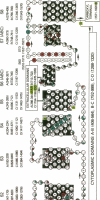
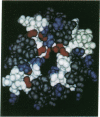
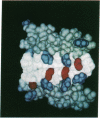
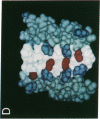
Abstract
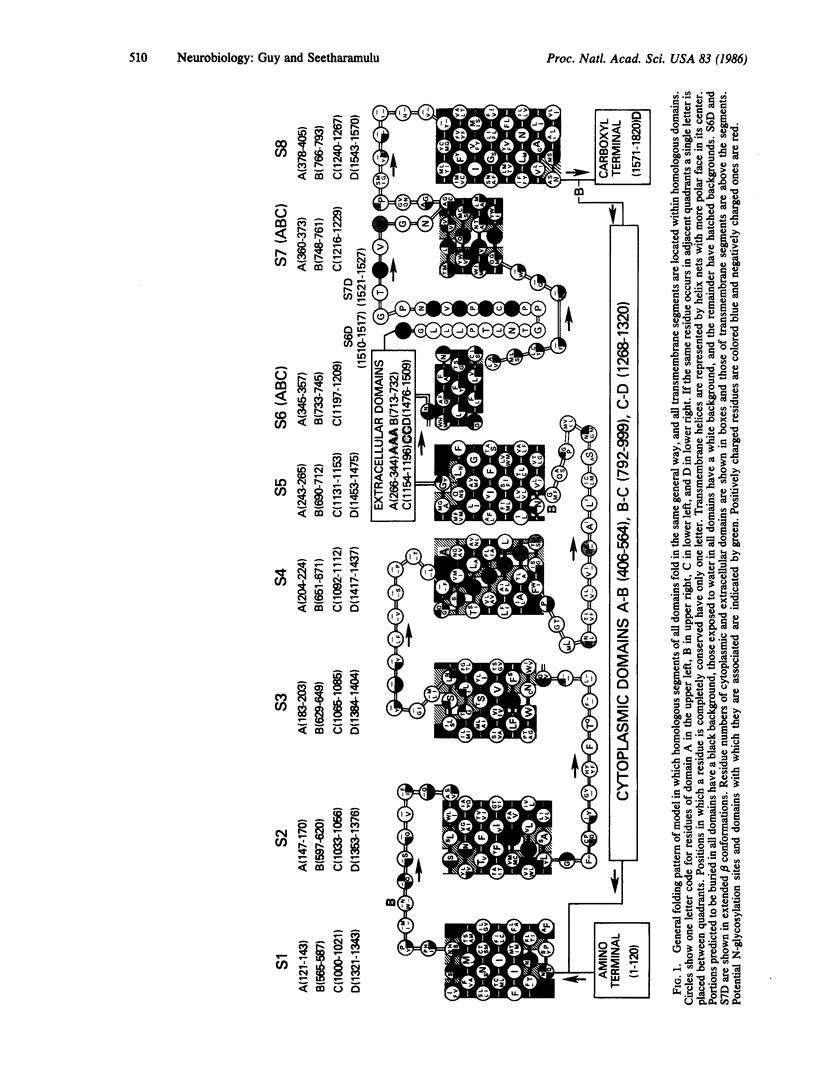
Secondary and tertiary structural models of sodium channel transmembrane segments were developed from its recently determined primary sequence in Electrophorus electricus. The model has four homologous domains, and each domain has eight homologous transmembrane segments, S1 through S8. Each domain contains three relatively apolar segments (S1, S2 and S3) and two very apolar segments (S5 and S8), all postulated to be transmembrane alpha-helices. S4 segments have positively charged residues, mainly arginines, at every third residue. The model channel lining is formed by four S4 transmembrane alpha-helices and four negatively charged S7 segments. S7 segments are postulated to be short, partially transmembrane amphipathic alpha-helices in three domains and a beta-strand in the last domain. S7 segments are preceded by short apolar segments (S6) postulated to be alpha-helices in three domains and a beta-strand in the last domain. Positively charged side chains of S4 form salt bridges with negatively charged side chains on S7 and near the ends of S1 and S3. Putative extracellular segments that contain 5 of the 10 potential N-glycosylation sites link S5 to S6. Channel activation may involve a 'helical screw' mechanism in which S4 helices rotate around their axes as they move toward the extracellular surface.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong C. M. Sodium channels and gating currents. Physiol Rev. 1981 Jul;61(3):644–683. doi: 10.1152/physrev.1981.61.3.644. [DOI] [PubMed] [Google Scholar]
- Chothia C., Levitt M., Richardson D. Helix to helix packing in proteins. J Mol Biol. 1981 Jan 5;145(1):215–250. doi: 10.1016/0022-2836(81)90341-7. [DOI] [PubMed] [Google Scholar]
- Criado M., Hochschwender S., Sarin V., Fox J. L., Lindstrom J. Evidence for unpredicted transmembrane domains in acetylcholine receptor subunits. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2004–2008. doi: 10.1073/pnas.82.7.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunker A. K., Zaleske D. J. Stereochemical considerations for constructing alpha-helical protein bundles with particular application to membrane proteins. Biochem J. 1977 Apr 1;163(1):45–57. doi: 10.1042/bj1630045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R. A structural model of the acetylcholine receptor channel based on partition energy and helix packing calculations. Biophys J. 1984 Jan;45(1):249–261. doi: 10.1016/S0006-3495(84)84152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R. Amino acid side-chain partition energies and distribution of residues in soluble proteins. Biophys J. 1985 Jan;47(1):61–70. doi: 10.1016/S0006-3495(85)83877-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic selectivity of Na and K channels of nerve membranes. Membranes. 1975;3:255–323. [PubMed] [Google Scholar]
- Hol W. G., Halie L. M., Sander C. Dipoles of the alpha-helix and beta-sheet: their role in protein folding. Nature. 1981 Dec 10;294(5841):532–536. doi: 10.1038/294532a0. [DOI] [PubMed] [Google Scholar]
- Miller J. A., Agnew W. S., Levinson S. R. Principal glycopeptide of the tetrodotoxin/saxitoxin binding protein from Electrophorus electricus: isolation and partial chemical and physical characterization. Biochemistry. 1983 Jan 18;22(2):462–470. doi: 10.1021/bi00271a032. [DOI] [PubMed] [Google Scholar]
- Mishina M., Tobimatsu T., Imoto K., Tanaka K., Fujita Y., Fukuda K., Kurasaki M., Takahashi H., Morimoto Y., Hirose T. Location of functional regions of acetylcholine receptor alpha-subunit by site-directed mutagenesis. 1985 Jan 31-Feb 6Nature. 313(6001):364–369. doi: 10.1038/313364a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Shimizu S., Tanabe T., Takai T., Kayano T., Ikeda T., Takahashi H., Nakayama H., Kanaoka Y., Minamino N. Primary structure of Electrophorus electricus sodium channel deduced from cDNA sequence. Nature. 1984 Nov 8;312(5990):121–127. doi: 10.1038/312121a0. [DOI] [PubMed] [Google Scholar]
- Wada A., Nakamura H. Nature of the charge distribution in proteins. Nature. 1981 Oct 29;293(5835):757–758. doi: 10.1038/293757a0. [DOI] [PubMed] [Google Scholar]