Abstract
Melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24), a unique member of the IL-10 gene family, displays a broad range of antitumor properties including cancer-specific induction of apoptosis, inhibition of tumor angiogenesis, and modulation of anti-tumor immune responses. Here we identify clusterin (CLU) as a MDA-7/IL-24 interacting protein in DU-145 cells and investigate the role of MDA-7/IL-24 in regulating CLU expression and mediating the antitumor properties of mda-7/IL-24 in prostate cancer. Ad.mda-7 decreased expression of soluble CLU (sCLU) and increased expression of nuclear CLU (nCLU). In the initial phase of Ad.mda-7 infection sCLU expression increased and CLU interacted with MDA-7/IL-24 producing a cytoprotective effect. Infection of stable clones of DU-145 prostate cancer cells expressing sCLU with Ad.mda-7 resulted in generation of nCLU that correlated with decreased cell viability and increased apoptosis. In the presence of mda-7/IL-24, sCLU-DU-145 cells displayed G2/M phase arrest followed by apoptosis. Similarly, Ad.mda-7 infection decreased cell migration by altering cytoskeleton in sCLU-DU-145 cells. Ad.mda-7-treated sCLU-DU-145 cells displayed a significant reduction in tumor growth in mouse xenograft models and reduced angiogenesis when compared to the vector control group. Tumor tissue lysates demonstrated enhanced nCLU generated from sCLU with increased apoptosis in the presence of MDA-7/IL-24. Our findings reveal novel aspects relative to the role of sCLU/nCLU in regulating the anticancer properties of MDA-7/IL-24 that may be exploited for developing enhanced therapies for prostate cancer.
Keywords: MDA-7/IL-24, soluble clusterin, nuclear clusterin, G2/M arrest, apoptosis
Introduction
CLU is a gene/protein highly conserved and expressed in several human tissues and fluids. Also known as testosterone repressed prostate message 2, apolipoprotein J, or sulfated glycoprotein 2, CLU regulates many biological functions including prostate carcinogenesis and tumor progression (Rosenberg et al., 1995; Jones et al., 2002). There are two known CLU protein isoforms generated in human cells. A nuclear form of CLU protein (nCLU) which is pro-apoptotic, and a secretory (soluble) form (sCLU) that is pro-survival (Scaltriti et al., 2004; Trougakos et al., 2005; Shannan et al., 2006; Moretti et al., 2007). CLU protein translation produces a protein precursor that is targeted by an initial signal peptide to the endoplasmic reticulum, where it is cleaved into two distinct peptides (α and β) held together by five disulfide bonds. The translation product is then glycosylated, and the mature heterodimeric glycoprotein, named sCLU, is secreted (Bettuzzi et al., 1989; Purrello et al., 1991; Jones et al., 2002). An alternative isoform of CLU has recently been identified and named nCLU, which is synthesized from a second in-frame AUG codon located in position 152. AUG152 becomes functional by (a) alternative splicing of CLU mRNA or (b) alternative initiation of translation. As a consequence, nCLU lacking the leader peptide does not enter the endoplasmic reticulum, thus skipping α/β cleavage and glycosylation, mainly localizing in the nucleus (Jones et al., 2002; Leskov et al., 2003; Trougakos et al., 2005; Yang et al., 2009).
Prostate cancer is the second leading cause of male cancer deaths in Western countries (Nelen, 2007). The disease is initially androgen-dependent, and androgen-deprivation therapy represents the first-line treatment for prostate cancer patients (Labrie et al., 2005). After an initial remission, prostate carcinoma progresses toward hormone-resistance, characterized by high proliferation rate, strong metastatic behavior, and refractoriness to classic chemotherapy (Lee and Tenniswood, 2004). CLU expression levels have been found to be either down-regulated or up-regulated in prostate cancer specimens (Bettuzzi et al., 2000; July et al., 2002; Caporali et al., 2004; Scaltriti et al., 2004). Antisense oligonucleotides and small interfering RNAs (siRNA) targeting the full-length CLU have been reported to induce apoptosis and chemosensitivity in vitro as well as in vivo in prostate cancer xenografts and in prostate cancer patients (Gleave et al., 2001; Trougakos et al., 2004; Gleave and Miyake, 2005; Springate et al., 2005). On the other hand, nCLU accumulation has been observed in dying prostate cells challenged with pro-apoptotic stimuli (Leskov et al., 2003). Overexpression of nCLU has been reported to induce G2/M phase arrest and caspase-dependent apoptosis in prostate cancer cells (Scaltriti et al., 2004). Similarly, previous studies showed that transfection with “full-length” CLU cDNA produced both sCLU and nCLU in nonmalignant PNT1A cells, whereas only sCLU was found in cancer cells (Moretti et al., 2007). This escape mechanism limiting production of the pro-apoptotic nCLU from the full-length CLU gene might play a crucial role in prostate tumorigenesis.
Using subtraction hybridization combined with induction of cancer cell terminal differentiation, our laboratory cloned melanoma differentiation-associated gene-7/interleukin-24 (mda-7/IL-24), a unique member of the IL-10–related cytokine gene family (Jiang et al., 1995; Pestka et al., 2004; Fisher et al., 2003; Dash et al., 2010). mda-7/IL-24 expression decreases during the process of melanoma progression (Jiang et al., 1995). Subsequent experiments documented that mda-7/IL-24 has nearly ubiquitous antitumor properties in vitro and in vivo, which resulted in successful entry into the clinic where safety and clinical efficacy when administered by adenovirus (Ad.mda-7; INGN 241) has been shown in a phase I clinical trial in humans with advanced cancers (Fisher et al., 2003; Fisher, 2005; Cunningham et al., 2005; Tong et al., Lebedeva et al., 2007; Sarkar et al., 2007). Forced mda-7/IL-24 expression in cancer cells in vitro and in vivo inhibits angiogenesis (Ramesh et al., 2003); stimulates an antitumor immune response (Miyahara et al., 2006; Gao et al., 2008); sensitizes cancer cells to radiation-, chemotherapy-, and antibody-induced killing; and elicits potent antitumor “bystander” antitumor activity (Su et al., 2005; Su et al., 2006; Yacoub et al., 2008; Sauane et al., 2008;). Following ectopic expression of mda-7/IL-24 by an adenovirus the MDA-7/IL-24 protein interacts with the endoplasmic reticulum (ER) chaperone protein BiP/GRP78 and initiates a cascade of “unfolded protein response” (UPR) events in tumor cells that culminates in apoptosis (Gupta et al., 2006). Additionally, overexpression of mda-7/IL-24 induces autophagic death in glioma as well as an initial cytoprotective autophagy in specific target cells, which switches to apoptosis in prostate cancer (Park et al., 2008; Bhutia et al., 2010; Bhutia et al., 2011). Recently, we reported that mda-7/IL-24 induces cancer cell-selective antitumor activity through ER stress by inhibiting the translation of the anti-apoptotic protein Mcl-1 in prostate cancer (Dash et al., 2010).
To enhance our understanding of how MDA-7/IL-24 induces cancer-specific killing we screened for MDA-7/IL-24 interacting proteins in DU-145 cells and through this analysis CLU was identified as an interacting molecule. Based on these findings, we investigated the role of mda-7/IL-24 in regulating CLU expression and the impact of CLU expression on mda-7/IL-24 antitumor activity in prostate cancer. Ad.mda-7 decreased expression of sCLU and increased nCLU in prostate cancer cells, which promoted G2/M phase arrest followed by apoptosis. Additionally, we demonstrated that expression of Ad.mda-7 enhances antitumor potential in prostate cells with high expression of sCLU.
Materials and Methods
Cell Lines, culture conditions, viability and colony forming assays
Human prostate carcinoma cells DU-145 and PC-3 were obtained from the American Type Culture Collection and cultured in DMEM supplemented with 10% fetal bovine serum, 1% sodium pyruvate, and nonessential amino acids. P69, an SV40-immortalized human prostate epithelial cell line was kindly provided by Dr. Joy Ware (Virginia Commonwealth University, Richmond, VA) and grown under serum-free conditions. Cells were infected with 100 plaque forming units (pfu)/cell of Ad.mda-7 or Ad.vec and analyzed as described (Bhutia et al., 2010). Cell viability was determined by standard MTT (3-(4, 5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assays. For colony formation assays, cells were seeded in 6-cm plates (2000 cells/plate) after treatment with Ad.mda-7 and colonies of >50 cells were scored after 2 weeks.
Expression Vectors and generation of stable clones
Full-length human CLU cDNA was generated as described (Moretti et al., 2007). Briefly, reverse transcription-PCR of normal human fibroblast total RNA was done using the primers 5′-GACTCCAGAATTGGAGGCATG-3′ (forward) and 5′-ATCTCACTCCTCCCGGTGCT-3′ (reverse). The cDNA was cloned into pGEM-T Easy (Promega). CLU full-length cDNA was then subcloned into the bicistronic expression vector pIRES-hyg (Clontech) to produce the pIRES-sCLU vector. A truncated CLU cDNA fragment was then amplified from the full-length expression vector using the primers 5′-GTCTCAGACAATGGGATCCAGGA-3′ (forward) and 5′-GACCTGCAGGCGGCCGCGAAT-3′ (reverse). Truncated cDNA was inserted in pIRES-hyg to generate the pIRES-nCLU vector. DU-145 ectopically expressing sCLU clones were generated as described previously (Dash et al., 2010).
Co-immunoprecipitation and mass spectrometry
Ad-mda-7-His expressing virus was used to infect DU-145 cells and cell lysates were immunoprecipitated with anti-His-tag antibody (Cell Signaling Tech.). The eluted lysates were run on SDS-PAGE and stained with Coomassie Blue. The stained bands were collected and sent to Taplin Mass Spectrometry Facility at Harvard Medical School (Boston, MA) for analysis using mass spectrometry.
Western blotting analysis and immunoprecipitation
Cells were cultured in 60-mm plates and protein extracts were prepared with radio-immunoprecipitation assay buffer containing a cocktail of protease inhibitors. Fifty micrograms of protein were resolved in SDS/PAGE, transferred to nitrocellulose membranes. The membranes were probed with anti-MDA-7/IL-24 (1:1,000, mouse monoclonal, GenHunter Corp., Nashville, TN), anti-clusterin (1:1000, mouse monoclonal, Milipore), anti-cyclin B1 (1:100, mouse monoclonal, BD Pharmogen, USA), anti-cdk1 (1:1000, mouse monoclonal, BD Pharmogen, USA). The Rabbit anti-PARP was obtained from Cell Signaling Technology. Actin (Sigma Aldrich, St. Louis, MO, USA) and Lamin B (Santa Cruz Biotechnology, CA) was used as loading control for total and nuclear protein, respectively. For coimmunoprecipitations, equivalent amounts of cell lysates were incubated with 20 μL of 50% protein A agarose at 4°C for 1 h followed by anti-MDA-7/IL-24 or anti-Clusterin monoclonal antibodies overnight at 4°C. Immunocomplexes were precipitated with 20 μL of 50% protein A agarose for 2 h. Immunoprecipitates were extensively washed, and the eluted precipitates were resolved by SDS/7% PAGE, transferred, and probed with the appropriate antibodies (Bhutia et al., 2010).
RT-PCR of CLU
Based on probable splice sites, the following primers were used to investigate whether alternative splice forms of CLU could be found: hCLU5′, 5′-ACAGGGTGCCGCTGACC-3′ and hCLUN term-rev, 5′-TTAGAGCTCCTTCAGCTTTGTC TCTG-3′. Total RNA from sCLU-DU-145 cells was extracted using RNAsol B (Invitrogen). Reverse transcription reactions were performed using the oligo(dT) primer (Promega) and Superscript II enzyme system (Invitrogen). The PCR step was performed using Taq DNA polymerase with PCR conditions were as follows: 95 °C for 30 s, 62 °C for 45 s, and 72 °C for 45 s. RT-PCR products were resolved using 2% agarose gel electrophoresis (Leskov et al., 2003).
Caspase assays
Caspase activity was measured using Caspase-Glo 3/7 assay following the manufacturer’s protocol (Promega Corp., Madison, WI) (Bhutia et al., 2010).
Analysis of CLU/MDA-7/IL-24 colocalization by immunofluoresdcence
DU-145 Cells were seeded onto chamber slides (Falcon; BD Biosciences, San Jose, CA) and maintained in DMEM with 10% fetal bovine calf serum, 24 h post-infection, cells were fixed with 2% paraformaldehyde, permeabilized by 0.1% Triton X-100, and then incubated with primary antibodies: mouse anti-MDA-7/IL-24 (1:100, mouse monoclonal, GenHunter Corp., Nashville, TN), Rabbit anti-clusterin (1:50, Goat polyclonal, Santa Cruz Biotechnology Inc. CA). Controls were incubated with only the secondary antibodies under the same experimental conditions. FITC-conjugated donkey anti-mouse IgG or anti-rabbit IgG (Molecular Probes) were used for visualization on a confocal microscope (Bhutia et al., 2010).
Detection of CLU by immunofluorescence
Fixed cells were incubated with the unlabeled monoclonal anti-clusterin primary antibody (1:100) followed by FITC-conjugated goat anti-mouse secondary antibody (Alexa Fluor 488) (Moretti et al., 2007).
Detection of actin by immunofluorescence
Cells were fixed with 3.7% formaldehyde and stained with FITC-phalloidin (1:2,000) (Sigma) and observed in a confocal microscope as described (Bhutia et al., 2010).
In vitro cell invasion assays
Cell invasion was determined in a modified Boyden chamber (BD Bioscience, Bedford, MA, USA) according to the manufacture’s instructions.
Human prostate cancer xenografts in athymic nude mice
sCLU-DU-145 and Vec Con-DU-145 cells (3 × 106) were injected s.c. in 100 μL of PBS in the left flank of male athymic nude mice. After establishment of visible tumors of ~100 mm3, requiring approximately 8–10 days, intratumoral injections of different Ads were administered to the tumor site at a dose of 4.5 × 108 p.f.u. (plaque forming units) in 100 μl. The injections were administered 3X in the first week and then 2X a week for 2 more weeks for a total of seven injections. A minimum of five animals was used per experimental condition. Tumor volume was measured twice weekly using a caliper and calculated using the formula: π/6 × larger diameter × (smaller diameter) 2. At the end of the experiment, animals were sacrificed, and the tumors were removed and weighed (Dash et al., 2010).
Immunohistochemical staining
For immunohistochemical analysis, formalin-fixed and paraffin-embedded specimens of 3–4-mm thickness were sectioned. Sections were deparaffinized, re-hydrated and then quenched in 3% H2O2 for 20 min. Sections were washed with PBS and blocked in PBS containing 1% bovine serum albumin for 20 min at 37 °C. Monoclonal anti-MDA-7/IL-24 (1:200), monoclonal anti-CD31 (1:200) (Dako Corporation, Carpenteria, CA) and Ki67 (1:50) (BD Pharmingen, San Diego, CA), were incubated for 3 h at room temperature and then washed 3X in PBS. Sections were incubated with an avidin–biotin–peroxidase complex (Vectastain Elite ABC Kit, Vector Laboratories, Burlingame, CA) and then washed 2X in PBS. Immunoreactivity was determined using diaminobenzidine as the final chromogen. Finally, sections were counterstained with Meyer’s hematoxylin, dehydrated through a sequence of increasing concentrations of alcohol, cleared in xylene and mounted with epoxydic medium. During the immunohistochemical assay, proof slides were coupled with negative control slides on which the primary antibody was omitted. Sections were also processed for hematoxylin and eosin staining (Dash et al., 2010).
Preparation of tumor tissue extracts
Whole-cell extracts of tumor samples were obtained by tissue homogenizations. Cells were lysed in ice-cold 1% Triton lysis solution (50 mM HEPES (pH 7.4), 150 mM NaCl, 10% glucose, 1% Triton X-100, 0.1% SDS, 15 mM MgCl2, 1 mM EDTA, 1 mM PMSF, 10 mg/ml leupeptin) for 30 min on ice. Insoluble matter was removed by centrifugation, and the protein concentration was measured by Bradford (Bio-Rad, Richmond, CA, USA) assays (So et al., 2005).
Statistical analysis
Data are presented as the mean ± standard error of mean (SEM) and analyzed for statistical significance using one-way analysis of variance (ANOVA) followed by Newman-Keuls test as a post-hoc test. A P value of <0.05 was considered significant.
Results
Effect of Ad.mda-7 on sCLU and nCLU in prostate cancer cells
To identify potential MDA-7/IL-24 interacting proteins, we purified MDA-7/IL-24 protein from human DU-145 cells overexpressing this protein tagged with His. From this purification, we identified several potential MDA-7/IL-24 interacting molecules, and mass spectrometry analysis revealed that one of them is CLU (data not shown). Immunoprecipitation assays confirmed that CLU interacts with MDA-7/IL-24 at 24 h post Ad.mda-7 infection (Fig. 1A; left panel; Supplementary Fig. S1). The interaction between MDA-7/IL-24 and CLU was further confirmed by double immunofluorescence analysis demonstrating intense yellow staining when these two proteins co-localized (Fig. 1A; right panel). However, we were unable to detect this interaction at 48 h and, intriguingly, found a decrease in CLU expression at this later time point (Fig. 1B). We further examined the effect of Ad.mda-7 on CLU expression in DU-145 and PC-3 cells. Western blotting indicated that 48 h post-infection of Ad.mda-7 the expression of sCLU was decreased in a dose-dependent manner in both prostate cancer cell lines (Fig. 1B and Supplementary Fig. S2A). Temporal studies indicated that Ad.mda-7 infection resulted in increased CLU expression at 24 h and then decreased expression at 48 h when apoptotic cell death was initiated (Fig. 1C) (Bhutia et al., 2010). The increase in expression of CLU at 24 h might provide a cytoprotective effect. To clarify the potential cytoprotective role of CLU on Ad.mda-7-induced apoptosis, we knocked down CLU expression with siRNA in DU-145 cells (Supplementary Fig. S2B) and examined Ad.mda-7 sensitivity by MTT assays. CLU-deficient D U-145 cells exhibited increased sensitivity toward Ad.mda-7 when administered at 100 pfu/cell (Fig. 1D), confirming the protective nature of sCLU. These results indicate that MDA-7/IL-24 directly interacts with CLU downregulating its expression, suggesting that the decrease in expression of CLU by Ad.mda-7 creates an unfavorable environment for the interaction of these two molecules and concomitant loss of the cytoprotective effect culminating in apoptosis.
Fig. 1. MDA-7/IL-24 regulates sCLU expression.
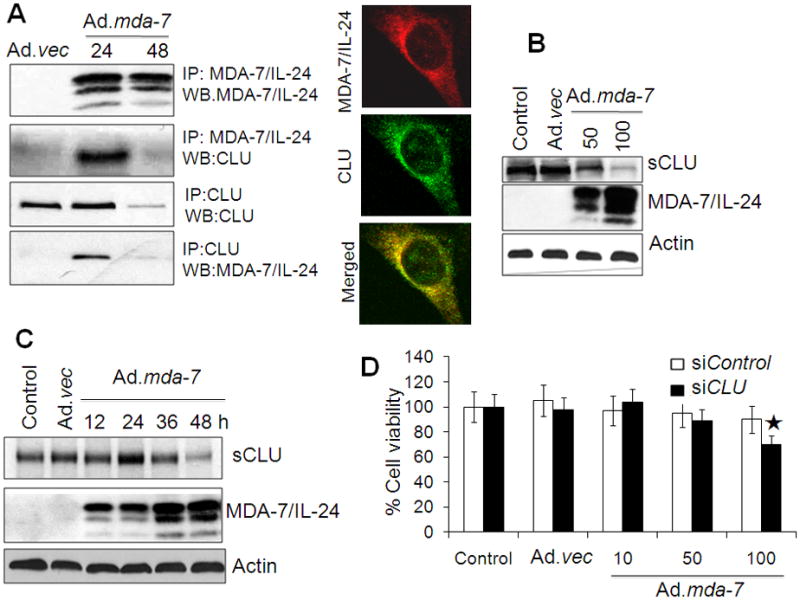
A, DU-145 cells were infected with 100 pfu/cell of Ad.vec or Ad.mda-7 for different times and immunoprecipitated with anti-MDA-7/IL-24 or anti-clusterin antibodies followed by immunoblotting with anti-clusterin or anti-MDA-7/IL-24 antibodies (left panel). Fluorescent confocal micrographs of DU-145 showing co-immunolocalization of CLU and MDA-7/IL-24 (right panel). B, DU-145 cells were infected with Ad.mda-7 or Ad.vec and after 48 h CLU expression was analyzed by Western blotting. C, DU-145 cells were infected with 100 pfu/cell of Ad.vec or Ad.mda-7 and after different time periods CLU expression was analyzed by Western blotting. D, DU-145 cells were transfected with CLU siRNAs and cell viability was determined by MTT assay 24 h after Ad.mda-7 infection.
As previously reported, transfection of a full-length CLU cDNA produces sCLU (70kDa) and nCLU (49kDa) in nonmalignant PNT1A (Moretti et al., 2007) as well as in P69 immortal normal human prostate epithelial cells (Supplementary Fig. S2C). We were unable to detect nCLU expression in DU-145 or PC-3 prostate cancer cells. Stable clones of DU-145 cells were generated using CLU cDNA and these clones produced only sCLU (sCLU-DU-145) (Supplementary Fig. S2D). These clones were infected with Ad.mda-7 for 48 h and the expression profile of CLU was analyzed by Western blotting and RT-PCR with subsequent experiments performed with clone 1. As compared with control or Ad.vec, infection with Ad.mda-7 resulted in a decrease in sCLU expression in parental DU-145 cells and an extra band of nCLU of ~49-kDa (Fig. 2A). RT-PCR analysis also documented a decrease in sCLU mRNA and an increase in nCLU mRNA in sCLU-DU-145 cells upon Ad.mda-7 infection (Fig. 2B). Similar results were also obtained in PC-3 cells transiently transfected with a CLU cDNA and infected with Ad.mda-7 (Supplementary Fig. S2E). These findings suggest that MDA-7/IL-24 regulates both sCLU and nCLU ultimately facilitating apoptosis induction, although the initial increase in sCLU might initially provide a cytoprotective effect.
Fig. 2. MDA-7/IL-24 regulates nCLU expression.
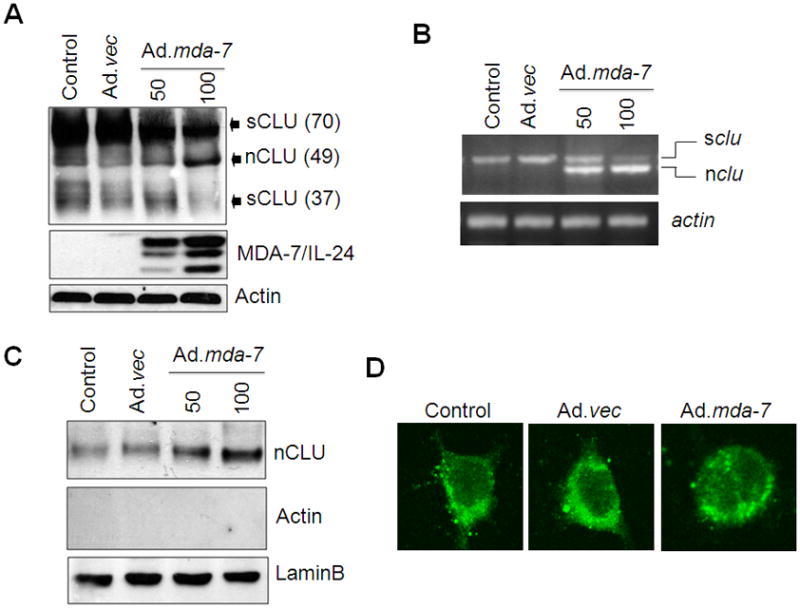
sCLU-DU-145 cells were infected with Ad.mda-7 or Ad.vec (100 pfu/cell) for 48 h and CLU expression was analyzed by Western blotting (A) and RT-PCR (B). C, sCLU-DU-145 cells were infected with Ad.mda-7 or Ad.vec (100 pfu/cell) for 48 h and CLU expression was detected in nuclear extracts by staining with Lamin B and Western blotting. D, Immunofluorescence detection of CLU staining in the nucleus 48 h after Ad.vec or Ad.mda-7 infection (100 pfu/cell).
To further confirm this hypothesis, the intracellular localization of nCLU was analyzed in sCLU-DU-145 cells by immunofluorescence and Western blotting analysis of nuclear extracts. Western blotting analysis confirmed that the Ad.mda-7-treated cells produced a protein band of ~49-kDa corresponding to the molecular weight of nCLU (Fig. 2C). CLU was localized in the cytoplasm of control cells that had a normal flattened shape. In Ad.mda-7-treated cells the protein was localized both in the cytosol as well as in the nucleus. These cells showed a rounded morphology, typical of cells undergoing apoptosis and detaching from the substrate, and with condensed nuclei (Fig. 2D). This study demonstrates that the nCLU isoform was produced in the presence of MDA-7/IL-24 from full-length CLU and translocated into the nucleus.
Ad.mda-7 enhances cell death resulting in G2/M arrest followed by apoptosis in sCLU-DU-145 cells
Previous studies suggested a pro-apoptotic role of nCLU, which specifically affects cell cycle regulation through the cyclin B1/CDK1 complex (Scaltriti et al., 2004). Based on this consideration, we analyzed the biological effects of nCLU produced from full-length CLU in the presence of MDA-7/IL-24. To clarify the role of nCLU in apoptosis, we examined Ad.mda-7 sensitivity in sCLU-DU-145 cells by cell viability (MTT) and caspase-3/7 activity assays 48 h after infection. We found that the viability of sCLU-DU-145 cells was significantly decreased as compared to control clones of DU-145 cells (Vec Con-DU-145) upon Ad.mda-7 infection (Fig. 3A). The caspase-3/7 assays and PARP activation further confirmed enhanced sensitivity of sCLU-DU-145 cells towards apoptosis in the presence of MDA-7/IL-24 (Fig. 3B and 3D). Colony-forming assays confirmed the increased sensitivity of sCLU-DU-145 cells toward Ad.mda-7 as compared with Vec Con-DU-145 cells (Fig. 3C). These findings suggest that although sCLU shows anti-apoptotic properties, Ad.mda-7 infection at 48 h enhanced apoptosis, which might be mediated by nCLU. Infection with Ad.mda-7 decreased cyclinB1 and cdk1 in sCLU-DU-145 cells as compared to Vec Con-DU-145 cells through a G2/M arrest (Fig. 3D). Additionally, inhibition of apoptosis by z-VAD-fmk (caspase 3 inhibitor) enhanced G2/M phase arrest in sCLU-DU-145 clone when compared to the Vec Con-DU-145 clone (Supplementary Table 1). These results demonstrate that Ad.mda-7 infection causes G2/M arrest followed by apoptosis in CLU-overexpressing DU-145 cells.
Fig. 3. Over expression of sCLU and MDA-7/IL-24 enhances G2/M arrest followed by apoptosis.
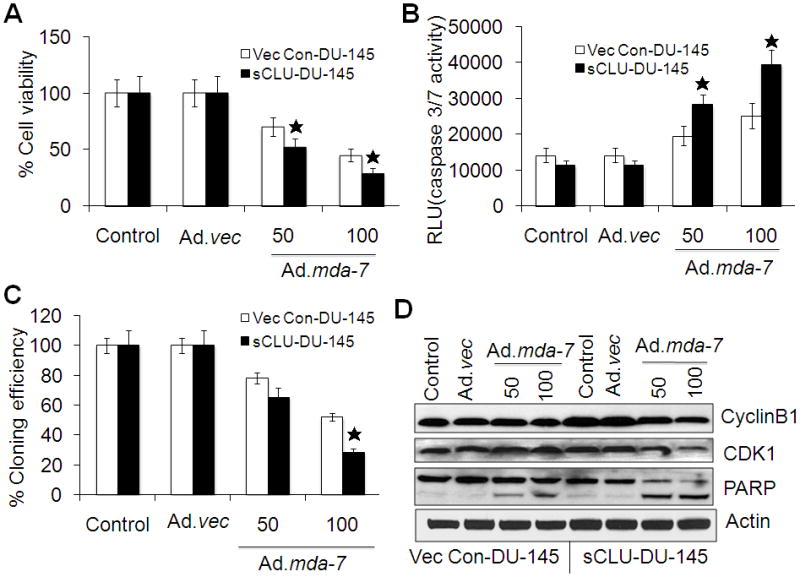
Vec Con-DU-145 and sCLU-DU-145 cells were infected with Ad.mda-7 (50 or 100 pfu/cell) or Ad.vec (100 pfu/cell) and after 48 h cell viability by MTT assay (A) and Caspase-Glo(R) 3/7 assay for caspase-3 expression (B) were determined. C, Colony formation assays in monolayer culture. Colonies were fixed, stained and counted (>50 cells) 2 wks after plating. Values are the mean ± S.D. of three independent experiments. *, P < 0.05 versus Vec Con-DU-145 Ad.mda-7-infected cells. D, After 48 h of infection with Ad.mda-7 or Ad.vec the expression of cyclin B1, cdk1 and PARP was analyzed by Western blotting in Vec Con-DU-145 and sCLU-DU-145 cells.
Effect of Ad.mda-7 on cell migration and cytoskeleton organization in sCLU-DU-145 cells
sCLU-DU-145 cells infected with Ad.mda-7 were evaluated using in vitro cellular invasion assays. Vec Con-DU-145 and sCLU DU-145 cells displayed very high invasion activity. Although Ad.mda-7 infection decreased invasion ability of both clones significantly, the inhibitory effect on sCLU-DU-145 clone was more pronounced than that in the Vec Con-DU-145 clone (Fig. 4A and 4B).
Fig. 4. Over expressed sCLU and MDA-7/IL-24 disrupts cytoskeleton organization and decreases cell migration.
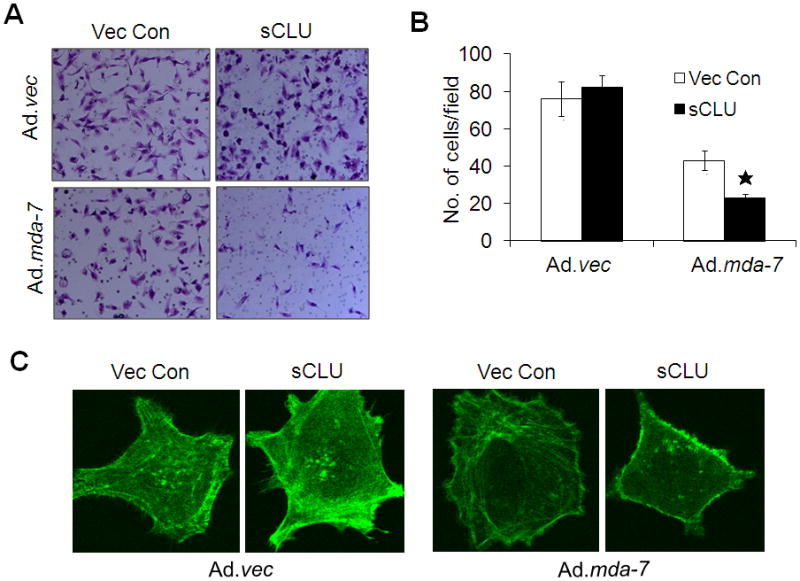
A, Vec Con-DU-145 and sCLU-DU-145 cells were infected with Ad.mda-7 or Ad.vec (100 pfu/cell) and after 12 h cells (5 × 104) were seeded onto the upper chamber of a Matrigel invasion chamber system in the absence of serum. Four h after seeding, the filters were fixed, stained, and photographed. (B) Quantitation of the invasion assay. The data expressed in the graph was the mean ± S.D. of three independent experiments. *, P < 0.05 vs. Vec Con-DU-145 Ad.mda-7-infected cells. C, After 48 h treatment with Ad.mda-7 or Ad.vec (100 pfu/cell) cells were fixed and stained with FITC-phalloidin and alterations in cytoskeleton were observed with a confocal microscope.
Immunofluorescence staining for F-actin revealed that Vec Con-DU-145 and sCLU-DU-145 cells displayed a diffuse actin cytoskeleton with intense actin staining at the level of stress fibers and under the cell cortex. Ad.mda-7 infection of the Vec Con-DU-145 clone did not induce any significant modifications in the actin cytoskeleton. However, Ad.mda-7 infection of sCLU-DU-145 cells resulted in a dramatic change in cell morphology and a decrease in intracellular actin staining, suggesting a complete disorganization of the actin cytoskeleton (Fig. 4C).
Ad.mda-7 displays enhanced antitumor activity in sCLU-DU-145-induced tumors in nude mice
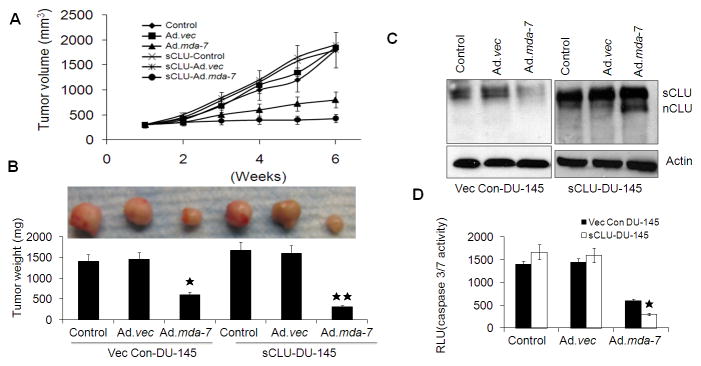
To determine whether the in vitro enhanced activity of Ad.mda-7 toward sCLU-DU-145 cells also occurred in the in vivo setting, subcutaneous xenografts were established in the left flanks of athymic nude mice using Vec Con-DU-145 cells and sCLU-DU-145 cells. After palpable tumors of ~100 mm3 developed, in approximately 8–10 days, the animals received 7 intratumoral injections over a 4-week period with 4.5 × 108 p.f.u. of Ad.vec or Ad.mda-7. Both Vec Con-DU-145 and sCLU-DU-145 cells formed large, aggressive and actively proliferating tumors in untreated and in Ad.vec-injected animals. Ad.mda-7 injection markedly inhibited growth of both groups of tumors, although the growth inhibition was more pronounced in tumors arising from sCLU-DU-145 cells compared to Vec Con-DU-145 cells (Fig. 5A and 5B).
Fig. 5. Ad.mda-7 displays an enhanced antitumor response in prostate cancer cells overexpressing sCLU.
Tumor xenografts from Vec Con-DU-145 and sCLU-DU-145 cells established in the left flanks of athymic nude mice were injected with phosphate-buffered saline (PBS) (control) or with the indicated Ad over a 4-wk period (total of seven injections). (A) Measurements of tumor xenograft volumes at different time points: average volume (minimum of five mice in each group), mean ± S.D. (*P< 0.05 between the indicated groups). (B) Photograph of the excised xenografted tumor with measurement of tumor weight at the end of the study; columns, mean (at least five mice in each group) ± S.D. sCLU and nCLU levels (C) and Caspase-Glo(R) 3/7 assay analyses for caspase-3 expression (D) were measured in tumor tissue lysates. The data expressed in the graph was the mean ± S.D. of three independent experiments. *, P < 0.05 vs. control and Ad.vec group; **, P < 0.05 vs. Vec Con-DU-145 Ad.mda-7-infected group.
We next analyzed tumor tissue lysates by Western blotting which indicated that sCLU expression was decreased in the Ad.mda-7-treated Vec Con-DU-145 and sCLU-DU-145 cells and additionally nCLU was detected in sCLU-DU-145 cells infected with Ad.mda-7 which was similar to the in vitro findings (Fig. 5C). Caspase-3/7 activity was measured by caspase 3/7-Glo assay in xenograft tissue lysates from each treatment group to assess whether MDA-7/IL-24-induced suppression of sCLU with increased nCLU enhanced apoptotic rates in vivo (Fig. 5D). Ad.mda-7-infected sCLU-DU-145 tumors had higher rates of apoptosis when compared with Vec Con-DU-145-induced tumors infected with Ad.mda-7 (Fig. 5D). These results suggest that the delay in tumor progression in the sCLU-DU-145 group resulted from increased apoptosis induced by the change in ratio of sCLU/nCLU in the tumor tissue. Expression of MDA-7/IL-24, Ki-67 (proliferation marker) and CD31 (angiogenesis marker) in tumor tissues was analyzed by immunohistochemistry. Ad.mda-7 infection of both Vec Con-DU-145 and sCLU-DU-145 groups showed significant expression of MDA-7/IL-24 and reduction in CD31 and Ki-67 staining (Dash et al., 2010). However, both Ki-67 and CD31 staining decreased more significantly in the sCLU-DU-145 group when compared to the Vec Con-DU-145 group (Fig. 6). These findings indicate that, Ad.mda-7 can efficiently generate nCLU from sCLU in cells with higher expression of CLU leading to inhibition of cell proliferation and angiogenesis, thereby resulting in tumor growth inhibition.
Fig. 6. Immuohistochemistry analysis of Vec Con-DU-145- and sCLU-DU-145-induced tumor xenografts.
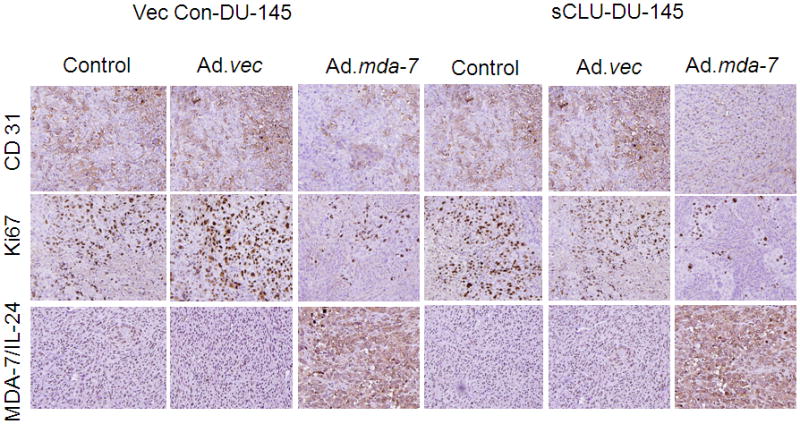
Tumor tissues were harvested and formalin-fixed paraffin-embedded sections were immunostained for MDA-7/IL-24, CD31 and Ki-67.
Discussion
mda-7/IL-24 has considerable potential as an anti-cancer therapeutic because of its diverse anti-tumor properties, its lack of toxicity toward normal cells and tissues, and its safety and efficacy as observed in a clinical trial in patients with advanced cancers (Fisher et al., 2003; Fisher, 2005; Cunningham et al., 2005; Tong et al., Lebedeva et al., 2007; Sarkar et al., 2007). Comprehending the molecular mechanism(s) by which mda-7/IL-24 promotes its diverse effects on cancer cells offers potential to provide rational approaches for enhancing the therapeutic activity of this novel cytokine (Fisher, 2005).
In the present study, we document that MDA-7/IL-24 differentially regulates sCLU and nCLU expression in prostate cancer cells. Transfection of sCLU into cancer cells increased survival in the presence of cytotoxic drugs (Hara et al., 2001; Hoeller et al., 2005), whereas down-regulation of sCLU by means of antisense oligonucleotides decreased drug resistance in cancer models (Gleave et al., 2001; Lee et al., 2002; So et al., 2005;). Our present results show that treatment of sCLU-overexpressing DU-145 cells with MDA-7/IL-24 decreases expression of sCLU and increases expression of nCLU resulting in enhanced in vitro and in vivo antitumor activity.
Our experiments document that Ad.mda-7 infection resulted in down regulation of sCLU expression and up regulation of nCLU in multiple human prostate cancer cell lines. Overexpression of sCLU in normal cells produced both sCLU and nCLU, whereas only sCLU was found in cancer cells indicating that a differential molecular mechanism may exist in normal cells, not operational in cancer cells that are initiated uniquely in tumor cells upon infection with Ad.mda-7. The precise process underlying CLU isoform production remains to be defined. However, since cancer progression results in downregulation of both MDA-7/IL-24 and nCLU with upregulation of sCLU, our present studies suggest a potential direct relationship between these changes in prostate cancer and cancer-specific apoptosis-induction. A previous study demonstrated that CLU isoforms were modulated with all-trans retinoic acid–induced proliferative arrest and apoptosis of intimal smooth muscle cells (Orlandi et al., 2005). Another point worth emphasizing is that at early phases of Ad.mda-7 infection, we observed an increase in the expression of sCLU that interacted with MDA-7/IL-24, which might provide a survival advantage from ER stress caused by Ad.mda-7. This hypothesis is supported by a previous report that high levels of CLU expression increase paclitaxel resistance in ovarian cancer cells by physically binding to paclitaxel, which may prevent paclitaxel from interacting with microtubules to induce apoptosis (Park et al., 2008).
nCLU is implicated in various cell functions, including DNA repair, cell cycle regulation, cell motility and apoptotic cell death (Scaltriti et al., 2004; Shannan et al., 2006). nCLU induced by MDA-7/IL-24 regulates cell fate by modifying cell cycle and apoptosis. A previous study showed that overexpression of nCLU induces G2/M arrest, leading to apoptosis through cyclin B1/cdk1 complex dysregulation in PC-3 cells (Scaltriti et al., 2004). Accordingly, we document that nCLU production by MDA-7/IL-24 caused G2/M phase arrest followed by apoptosis. Furthermore, it has been reported that nCLU has potent anti-migratory activity through its binding to α-actinin (Moretti et al., 2007) and our present results revealed that nCLU generated by MDA-7/IL-24 is involved in altering cytoskeleton and resulted in decreased cell migration. Moreover, MDA-7/IL-24 decreased cell proliferation and colony formation with apoptosis induction in sCLU-overexpressing prostate cancer cells. Our study suggests that MDA-7/IL-24 would be a potent potential anticancer agent for resistant prostate cancers, or other tumors, displaying high levels of sCLU expression.
It is well documented that increased expression of secreted sCLU is associated with chemoresistance, radioresistance, and hormone-resistance, making secreted sCLU an attractive target for developing antitumor therapeutics (Hara et al., 2001; July et al., 2002; Gleave et al., 2005;). However, it is crucial that any targeting of secreted sCLU should have minimal effect on nCLU and in this framework, it is important to decipher the effect of nCLU at inhibiting sCLU expression with current antisense oligonucleotides being used for effective tumor therapy in prostate cancer. We showed that MDA-7/IL-24 caused suppression of secreted sCLU with increased nCLU levels in sCLU-DU-145 xenografts. This unique property of MDA-7/IL-24 is associated with increased apoptotic rates and delayed tumor progression in vivo. Moreover, in vivo administration of a CLU antisense oligonucleotide (OGX-011) with paclitaxel results in elevated nCLU expression and significantly delayed MCF-7 tumor growth (So et al., 2005) providing pre-clinical proof-of principle for the role of nCLU in developing multimodality therapies for breast cancer. Our results suggest that prostate cancer patients would benefit from knockdown of sCLU with concomitant upregulation of nCLU. Lowering the sCLU/nCLU ratio with a cancer-specific apoptosis-inducing molecule like MDA-7/IL-24 might provide a very efficient strategy to control prostate cancer cell outgrowth and metastatic spread.
Supplementary Material
Acknowledgments
The present study was supported in part by National Institutes of Health grant R01 CA097318 (P.B.F.), P01 CA104177 (P.B.F., P.D., S.G., D.T.C.) and R01 CA138540 (D.S.), the National Foundation for Cancer Research (P.B.F.), the Dana Foundation (D.S.) and the Korea Ministry of Education, Science and Technology through National Research Foundation (2009-0063466) (S.-G.L.) and Fondazione Cariparma (2010.0445), PRIN 2008 (F.R., S.B.). D.S. and X.-Y.W. are Harrison Endowed Scholars in Cancer Research in the VCU Massey Cancer Center. P.B.F. holds the Thelma Newmeyer Corman Chair in Cancer Research in the VCU Massey Cancer Center.
References
- Bettuzzi S, Davalli P, Astancolle S, Carani C, Madeo B, Tampieri A, Corti A. Tumor progression is accompanied by significant changes in the levels of expression of polyamine metabolism regulatory genes and clusterin (sulphated glycoprotein2) in human prostate cancer specimens. Cancer Res. 2000;60:28–34. [PubMed] [Google Scholar]
- Bettuzzi S, Hiipakka RA, Gilna P, Liao ST. Identification of an androgen-repressed mRNA in rat ventral prostate as coding for sulphated glycoprotein 2 by cDNA cloning and sequence analysis. Biochem J. 1989;257:293–296. doi: 10.1042/bj2570293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Mechanism of autophagy to apoptosis switch triggered in prostate cancer cells by antitumor cytokine melanoma differentiation-associated gene 7/interleukin-24. Cancer Res. 2010;70:3667–3676. doi: 10.1158/0008-5472.CAN-09-3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhutia SK, Dash R, Das SK, Azab B, Su ZZ, Lee SG, Grant S, Yacoub A, Dent P, Curiel DT, Sarkar D, Fisher PB. Autophagy switches to apoptosis in prostate cancer cells infected with melanoma differentiation associated gene-7/interleukin-24 (MDA-7/IL-24) Autophagy. 2011 doi: 10.4161/auto.7.9.16163. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporali A, Davalli P, Astancolle S, D’Arca D, Brausi M, Bettuzzi S, Corti A. The chemopreventive action of catechins in the TRAMP mouse model of prostate carcinogenesis is accompanied by clusterin. Carcinogenesis. 2004;25:2217–2224. doi: 10.1093/carcin/bgh235. [DOI] [PubMed] [Google Scholar]
- Cunningham CC, Chada S, Merritt JA, Tong A, Senzer N, Zhang Y, Mhashilkar A, Parker K, Vukelja S, Richards D, Hood J, Coffee K, Nemunaitis J. Clinical and local biological effects of an intratumoral injection of mda-7 (IL24; INGN 241) in patients with advanced carcinoma: a phase I study. Mol Ther. 2005;11:149–159. doi: 10.1016/j.ymthe.2004.09.019. [DOI] [PubMed] [Google Scholar]
- Dash R, Bhutia SK, Azab B, Su ZZ, Quinn BA, Kegelmen TP, Das SK, Kim K, Lee SG, Park MA, Yacoub A, Rahmani M, Emdad L, Dmitriev IP, Wang XY, Sarkar D, Grant S, Dent P, Curiel DT, Fisher PB. mda-7/IL-24: a unique member of the IL-10 gene family promoting cancer-specific toxicity. Cytokine Growth Factor Rev. 2011;21:381–391. doi: 10.1016/j.cytogfr.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash R, Richards JE, Su ZZ, Bhutia SK, Azab B, Rahmani M, Dasmahapatra G, Yacoub A, Dent P, Dmitriev IP, Curiel DT, Grant S, Pellecchia M, Reed JC, Sarkar D, Fisher PB. Mechanism by which Mcl-1 regulates cancer-specific apoptosis triggered by mda-7/IL-24, an IL-10-related cytokine. Cancer Res. 2010;70:5034–5045. doi: 10.1158/0008-5472.CAN-10-0563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher PB, Gopalkrishnan RV, Chada S, Ramesh R, Grimm EA, Rosenfeld MR, Curiel DT, Dent P. mda-7/IL-24, a novel cancer selective apoptosis inducing cytokine gene: from the laboratory into the clinic. Cancer Biol Ther. 2003;2:S23–S37. [PubMed] [Google Scholar]
- Fisher PB. Is mda-7/IL-24 a “magic bullet” for cancer? Cancer Res. 2005;65:10128–10138. doi: 10.1158/0008-5472.CAN-05-3127. [DOI] [PubMed] [Google Scholar]
- Gao P, Sun X, Chen X, Wang Y, Foster BA, Subjeck J, Fisher PB, Wang XY. Secretable chaperone Grp170 enhances therapeutic activity of a novel tumor suppressor, mda-7/IL-24. Cancer Res. 2008;68:3890–3898. doi: 10.1158/0008-5472.CAN-08-0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleave M, Miyake H. Use of antisense oligonucleotides targeting the cytoprotective gene, clusterin, to enhance androgen- and chemo-sensitivity in prostate cancer. World J Urol. 2005;23:38–46. doi: 10.1007/s00345-004-0474-0. [DOI] [PubMed] [Google Scholar]
- Gleave ME, Miyake H, Zellweger T, Chi K, July L, Nelson C, Rennie P. Use of antisense oligonucleotides targeting the antiapoptotic gene, clusterin/testosterone-repressed prostate message 2, to enhance androgen sensitivity and chemosensitivity in prostate cancer. Urology. 2001;58:39–49. doi: 10.1016/s0090-4295(01)01241-9. [DOI] [PubMed] [Google Scholar]
- Gupta P, Walter MR, Su ZZ, Lebedeva IV, Emdad L, Randolph A, Valerie K, Sarkar D, Fisher PB. BiP/GRP78 is an intracellular target for MDA-7/IL-24 induction of cancer-specific apoptosis. Cancer Res. 2006;66:8182–8191. doi: 10.1158/0008-5472.CAN-06-0577. [DOI] [PubMed] [Google Scholar]
- Hara I, Miyake H, Gleave ME, Kamidono S. Introduction of clusterin gene into human renal cell carcinoma cells enhances their resistance to cytotoxic chemotherapy through inhibition of apoptosis both in vitro and in vivo. Jpn J Cancer Res. 2001;92:1220–1224. doi: 10.1111/j.1349-7006.2001.tb02143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoeller C, Pratscher B, Thallinger C, Winter D, Fink D, Kovacic B, Sexl V, Wacheck V, Gleave ME, Pehamberger H, Jansen B. Clusterin regulates drug-resistance in melanoma cells. J Invest Dermatol. 2005;124:1300–1307. doi: 10.1111/j.0022-202X.2005.23720.x. [DOI] [PubMed] [Google Scholar]
- Jiang H, Lin JJ, Su ZZ, Goldstein NI, Fisher PB. Subtraction hybridization identifies a novel melanoma differentiation associated gene, mda-7, modulated during human melanoma differentiation, growth and progression. Oncogene. 1995;11:2477–2486. [PubMed] [Google Scholar]
- Jones SE, Jomary C. Clusterin. Int J Biochem Cell Biol. 2002;34:427–431. doi: 10.1016/s1357-2725(01)00155-8. [DOI] [PubMed] [Google Scholar]
- July LV, Akbari M, Zellwegger T, Jones EC, Goldenberg SL, Gleave ME. Clusterin expression is significantly enhanced in prostate cancer cells following androgen withdrawal therapy. Prostate. 2002;50:179–188. doi: 10.1002/pros.10047. [DOI] [PubMed] [Google Scholar]
- Labrie F, Bélanger A, Luu-The V, Labrie C, Simard J, Cusan L, Gomez J, Candas B. Gonadotropin releasing hormone agonists in the treatment of prostate cancer. Endocr Rev. 2005;26:361–379. doi: 10.1210/er.2004-0017. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Emdad L, Su ZZ, Gupta P, Sauane M, Sarkar D, Staudt MR, Liu SJ, Taher MM, Xiao R, Barral P, Lee SG, Wang D, Vozhilla N, Park ES, Chatman L, Boukerche H, Ramesh R, Inoue S, Chada S, Li R, De Pass AL, Mahasreshti PJ, Dmitriev IP, Curiel DT, Yacoub A, Grant S, Dent P, Senzer N, Nemunaitis JJ, Fisher PB. mda-7/IL-24, novel anticancer cytokine: focus on bystander antitumor, radiosensitization and antiangiogenic properties and overview of the phase I clinical experience. Int J Oncol. 2007;31:985–1007. [PubMed] [Google Scholar]
- Lee CH, Jin RJ, Kwak C, Jeong H, Park MS, Lee NK, Lee SE. Suppression of clusterin expression enhanced cisplatin-induced cytotoxicity on renal cell carcinoma cells. Urology. 2002;60:516–520. doi: 10.1016/s0090-4295(02)01806-x. [DOI] [PubMed] [Google Scholar]
- Lee ECY, Tenniswood MPR. Emergence of metastatic hormone-refractory disease in prostate cancer after anti-androgen therapy. J Cell Biochem. 2004;91:662–670. doi: 10.1002/jcb.20040. [DOI] [PubMed] [Google Scholar]
- Leskov K, Klokov D, Li J, Kinsella TJ, Boothman DA. Synthesis and functional analyses of nuclear clusterin: a cell death protein. J Biol Chem. 2003;278:11590–11600. doi: 10.1074/jbc.M209233200. [DOI] [PubMed] [Google Scholar]
- Miyahara R, Banerjee S, Kawano K, Efferson C, Tsuda N, Miyahara Y, Ioannides CG, Chada S, Ramesh R. Melanoma differentiation associated gene-7 (mda-7)/interleukin (IL)-24 induces anticancer immunity in a syngeneic murine model. Cancer Gene Ther. 2006;13:753–761. doi: 10.1038/sj.cgt.7700954. [DOI] [PubMed] [Google Scholar]
- Moretti RM, Marelli MM, Mai S, Cariboni A, Scaltriti M, Bettuzzi S, Limonta P. Clusterin isoforms differentially affect growth and motility of prostate cells: possible implications in prostate tumorigenesis. Cancer Res. 2007;67:10325–10333. doi: 10.1158/0008-5472.CAN-07-0516. [DOI] [PubMed] [Google Scholar]
- Nelen V. Epidemiology of prostate cancer. Recent Results Cancer Res. 2007;175:1–8. doi: 10.1007/978-3-540-40901-4_1. [DOI] [PubMed] [Google Scholar]
- Orlandi A, Pucci S, Ciucci A, Pichiorri F, Ferlosio A, Spagnoli LG. Modulation of clusterin isoforms is associated with all-trans retinoic acid-induced proliferative arrest and apoptosis of intimal smooth muscle cells. Arterioscler Thromb Vasc Biol. 2005;25:348–353. doi: 10.1161/01.ATV.0000152609.28569.e1. [DOI] [PubMed] [Google Scholar]
- Park DC, Yeo SG, Wilson MR, Yerbury JJ, Kwong J, Welch WR, Choi YK, Birrer MJ, Mok SC, Wong KK. Clusterin interacts with Paclitaxel and confer Paclitaxel resistance in ovarian cancer. Neoplasia. 2008;10:964–972. doi: 10.1593/neo.08604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MA, Yacoub A, Sarkar D, Emdad L, Rahmani M, Spiegel S, Koumenis C, Graf M, Curiel DT, Grant S, Fisher PB, Dent P. PERK-dependent regulation of MDA-7/IL-24-induced autophagy in primary human glioma cells. Autophagy. 2008;4:513–515. doi: 10.4161/auto.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB. Interleukin-10 and related cytokines and receptors. Annu Rev Immunol. 2004;22:929–979. doi: 10.1146/annurev.immunol.22.012703.104622. [DOI] [PubMed] [Google Scholar]
- Purrello M, Bettuzzi S, Di Pietro C, Mirabile E, Di Blasi M, Rimini R, Grzeschik KH, Ingletti C, Corti A, Sichel G. The gene for SP-40,40, human homolog of rat sulphated glycoprotein 2, rat clusterin, and testosterone-repressed prostate message 2, maps to chromosome 8. Genomics. 1991;10:151–156. doi: 10.1016/0888-7543(91)90495-z. [DOI] [PubMed] [Google Scholar]
- Ramesh R, Mhashilkar AM, Tanaka F, Saito Y, Branch CD, Sieger K, Mumm JB, Stewart AL, Boquoi A, Dumoutier L, Grimm EA, Renauld JC, Kotenko S, Chada S. Melanoma differentiation-associated gene 7/interleukin (IL)-24 is a novel ligand that regulates angiogenesis via the IL-22 receptor. Cancer Res. 2003;63:5105–5113. [PubMed] [Google Scholar]
- Rosenberg ME, Silkensen J. Clusterin: physiologic and pathophysiologic considerations. Int J Biochem Cell Biol. 1995;27:633–645. doi: 10.1016/1357-2725(95)00027-m. [DOI] [PubMed] [Google Scholar]
- Sarkar D, Lebedeva IV, Gupta P, Emdad L, Sauane M, Dent P, Curiel DT, Fisher PB. Melanoma differentiation associated gene-7 (mda-7)/IL-24: a ‘magic bullet’ for cancer therapy? Expert Opin Biol Ther. 2007;7:577–586. doi: 10.1517/14712598.7.5.577. [DOI] [PubMed] [Google Scholar]
- Sauane M, Su ZZ, Gupta P, Lebedeva IV, Dent P, Sarkar D, Fisher PB. Autocrine regulation of mda-7/IL-24 mediates cancer-specific apoptosis. Proc Natl Acad Sci U S A. 2008;105:9763–9768. doi: 10.1073/pnas.0804089105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaltriti M, Brausi M, Amorosi A, Caporali A, D’Arca D, Astancolle S, Corti A, Bettuzzi S. Clusterin (SGP-2, ApoJ) expression is down-regulated in low- and high grade human prostate cancer. Int J Cancer. 2004;108:23–30. doi: 10.1002/ijc.11496. [DOI] [PubMed] [Google Scholar]
- Scaltriti M, Santamaria A, Paciucci R, Bettuzzi S. Intracellular clusterin induces G2-M phase arrest and cell death in PC-3 prostate cancer cells. Cancer Res. 2004;64:6174–6182. doi: 10.1158/0008-5472.CAN-04-0920. [DOI] [PubMed] [Google Scholar]
- Shannan B, Seifert M, Leskov K, Willis J, Boothman D, Tilgen W, Reichrath J. Challenge and promise: roles for clusterin in pathogenesis, progression and therapy of cancer. Cell Death Differ. 2006;13:12–19. doi: 10.1038/sj.cdd.4401779. [DOI] [PubMed] [Google Scholar]
- So A, Sinnemann S, Huntsman D, Fazli L, Gleave ME. Knockdown of the cytoprotective chaperone, clusterin, chemosensitizes human breast cancer cells both in vitro and in vivo. Mol Cancer Ther. 2005;4:1837–1849. doi: 10.1158/1535-7163.MCT-05-0178. [DOI] [PubMed] [Google Scholar]
- Springate CM, Jackson JK, Gleave ME, Burt HM. Efficacy of an intratumoral controlled release formulation of clusterin antisense oligonucleotide complexed with chitosan containing paclitaxel or docetaxel in prostate cancer xenograft models. Cancer Chemother Pharmacol. 2005;56:239–247. doi: 10.1007/s00280-004-0997-5. [DOI] [PubMed] [Google Scholar]
- Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, James CD, Randolph A, Valerie K, Walter MR, Dent P, Fisher PB. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- Su ZZ, Lebedeva IV, Sarkar D, Emdad L, Gupta P, Kitada S, Dent P, Reed JC, Fisher PB. Ionizing radiation enhances therapeutic activity of mda-7/IL-24: overcoming radiation- and mda-7/IL-24-resistance in prostate cancer cells overexpressing the antiapoptotic proteins bcl-xL or bcl-2. Oncogene. 2006;25:2339–2348. doi: 10.1038/sj.onc.1209271. [DOI] [PubMed] [Google Scholar]
- Tong AW, Nemunaitis J, Su D, Zhang Y, Cunningham C, Senzer N, Netto G, Rich D, Mhashilkar A, Parker K, Coffee K, Ramesh R, Ekmekcioglu S, Grimm EA, van Wart Hood J, Merritt J, Chada S. Intratumoral injection of INGN 241, a nonreplicating adenovector expressing the melanomadifferentiation associated gene-7 (mda-7/IL24): biologic outcome in advanced cancer patients. Mol Ther. 2005;11:160–172. doi: 10.1016/j.ymthe.2004.09.021. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, Lourda M, Agiostratidou G, Kletsas D, Gonos EF. Differential effects of clusterin/apolipoprotein J on cellular growth and survival. Free Rad Biol Med. 2005;38:436–449. doi: 10.1016/j.freeradbiomed.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Trougakos IP, So A, Jansen B, Gleave ME, Gonos ES. Silencing expression of the clusterin/apolipoprotein J gene in human cancer cells using small interfering RNA induces spontaneous apoptosis, reduced growth ability, and cell sensitization to genotoxic and oxidative stress. Cancer Res. 2004;64:1834–1842. doi: 10.1158/0008-5472.can-03-2664. [DOI] [PubMed] [Google Scholar]
- Yacoub A, Hamed H, Emdad L, Dos Santos W, Gupta P, Broaddus WC, Ramakrishnan V, Sarkar D, Shah K, Curiel DT, Grant S, Fisher PB, Dent P. MDA-7/IL-24 plus radiation enhance survival in animals with intracranial primary human GBM tumors. Cancer Biol Ther. 2008;7:917–933. doi: 10.4161/cbt.7.6.5928. [DOI] [PubMed] [Google Scholar]
- Yang CR, Leskov K, Hosley-Eberlein K, Criswell T, Pink JJ, Kinsella TJ, Boothman DA. Nuclear clusterin/XIP8, an X-ray-induced Ku70-binding protein that signals cell death. Nucleic Acid Res. 2000;97:5907–5912. doi: 10.1073/pnas.97.11.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.