments
The present study was supported in part by NIH grants R01 CA097318, R01 CA127641 and P01 CA104177, to P.B. Fisher; NIH grant R01 CA138540-01, and grants from the Dana Foundation and James S. McDonnell Foundation to D. Sarkar. D. Sarkar and X.-Y. Wang are Harrison Scholars in Cancer Research, D. Sarkar is a Blick scholar, and P.B. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research in the Massey Cancer Center.
References
- 1.Fong Y, Kemeny N, Paty P, Blumgart LH, Cohen AM. Treatment of colorectal cancer: hepatic metastasis. Semin Surg Oncol. 1996;12(4):219–252. doi: 10.1002/(SICI)1098-2388(199607/08)12:4<219::AID-SSU3>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Lynch HT, de la Chapelle A. Hereditary colorectal cancer. N Engl J Med. 2003;348(10):919–932. doi: 10.1056/NEJMra012242. in eng. [DOI] [PubMed] [Google Scholar]
- 3.Chu RL, Post DE, Khuri FR, Van Meir EG. Use of replicating oncolytic adenoviruses in combination therapy for cancer. Clinical Cancer Res. 2004;10(16):5299–5312. doi: 10.1158/1078-0432.CCR-0349-03. [DOI] [PubMed] [Google Scholar]
- 4.Reid T, et al. Hepatic arterial infusion of a replication-selective oncolytic adenovirus (dl1520): phase II viral, immunologic, and clinical endpoints. Cancer Res. 2002;62(21):6070–6079. [PubMed] [Google Scholar]
- 5.Korn WM, et al. Expression of the coxsackievirus- and adenovirus receptor in gastrointestinal cancer correlates with tumor differentiation. Cancer Gene Ther. 2006;13(8):792–797. doi: 10.1038/sj.cgt.7700947. [DOI] [PubMed] [Google Scholar]
- 6.Glasgow JN, Bauerschmitz GJ, Curiel DT, Hemminki A. Transductional and transcriptional targeting of adenovirus for clinical applications. Curr Gene Ther. 2004;4(1):1–14. doi: 10.2174/1566523044577997. [DOI] [PubMed] [Google Scholar]
- 7.Pandha HS, et al. Coxsackie B and adenovirus receptor, integrin and major histocompatibility complex class I expression in human prostate cancer cell lines: implications for gene therapy strategies. Prostate Cancer and Prostatic Diseases. 2003;6(1):6–11. doi: 10.1038/sj.pcan.4500611. [DOI] [PubMed] [Google Scholar]
- 8.Sachs MD, et al. Integrin alpha(v) and coxsackie adenovirus receptor expression in clinical bladder cancer. Urology. 2002;60(3):531–536. doi: 10.1016/s0090-4295(02)01748-x. [DOI] [PubMed] [Google Scholar]
- 9.Rauen KA, et al. Expression of the coxsackie adenovirus receptor in normal prostate and in primary and metastatic prostate carcinoma: potential relevance to gene therapy. Cancer Res. 2002;62(13):3812–3818. [PubMed] [Google Scholar]
- 10.Anders M, et al. Disruption of 3D tissue integrity facilitates adenovirus infection by deregulating the coxsackievirus and adenovirus receptor. Proc Natl Acad Sci U S A. 2003;100(4):1943–1948. doi: 10.1073/pnas.0337599100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paul CP, et al. Characterization of infectivity of knob-modified adenoviral vectors in glioma. Cancer Biol Ther. 2008;7(5):786–793. doi: 10.4161/cbt.7.5.5421. [DOI] [PubMed] [Google Scholar]
- 12.Tsuruta Y, et al. A mosaic fiber adenovirus serotype 5 vector containing reovirus sigma 1 and adenovirus serotype 3 knob fibers increases transduction in an ovarian cancer ex vivo system via a coxsackie and adenovirus receptor-independent pathway. Clin Cancer Res. 2007;13(9):2777–2783. doi: 10.1158/1078-0432.CCR-06-2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8(8):573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mathis JM, Stewart PL, Zhu ZB, Curiel DT. Advanced generation adenoviral virotherapy agents embody enhanced potency based upon CAR-independent tropism. Clinical Cancer Res. 2006;12(9):2651–2656. doi: 10.1158/1078-0432.CCR-06-0497. Translated from eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11(12):2477–2486. [PubMed] [Google Scholar]
- 16.Jiang H, et al. The melanoma differentiation associated gene mda-7 suppresses cancer cell growth. Proc Natl Acad Sci U S A. 1996;93(17):9160–9165. doi: 10.1073/pnas.93.17.9160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang EY, et al. Genomic structure, chromosomal localization and expression profile of a novel melanoma differentiation associated (mda-7) gene with cancer specific growth suppressing and apoptosis inducing properties. Oncogene. 2001;20(48):7051–7063. doi: 10.1038/sj.onc.1204897. [DOI] [PubMed] [Google Scholar]
- 18.Parrish-Novak J, et al. Interleukins 19, 20, and 24 signal through two distinct receptor complexes. Differences in receptor-ligand interactions mediate unique biological functions. J Biol Chem. 2002;277(49):47517–47523. doi: 10.1074/jbc.M205114200. [DOI] [PubMed] [Google Scholar]
- 19.Caudell EG, et al. The protein product of the tumor suppressor gene, melanoma differentiation-associated gene 7, exhibits immunostimulatory activity and is designated IL-24. J Immunol. 2002;168(12):6041–6046. doi: 10.4049/jimmunol.168.12.6041. [DOI] [PubMed] [Google Scholar]
- 20.Fickenscher H, et al. The interleukin-10 family of cytokines. Trends Immunol. 2002;23(2):89–96. doi: 10.1016/s1471-4906(01)02149-4. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P, et al. mda-7/IL-24: multifunctional cancer-specific apoptosis-inducing cytokine. Pharmacol Ther. 2006;111(3):596–628. doi: 10.1016/j.pharmthera.2005.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65(22):10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- 23.Emdad L, et al. Historical perspective and recent insights into our understanding of the molecular and biochemical basis of the antitumor properties of mda-7/IL-24. Cancer Biol Ther. 2009;8(5):391–400. doi: 10.4161/cbt.8.5.7581. [DOI] [PubMed] [Google Scholar]
- 24.Su Z, et al. A combinatorial approach for selectively inducing programmed cell death in human pancreatic cancer cells. Proc Natl Acad Sci U S A. 2001;98(18):10332–10337. doi: 10.1073/pnas.171315198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Su ZZ, et al. The cancer growth suppressor gene mda-7 selectively induces apoptosis in human breast cancer cells and inhibits tumor growth in nude mice. Proc Natl Acad Sci U S A. 1998;95(24):14400–14405. doi: 10.1073/pnas.95.24.14400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cunningham CC, et al. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11(1):149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 27.Tong AW, et al. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanoma-differentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11(1):160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- 28.Ramesh R, et al. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63(16):5105–5113. [PubMed] [Google Scholar]
- 29.Su Z, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24(51):7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 30.Dash R, et al. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70(12):5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yacoub A, et al. Cisplatin enhances protein kinase R-like endoplasmic reticulum kinase- and CD95-dependent melanoma differentiation-associated gene-7/interleukin-24-induced killing in ovarian carcinoma cells. Mol Pharmacol. 2010;77(2):298–310. doi: 10.1124/mol.109.061820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamed HA, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18(6):1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Wei J, et al. BI-97C1, an optically pure Apogossypol derivative as pan-active inhibitor of antiapoptotic B-cell lymphoma/leukemia-2 (Bcl-2) family proteins. J Med Chem. 2010;53(10):4166–4176. doi: 10.1021/jm1001265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dash R, et al. Apogossypol derivative BI-97C1 (Sabutoclax) targeting Mcl-1 sensitizes prostate cancer cells to mda-7/IL-24-mediated toxicity. Proc Natl Acad Sci U S A. 2011;108(21):8785–8790. doi: 10.1073/pnas.1100769108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lebedeva IV, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda-7/IL-24. Oncogene. 2003;22(54):8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 36.Dash R, et al. Enhanced delivery of mda-7/IL-24 using a serotype chimeric adenovirus (Ad.5/3) improves therapeutic efficacy in low CAR prostate cancer cells. Cancer Gene Ther. 2010;17(7):447–456. doi: 10.1038/cgt.2009.91. [DOI] [PubMed] [Google Scholar]
- 37.Lebedeva IV, et al. The cancer growth suppressing gene mda-7 induces apoptosis selectively in human melanoma cells. Oncogene. 2002;21(5):708–718. doi: 10.1038/sj.onc.1205116. [DOI] [PubMed] [Google Scholar]
- 38.Sarkar D, et al. Dual cancer-specific targeting strategy cures primary and distant breast carcinomas in nude mice. Proc Natl Acad Sci U S A. 2005;102(39):14034–14039. doi: 10.1073/pnas.0506837102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarkar D, et al. Targeted virus replication plus immunotherapy eradicates primary and distant pancreatic tumors in nude mice. Cancer Res. 2005;65(19):9056–9063. doi: 10.1158/0008-5472.CAN-05-1261. [DOI] [PubMed] [Google Scholar]
- 40.Ulasov IV, Tyler MA, Zheng S, Han Y, Lesniak MS. CD46 represents a target for adenoviral gene therapy of malignant glioma. Human Gene Ther. 2006;17(5):556–564. doi: 10.1089/hum.2006.17.556. [DOI] [PubMed] [Google Scholar]
- 41.Eulitt PJ, et al. Enhancing mda-7/IL-24 therapy in renal carcinoma cells by inhibiting multiple protective signaling pathways using sorafenib and by Ad.5/3 gene delivery. Cancer Biol & Ther. 2011;10(12):1290–1305. doi: 10.4161/cbt.10.12.13497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hamed HA, et al. Inhibition of multiple protective signaling pathways and Ad.5/3 delivery enhances mda-7/IL-24 therapy of malignant glioma. Mol Ther. 2010;18(6):1130–1142. doi: 10.1038/mt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 43.Kanerva A, et al. Targeting adenovirus to the serotype 3 receptor increases gene transfer efficiency to ovarian cancer cells. Clinical Cancer Res. 2002;8(1):275–280. [PubMed] [Google Scholar]
- 44.Haviv YS, et al. Adenoviral gene therapy for renal cancer requires retargeting to alternative cellular receptors. Cancer Res. 2002;62(15):4273–4281. [PubMed] [Google Scholar]
- 45.Volk AL, et al. Enhanced adenovirus infection of melanoma cells by fiber-modification: incorporation of RGD peptide or Ad5/3 chimerism. Cancer Biol & Ther. 2003;2(5):511–515. doi: 10.4161/cbt.2.5.440. [DOI] [PubMed] [Google Scholar]


