Abstract
Histone modification determines epigenetic patterns of gene expression with methylation of histone H3 at lysine 4 (H3K4) often associated with active promoters. LSD1/KDM1 is a histone demethylase that suppresses gene expression by converting di-methylated H3K4 to mono- and un-methylated H3K4. LSD1 is essential for metazoan development but its pathophysiological functions in cancer remain mainly uncharacterized. In this study, we developed specific bioactive small inhibitors of LSD1 that enhance H3K4 methylation and derepress epigenetically suppressed genes in vivo. Strikingly, these compounds inhibited the proliferation of pluripotent cancer cells including teratocarcinoma, embryonic carcinoma, and seminoma or embryonic stem cells that express the stem cell markers Oct4 and Sox2, while displaying minimum growth inhibitory effects on non-pluripotent cancer or normal somatic cells. RNAi-mediated knockdown of LSD1 expression phenocopied these effects, confirming the specificity of small molecules and further establishing the high degree of sensitivity and selectivity of pluripotent cancer cells to LSD1 ablation. In support of these results, we found that LSD1 protein level is highly elevated in pluripotent cancer cells and in human testicular seminoma tissues that express Oct4. Using these novel chemical inhibitors as probes, our findings establish LSD1 and histone H3K4 methylation as essential cancer-selective epigenetic targets in pluripotent cancer cells that have stem cell properties.
Keywords: LSD1/KDM1, Histone H3 Methylation at K4 (H3K4), Histone Demethylase, Pluripotent Cancer Cell, Cancer Stem Cell
Introduction
Histone methylation is a major covalent modification of histones that epigenetically regulates cell-specific gene expression patterns (1, 2). While methylations of histone H3 at lysines 9 (H3K9) and 27 (H3K27) suppress gene expression, the methylations of lysine 4 (K4) in histone H3 (H3K4) usually associate with actively transcribed genes (1–3). Histone methylation is dynamically controlled by specific histone methyltransferases and demethylases (3). Specifically, the methylations at H3K4 are primarily catalyzed by histone methyltransferase complexes composed of the members of MLL (Mixed Lineage Leukemia) SET-domain methyltransferases, ASH2, WDR5, and RBBP5 (2, 4). The methyl groups in H3K4 are removed by histone demethylases LSD1 (also called KDM1, AOF2, or BHC110), LSD2, and JARID1A–1D (1, 3, 5, 6).
Lysine-specific demethylase 1 (LSD1) belongs to the flavin adenine dinucleotide (FAD)-dependent amine oxidase family, and specifically catalyzes the demethylation of di-and mono-methylated H3K4 through amine oxidation (1, 5, 7). In contrast to the JARID1 family of Jumonji C (JmjC) domain-containing demethylases that remove the methyl group from tri-, di-, and mono-methylated H3K4 (1), demethylation by LSD1 requires a protonated nitrogen in the methylated histone, precluding it from removing the methyl group from tri-methylated H3K4 (1, 5, 8). LSD1 is highly conserved in high eukaryotes and null mutation of LSD1 genes in the mouse causes embryonic lethality (9). However, its pathophysiological function remains unclear (9, 10). Because the catalytic domain of LSD1 shares significant similarity with other members of the amine oxidase family, most investigation on LSD1 function involves the use of non-selective amine oxidase inhibitors, originally developed against two major isoforms of monoamine oxidases, MAO-A and MAO-B, and act through the irreversible modification of the covalently bound FAD at high concentrations (millimolars)(5, 8, 11–15). However, these monoamine oxidase inhibitors induce substantial toxicity in vivo, causing difficulty and confusion for interpretation of LSD1 in vivo function.
Cancer stem cells are considered as the origin of various heterogeneous cancer populations due to their pluripotent or multipotent stem cell property (16–19). Development of therapeutic drugs that target cancer stem cells is an unmet medical demand since these cells appear to be more resistant to conventional chemo- or radio-therapy. They also often act as the source for metastasis or recurring drug resistant cancers after treatment (16, 19, 20). Teratoma and teratocarcinoma are pluripotent germ cell tumors caused by abnormal development of embryonic stem (ES) cells (21–23). Other pluripotent tumors include embryonic carcinomas, seminomas, choriocarcinomas, and tumors of yolk sac. These tumors often display pluripotent stem cell properties, express stem cell markers Oct4 and Sox2, and are capable of differentiating into various tissue types (21–24). It is well established that ES cells have distinct patterns of histone methylations and other epigenetic modifications for their maintenance and self-renewal (25). Re-programming of somatic cells into the induced pluripotent stem (iPS) cells by expression of Oct4 and Sox2 is associated with dramatic rearrangement of histone methylation (25, 26). However, it remains unclear the exact role of various histone methylases and demethylases in defining the pluripotency of ES cells or cancer stem cells.
Materials and Methods
Molecular modeling
The docking template structure of LSD1 was derived from the crystal structure of LSD1 bound to the substrate-like peptide (PDB code: 2v1d) (27). Docking was performed using the latest version AutoDock 4.0 (28). The illustrated structures were made by Pymol (29).
Cell lines
F9, NCCIT, NTERA-2, HeLa, 293, NIH3T3, and mouse embryonic stem (ES) cells were obtained from American Type Culture Collection (ATCC) within six months of the submission. All cells were maintained in Dulbecco’s Modified Eagle Medium with 10% fetal bovine serum (30). F9 cells were grown on petri dishes coated with 0.1% gelatin while mouse ES cells were cultured on irradiated mouse embryonic fibroblasts (30, 31). All cells were authenticated in Nevada Cancer Institute by specific markers such as Oct4, Sox2, p53, and p16Ink4a and by their cell morphology and used within three months.
In vitro demethylation assays
Human LSD1 and mouse LSD2 were expressed in bacteria and purified as GST fusion proteins (5, 30). Human LSD1 and JARID1A proteins were also obtained from BPS Biosciences. The recombinant proteins were used for demethylation assays using either the di-methylated H3K4 peptide or isolated histones from the nuclei of HeLa cells as reported (5, 32, 33)(see Supplemental Materials and Methods). The conversion efficiency of the di-methylated (Me2) H3K4 peptide to mono- (Me1) and non-methylated (Me0) H3K4 is calculated using the integrated product peak areas in MALDI TOF/TOF mass-spectrometry (Applied Biosystems 4800 Plus) and the following formula:
The in vitro IC50 of each CBB compound for LSD1 inhibition was calculated using the conversion efficiency and the GraphPad Prism5 software (5, 34).
Cell permeability assays
CBB1002, CBB1003 and 1007 were incubated with F9 cells for 2 hours. The extensively washed cells were lysed immediately and the lysates were mixed with 4 volume of acetone at −20 °C for 6 hours. The precipitated proteins/macromolecules were removed by centrifugation and the supernatant was lyophilized and dried pellets were dissolved in methanol. The presence of CBB compounds was analyzed by mass-spectrometry using the pure compounds and the lysates from untreated cells as m/z controls.
Results
Design and synthesis of novel LSD1 chemical inhibitors
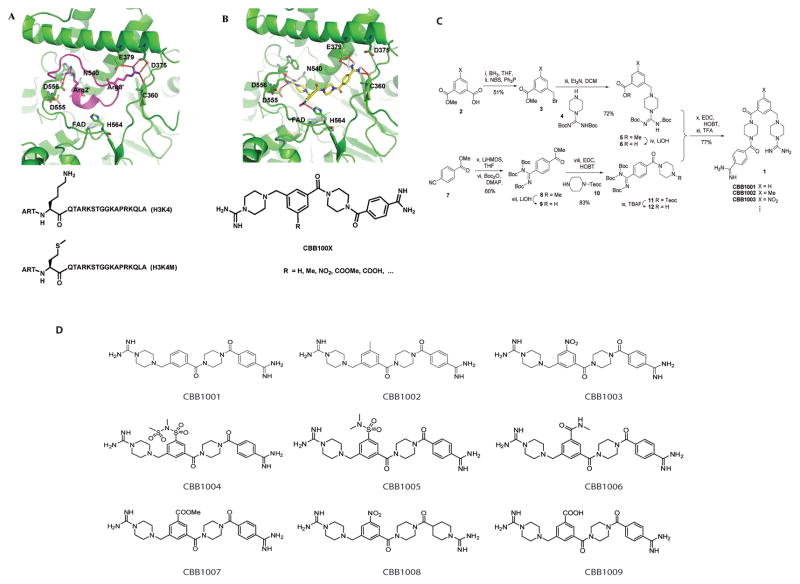
To understand the function of LSD1, we developed novel chemical compounds that specifically inhibit LSD1 demethylase. As a template for new LSD1 inhibitors, we used the protein crystal structure of LSD1 and a substrate-like peptide inhibitor, which was derived from the N-terminal 21-amino acid residues of histone H3 peptide in which lysine 4 (K4) is replaced by methionine (Figure 1A, H3K4M) that binds to LSD1 with high binding affinity (Ki = 0.05 μM)(14, 27, 35, 36). The positively charged residues (Arg2 and Arg8) of the peptide establish favorable electrostatic interactions with a cluster of negatively charged residues on LSD1 surface that involve Asp375, Glu379, Asp553, Asp555, Asp556, Asp557, and Glu559 (Figure 1A). The funneled channel that accesses to FAD is blocked by the peptide inhibitor. Based on the structural features of LSD1 active site, especially the highly acidic properties of the surface around the active site, we designed a non-peptide chemical scaffold that binds to LSD1 with similar non-covalent binding mode to that of the peptide inhibitor (Figure 1B). The guanidinium groups of the small molecules, which mimic the arginines, form strong hydrogen bonds with the negatively charged residues of LSD1. A small compound library composed of total 9 small molecules (CBB1001–1009) based on the original design (Figure 1B–1D) was synthesized (Figure 1D).
Figure 1.
Design and synthesis of novel chemical inhibitors that interact with histone demethylase LSD1.
A. The interactions between the substrate-like peptide and LSD1. The crystal structure of LSD1 was used as a template to design the binding of a substrate-like peptide H3K4M.
B. An illustration of the de novo designed non-peptide chemical scaffold that binds to LSD1 with similar mode to that of the H3K4M peptide. The guanidinium groups of the inhibitors form strong hydrogen bonds with the negatively charged residues of LSD1, and the hydrophobic substituents dock into the deep pocket that is close to FAD. Other interactions are also indicated. For example, the nitro group of CBB1003 is likely to form a hydrogen bonding interaction with His564.
C. The synthetic scheme of novel LSD1 inhibitors using CBB1001 as an example.
D. A small library of synthesized LSD1 inhibitory compounds CBB1001–CBB1009.
The synthesis of the individual LSD1 inhibitor employed conventional solution chemistry is shown in Scheme 1 using CBB1001 as an example (Figure 1C). Mono-methyl isophthalate was converted into the corresponding bromide 3 (51% yield) by a two-step sequence including selective reduction of the acid group in 2 followed by treatment with N-bromosuccinimide and triphenylphosphane. A condensation of bromide 3 with 1-[N,N′-bis(tert-butoxycarbonyl)amidino]piperazine 4 afforded the key intermediate 5 which was hydrolyzed to produce intermediate 6. Intermediate 11 was initiated by treatment of methyl 4-cyano-benzoate with lithium bis(trimethylsilyl)amide, followed by protection with di-tert-butyldicarbonate to produce ester 8, which was converted to acid and condensed with 2-(trimethylsilyl)ethyl piperazine-1-carboxylate to produce fragment 11 (83% yield). The 2-(trimethylsilyl)ethyl carbamate protecting group in 11 was removed by the action of tetra-n-butylammonium fluoride and the resulting free amine 12 was condensed with acid 6 to provide the corresponding coupling product. This was then treated with trifluoroacetic acid to effect a global deprotection to furnish CBB1001 (77% yield). The preparation of additional 8 LSD1 inhibitory compounds (CBB1002–CBB1009) was performed according to synthetic procedures identical to that of CBB1001 except modifications at the R position (Figure 1B–D).
LSD1 compounds can specifically inhibit the LSD1 demethylase activity in vitro
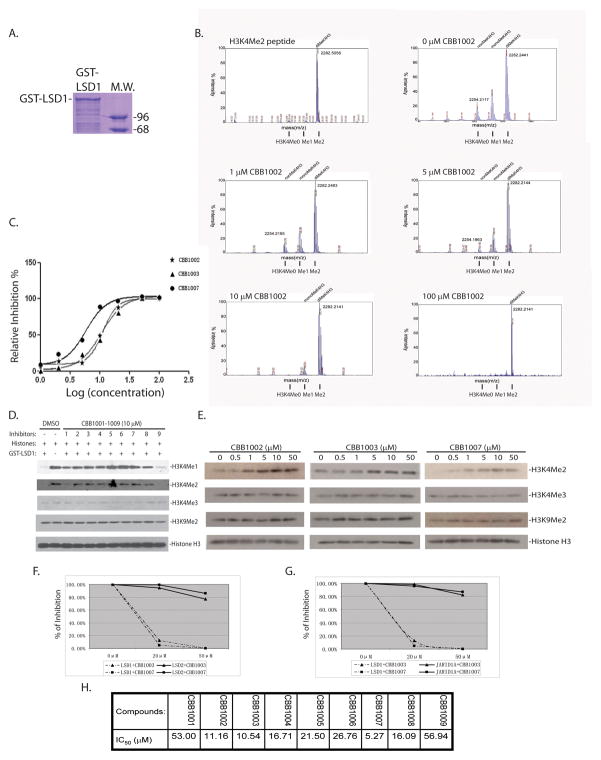
To test the potency of the compounds, the demethylation of a synthetic histone H3 amino-terminal substrate peptide that contains the di-methylated K4 by the recombinant human LSD1 protein was established (Figure 2A). The LSD1 protein displayed an intrinsic demethylase activity on this substrate in vitro, yielding the intermediate mono-methylated and final unmethylated H3K4 peptides (5), which can be separated, resolved, and semi-quantified by mass-spectrometry (Figure 2B). Analysis of CBB compounds at various dose-response concentrations revealed that most of CBB compounds could effectively inhibit LSD1 activity, with CBB1002, 1003, and 1007 displayed the best half maximal inhibitory concentration (IC50) at 11.16 μM, 10.54 μM, 5.27 μM, respectively (Figure 2B, 2C, and 2H).
Figure 2.
Analysis of CBB compounds on LSD1-dependent demethylation in vitro.
A. Purified recombinant GST-LSD1 protein used for the demethylation assay.
B–C. LSD1 inhibitors can inhibit LSD1 demethylase activity in vitro. Recombinant LSD1 was incubated at 30 °C for 1 hour with the di-methylated H3K4 peptide (H3K4Me2) and various concentrations of CBB1002 (B) or other CBB compounds (C). The demethylated products, mono-methylated (H3K4Me1) and non-methylated (H3K4me0), were analyzed by mass-spectrometry (MS) (B). The MS peak areas were integrated and used to calculate IC50 of CBB002, 1003, and 1007 at 0, 1, 2, 5, 10, 20, 50, 100 μM, respectively (C).
D–E. Methylated histones and LSD1 were assayed as in B with 10 μM CBB1001–1009 (D); or with various concentrations of CBB1002, 1003, and 1007 (E) as indicated. The in vitro inhibitory effects of CBB compounds on the mono-, di-, and tri-methylated histone H3K4, di-methylated H3K9, and histone H3 by LSD1 were analyzed by Western-blotting with specific antibodies.
F–G. LSD1, LSD2 and JARID1A demethylation reactions were analyzed using the di-methylated H3K4 substrate peptide in the presence of 0, 20, and 50 μM CBB1003 and 1007 as in B. The inhibitory effects were plotted and compared.
H. The in vitro IC50 of CBB1001–1009 for LSD1 using the MS method as in B and C.
We also used methylated histones as LSD1 substrates to analyze the effects of CBB compounds (5). As reported (5), LSD1 efficiently demethylated both mono- and di-methylated H3K4 in vitro but did not have detectable effects on tri-methylated H3K4 and di-methylated H3K9 (Figure 2D–E). At 10 μM, all CBB compounds except CBB1001 and 1009 significantly blocked the demethylase activity of LSD1 on mono- and di-methylated H3K4, but not tri-methylated H3K4 or di-methylated H3K9 (Figure 2D–E). Analysis of inhibitory concentrations of representative CBB1002, 1003, and 1007 suggests that their IC50 are similar to the assays by mass-spectrometry, with IC50 of CBB1002 and 1003 about 5–10 μM, and CBB1007 about 1–5 μM (Figure 2E).
We also examined the effects of CBB compounds on other histone demethylases such as LSD2 and JARID1A. LSD2 is a paralogue of LSD1 which shares 38% of identical amino acid residues and 56% of amino acid similarity with LSD1 protein in the conserved catalytic carboxy domain (6, 32). It can demethylate both mono- and di-methylated H3K4. JARID1A is a Jumonji domain-containing histone demethylase that specifically removes tri-, di-, and mono-methylated H3K4 (1, 33). Mass-spectrometry-based demethylation assays revealed that CBB compounds did not inhibit LSD2 and JARID1A demethylase activities (Figure 2F and 2G), suggesting CBB compounds specifically inhibit LSD1 but not other demethylases.
LSD1 compounds are active in cancer cells to inhibit LSD1 demethylation activity
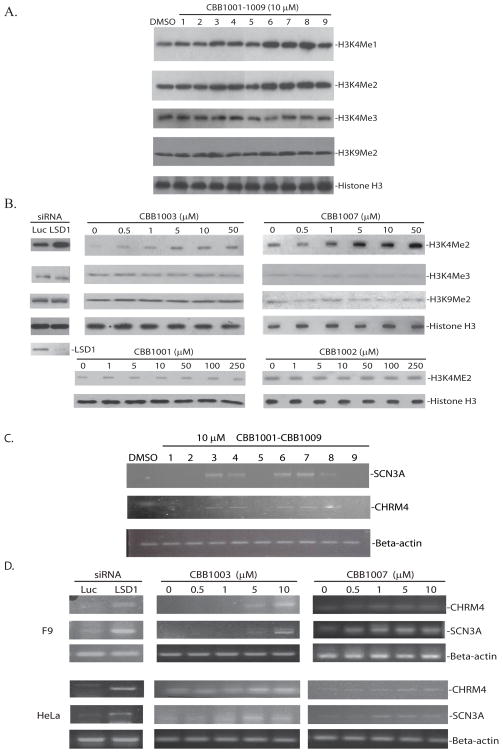
LSD1 is a demethylase for mono- and di-methylated H3K4 and loss of LSD1 in vivo causes the accumulation of these methylated forms of H3K4 (5, 11, 15). To determine the in vivo effects of new LSD1 inhibitors, we treated mouse F9 embryonic teratocarcinoma cells with CBB compounds and then monitored the accumulation of methylated H3K4 (Figure 3A). Notably, while CBB1001, CBB1002, CBB1005, and CBB1009 did not have significant effects at 10 μM, CBB1003, 1004, and 1006–1008 caused a reproducible increase of mono- and di-methylated H3K4 after treatment (Figure 3A) but had very little effects on tri-methylated H3K4 and di-methylated H3K9, suggesting that they are specific for LSD1 but not other histone demethylases. Analysis of the effects of two representative compounds CBB1003 and CBB1007 at various concentrations in F9 cells indicated that the IC50 of CBB1003 and CBB1007 is about 5–10 and 1–5 μM, respectively, (Figure 3B).
Figure 3. In vivo analysis of CBB compounds on LSD1 demethylation and gene activation.
A. In vivo effects of LSD1 compounds on methylation of histone H3. F9 cells were treated with 10 μM CBB1001–1009 for 24 hours. Total histones were extracted and the levels of methylated H3K4, H3K9, and histone H3 were monitored by Western blotting with specific antibodies, respectively. Only CBB1003, 1004, and 1006–1008 had significant effects on the mono- and di-methylated H3K4.
B. Dose effects of CBB1001, 1002, 1003, and 1007 on histone H3 methylation in F9 cells as assayed in 3A. The effects of LSD1 ablation by specific siRNA on histone methylation in F9 cells (left panels). The F9 cells were treated with 50 nM luciferase (Luc) or LSD1 specific siRNAs for 48 hours and the methylation of H3K4 was analyzed.
C. Effects of LSD1 inhibitors on epigenetic suppressed gene expression. F9 cells were treated with 10 μM CBB1001–1009 for 24 hours. The activation of epigenetically suppressed CHRM4 and SCN3A genes were monitored by quantitative RT-PCR using the beta-actin gene as a control. Only CBB1003, 1004, and 1006–1008 can activate the expression of CHRM4 and SCN3A genes.
D. Dose-dependent effects of CBB1003 and 1007 on the expression of CHRM4 and SCN3A genes in F9 and HeLa cells. The effects of LSD1 siRNAs in F9 cells were also included.
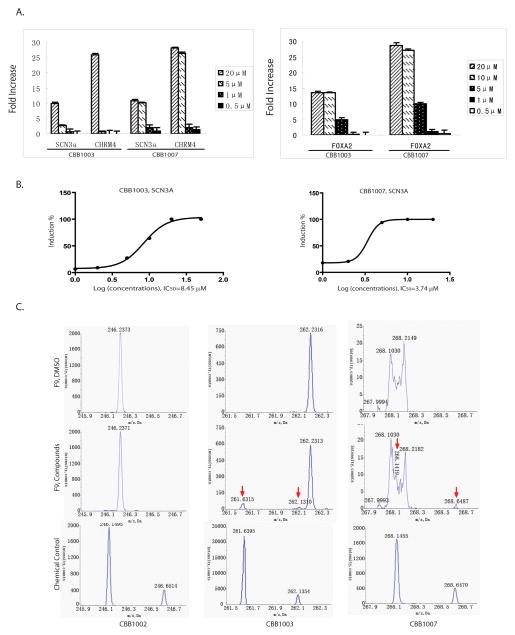
Loss of LSD1 can cause the activation of epigenetically suppressed genes, such as CHRM4/M4-ArchR and SCN3A, in cultured cells (5), as a consequence of increased levels of H3K4 methylation. To investigate whether CBB compounds can inhibit LSD1 and consequently induce epigenetically suppressed gene expression, we monitored the activation of CHRM4 and SCN3A genes by quantitative RT-PCR. While CBB1001, CBB1002, CBB1005, and CBB1009 could not activate the expression of these genes at 10 μM (Figure 3C), treatment of F9 cells with CBB1003, 1004, and 1006–1008 led to the activation of CHRM4 and SCN3A expression (Figure 3C). Notably, we can detect the activation of CHRM4 and SCN3A expression as low as 0.5–1 μM of CBB1007 and 5–10 μM of CBB1003 (Figure 3D). Real-time quantitative RT-PCR analysis at various dose-response concentrations of CBB1003 and 1007 revealed that the IC50 of CBB1003 or CBB1007 is 8.45 μM or 3.74 μM, respectively (Figure 4A and B). We also found that LSD1 inhibitors can markedly induce the expression of genes for differentiation (Figure 4A), such as FOXA2 (37), suggesting that inhibition of LSD1 may induce pluripotent F9 cells to differentiate.
Figure 4.
LSD1 inhibitors induce the expression of differentiation genes and are selectively permeable to cells.
A–B. F9 were cultured with various concentrations of CBB1003 and 1007 as indicated for 24 hours. The expression of SCN3A, RM4, and FOXA2 was quantified by real-time RT-PCR. The concentrations of CBB1003 or 1007 in B were the same as in the left or right panels of A, respectively.
C. CBB1003 and 1007 are permeable to cells. CBB1002, CBB1003 and 1007 were incubated with F9 cells for 2 hours, using dimethyl sulfoxide (DMSO) as a control. After extensive washing of treated cells, the compounds were extracted and their presence (arrows) was analyzed by mass-spectrometry using pure compounds as a control.
LSD1 compounds selectively inhibit the growth of pluripotent teratocarcinoma, embryonic carcinoma, seminoma, and embryonic stem cells
LSD1 is highly conserved in high eukaryotes but it can only demethylate di- and mono-methylated H3K4 (5), while the demethylases of JARID1 family (1A–1D) that contain the Jumonji C (JmjC) domain can remove tri-, di, and mono-methylated H3K4 (3). In previous reports, loss of LSD1 by siRNA-mediated ablation in many cultured cells usually did not cause any obvious inhibition of cell growth (5, 15), presumably due to the presence of other H3K4 demethylases (JARID1A–1D) in the same cell that may functionally substitute for the activity of LSD1 to demethylate H3K4.
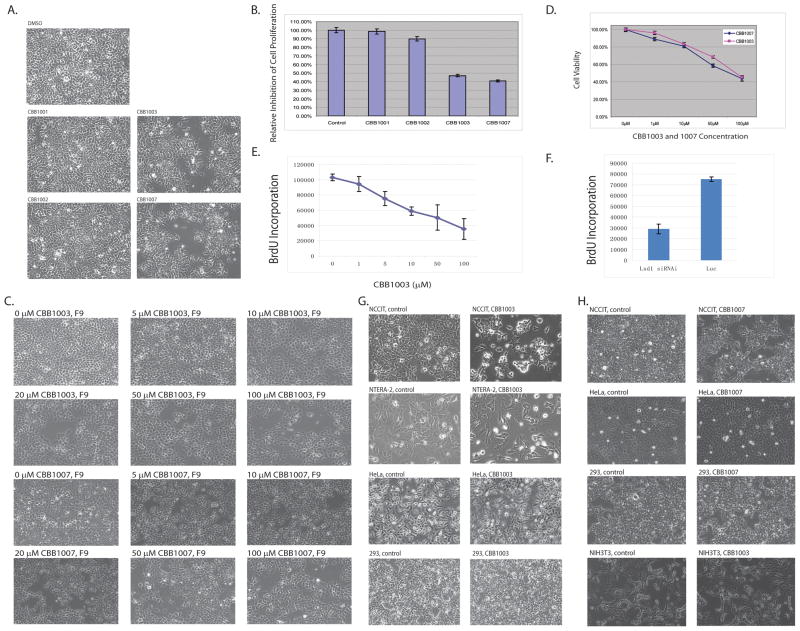
Strikingly, we have noticed that treatment of CBB1003 and CBB1007 led to significant growth inhibition of mouse embryonic teratocarcinoma F9 cells (Figure 5A–E), as analyzed by cell doublings, MTT proliferation assays, and bromodeoxyuridine (BrdU) incorporation. The growth inhibitory effects of CBB1003 and CBB1007 correlate with their ability to induce mono- and di-methylations of H3K4 and the activation of epigenetically suppressed gene expression (Figure 3–5), suggesting that the growth inhibition is a consequence of specific LSD1 inhibition in F9 cells. Since CBB1002 can effectively inhibit LSD1 activity in vitro with similar IC50 to that of CBB1003 and 1007, but it could not inhibit F9 cell growth and alter H3K4 methylation or gene expression in vivo, it is likely that only CBB1003 and 1007 can enter the cell while CBB1002 cannot. To test this possibility, we examined whether F9 cells can retain CBB1002, CBB1003 and 1007 after exposing to the compounds for 2 hours. We found that while CBB1002 is not detectable, both CBB1003 and 1007 can be readily found in F9 cells by mass-spectrometry. These results suggest that modification at the R position in the CBB chemical scaffold (Figure 1C and 1D) affects the cellular permeability of these compounds.
Figure 5.
LSD1 compounds selectively inhibit the growth of pluripotent embryonic carcinoma, teratocarcinoma and seminoma cells but not non-pluripotent cells.
A–B. Mouse F9 cells were treated with DMSO and 50 μM LSD1 compounds CBB1001–3 and 1007 for 30 hours and the cell numbers were counted. CBB1003 and 1007 significantly inhibit the growth of F9 cells but not CBB1001 and 1002. Percentage of compound-treated cells relative to the control is shown in B.
C. Dose-response of F9 cells to various concentrations of CBB1003 and 1007 as indicated for 30 hours.
D. Percentage of CBB1003- and 1007-treated cells relative to control cells, assayed by the MTT proliferation assay using indicated concentrations for 30 hours.
E. Inhibition of bromodexoyuridine (BrdU) incorporation in F9 cells treated with various indicated concentrations of CBB1003 for 24 hours.
F. Relative BrdU incorporation after treating F9 cells with 50 nM luciferase (Luc) and LSD1 siRNAs for 48 hours.
G and H. The growth of pluripotent NCCIT and NTERA-2 cancer cells was inhibited by 50 μM CBB1003 or CBB1007 for 30 hours but not non-pluripotent HeLa, 293, and NIH3T3 cells.
The inhibitory effects of CBB compounds on F9 cells also prompted us to examine whether LSD1 compounds can inhibit other cancer or normal cells. A screen of various cultured cell lines revealed that, consistent with previous reports (5, 15), many cultured cancer or established somatic cell lines, such as HeLa, 293, or NIH 3T3 (Figure 5G and 5H), did not display any significant growth inhibition after the treatment of CBB1003 or CBB1007, even at relatively high concentrations (100–200 μM).
The mouse F9 cell is a pluripotent embryonic stem cell-like teratocarcinoma cell because it expresses pluripotent stem cell markers Oct4 and Sox2, and Lin28. It also retains stem cell-like properties such as rapid spherical growth and the ability to differentiate (21, 24). Inoculation of F9 cells into the immunodeficient mouse can induce teratocarcinomas which can differentiate into a wide range of tissue types (24). However, HeLa, 293, and NIH3T3 cells do not express stem cell markers Oct4 and Sox2 and are incapable of differentiation and hence are considered non-stem cell lineage. To determine whether human teratocarcinoma cells or similar pluripotent germ cell tumors are also sensitive to LSD1 inhibitors, human pluripotent mediastinal mixed germ NCCIT cell, an intermediate cell between seminoma and embryonic carcinoma, and pluripotent human testicular embryonic carcinoma NTERA-2 cell were examined. We found that the growth of both NCCIT and NTERA-2 cells are highly sensitive to LSD1 inhibitors (Figure 5G and 5H). Since embryonic carcinoma and teratocarcinoma are derived from pluripotent embryonic stem (ES) cells, we also tested whether mouse ES cells are sensitive to LSD1 compounds. Treatment of mouse ES cells with CBB1003 and 1007 also led to substantial inhibition of the spherical growth of ES cells (Figure 6D). These studies indicate that embryonic carcinomas, teratocarcinoma, seminomas, possibly other stem cell-derived cells of pluripotent germ cell tumors (21), and ES cells are particularly sensitive to LSD1 inhibitors, suggesting that LSD1 plays an essential function in these pluripotent cancer cells or ES cells.
Figure 6.
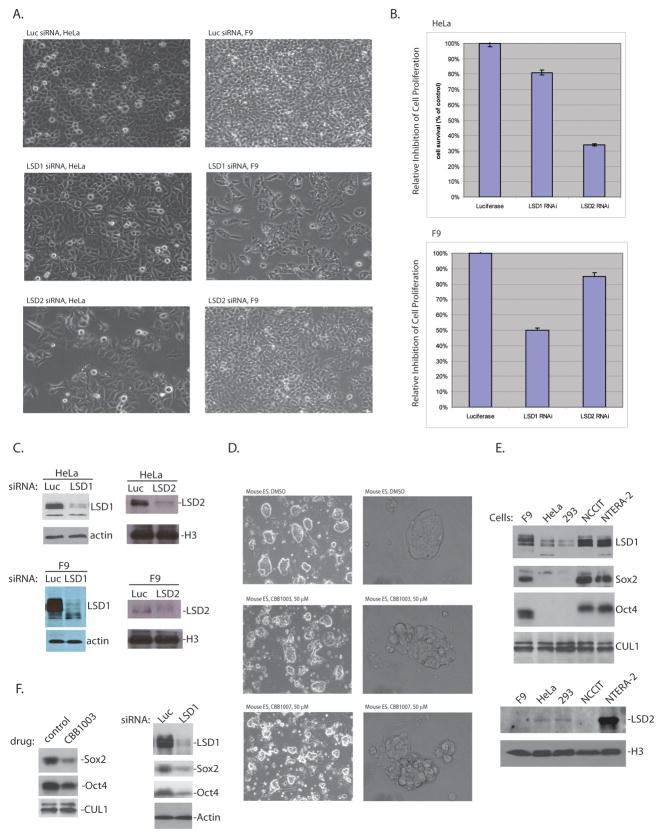
Inactivation of LSD1 blocks the growth of pluripotent F9 and ES cells but not HeLa cells.
A–C: HeLa and F9 cells were transfected with 50 nM of luciferase (Luc), LSD1 or LSD2 specific siRNAs for 48 hours. The cells were examined for growth inhibition (A and B) and for LSD1 and LSD2 protein levels by blotting with anti-LSD1 or LSD2 antibodies (C). Loss of LSD1 in F9 but not HeLa cells led to growth inhibition, while loss of LSD2 has an opposite effect on HeLa and F9 cells.
D. Mouse embryonic stem (ES) cells were treated with either DMSO or 50 μM CBB1003 or 1007 for 30 hours as indicated.
E. High protein levels of LSD1 in pluripotent F9, NCCIT, and NTERA-2 cells that also express Oct4 and Sox2 pluripotent stem cell markers. LSD2 expression was very low in F9 and NCCIT cells.
F. Effects of LSD1 inhibition or siRNA-based ablation on Sox2 and Oct4 expression.
LSD1 ablation selectively inhibits the growth of pluripotent cancer cells
To confirm the critical role of LSD1 in pluripotent cancer cells and the specificity of LSD1 inhibitors, we analyzed the effects of LSD1 ablation by siRNA in F9 and HeLa cells. After ablating LSD1 expression with a specific siRNA targeted at an mRNA region that is identical between mouse and human LSD1 genes, only F9 cells displayed marked inhibition of cell growth and BrdU incorporation but HeLa cells did not (Figure 6A–C, and 5F). We also ablated LSD2 by siRNA to determine whether it has any effects on the growth of these cells. While LSD1 demethylates the methylated H3K4 in certain promoter regions (1), LSD2 has a distinct function to demethylate mono- and di-methylated H3K4 in the highly transcribed coding region enriched with trimethylated of histone H3 at lysine 36 (H3K36)(6). We found that ablation of LSD2 by specific siRNAs caused the inhibition of HeLa cell proliferation but not that of F9 cells (Figure 6A–C). These observations suggest that LSD1 compounds are specifically target at LSD1, but not LSD2.
LSD1 expression is highly elevated in pluripotent cancer cells
To determine the mechanism by which pluripotent F9, NCCIT, and NTERA-2 cancer cells are selectively sensitive to LSD1 inhibition or inactivation, LSD1 protein levels were compared between these cells and HeLa and 293 cells. Our analysis revealed that F9, NCCIT, and NTERA-2 cells express high levels of LSD1 protein while the expression of LSD1 is much lower in HeLa and 293 cells (Figure 6E). The elevated levels of LSD1 are associated with the expression of pluripotent stem cell markers Oct4 and Sox2 (Figure 6E), which are not expressed in HeLa or 293 cells. In contrast, LSD2 is relatively highly expressed in HeLa, 293, and NTERA-2, but is very low in F9 and NCCIT cells (Figure 6E). Our observation suggests that the elevated levels of LSD1 may render F9, NCCIT, and NTERA-2 cancer cells more dependent on LSD1, which may consequently underlie the selective sensitivity of these pluripotent cancer cells towards the loss of LSD1. In addition, we also found that inactivation of LSD1 by treatment of LSD1 inhibitors or siRNAs caused the significant downregulation of Sox2 and Oct4 expression (Figure 6F), while the expression of other proteins, such as CUL1 or actin, was not altered. Thus, by regulating the stem-cell specific expression of Sox2 and Oct4 or probably other essential stem cell genes, LSD1 plays a unique and essential role in the proliferation of many stem cell-like pluripotent cancer cells.
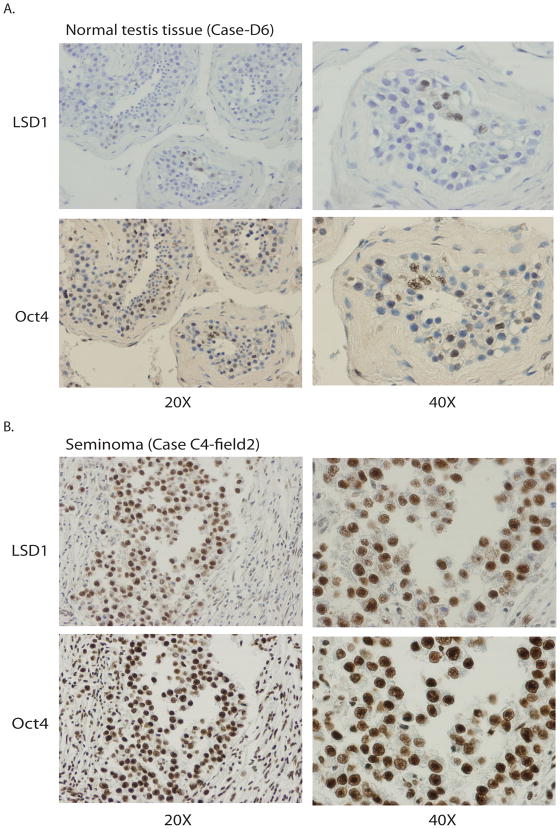
We also wondered whether LSD1 serves as a good protein marker for human teratocarcinomas, embryonic carcinomas, seminomas, and other pluripotent germ cell tumors in situ (21–23). By screening human seminoma tissue microarrays, we found that while normal human testicular tissues surrounding the tumors contain very low levels of LSD1 (Figure 7A), immuno-staining of tumor microarrays revealed that 6 out 6 human testicular seminomas (100%) displayed high levels of LSD1 protein (Figure 7B). These seminomas are likely to be pluripotent since they also express stem cell marker Oct4 (Figure 7B) (21–23). The marked elevation of LSD1 protein levels in human testicular seminomas is consistent with our observation that pluripotent F9, NCCIT, and NTERA-2 cancer cells also contain high levels of LSD1, Oct4, and Sox2 proteins and that these cells are highly sensitive to LSD1 inhibitors.
Figure 7.
Elevated LSD1 protein levels in human testicular seminomas that express Oct4.
A. LSD1 and Oct4 proteins are low or non-detectable in normal testis tissue. Immunohistological staining of human testicular normal tissues surrounding seminomas were stained with anti-LSD1 (top) or Oct4 (bottom) antibodies, three normal human testicular tissues were examined and all displayed low levels of LSD1 and Oct4. One of them is shown (Case D6).
B. Six human testicular seminomas were stained with anti-LSD1 and Oct4 antibodies. All of them displayed elevated protein levels of LSD1 and Oct4. One of the seminomas was shown (Case C4).
Discussion
In this report, we have developed a new class of chemical compounds that specifically inhibit histone demethylase LSD1. Unlike previous monoamine oxidase inhibitors which form a covalent chemical bond between FAD and the compounds and thus requires high structural constraints of the compounds (13, 14, 38), the new LSD1 inhibitors specifically interact with LSD1 and inhibit its activity without forming a covalent bond (Figure 1). The simplicity of compound synthesis and the low spatial requirement of compound interaction with LSD1 allow more flexible, efficient, and rapid chemical synthesis schemes for further diversified modifications to identify better compounds with high affinity, specificity, cell permeability, and stability. The development of our LSD1 inhibitory compounds thus represents a new strategy to inhibit the activity of LSD1 to modulate the epigenetic regulation of gene expression.
The specificity of new LSD1 inhibitors provides us a unique tool to explore the biological function of LSD1. By using these compounds as a probe, we unexpectedly found that inhibition of LSD1 selectively blocked the growth of pluripotent teratocarcinoma, embryonic carcinoma, seminoma, and ES cells, likely due to their highly elevated levels of LSD1 expression (Figures 5–7). Many tissue-specific cancer stem cells such as breast, ovarian, and lung cancer stem cells also express Oct4 and Sox2 (39–41). Whether these cancer stem cells are sensitive to our LSD1 inhibitors remains to be tested. One additional use of our novel LSD1 inhibitors is to remove teratomas/embryonic carcinomas during stem cell-based therapy. One major problem in stem/iPS cell-based therapy is the formation of embryonic carcinomas, teratomas, or teratocarcinomas by incomplete differentiation of ES/iPS cells in the organs of recipients (42). Removal of these tumors by LSD1 inhibitors may ensure the successful application of stem cell-based therapy. Our work thus suggests that LSD1 and methylation at H3K4 serve as novel targets for growth inhibition of stem cell-like tumors for cancer therapy. While epigenetic regulators constitute some appealing targets for therapeutic inhibition, this study reveals the first target for which cancer stem cell selectivity may be achievable.
Supplementary Material
Acknowledgments
This work was supported by grants from Hong Kong Polytechnic University (PolyU 5636/08M), Hong Kong Research Grants Council (PolyU 5638/07M), Fong Shu Fook Tong Foundation and Joyce M. Kuok Foundation, Shenzhen Bureau of Science, Technology and Information (JC200903160367A, ED200609150042A, and ZD200806180051A), National Natural Science Foundation of China (NSFC30971616), and National Institutes of Health (R01CA989550).
Footnotes
Financial Disclosure
This work was supported by grants from Hong Kong Polytechnic University (PolyU 5636/08M), Hong Kong Research Grants Council (PolyU 5638/07M), Fong Shu Fook Tong Foundation and Joyce M. Kuok Foundation to T. Y., and funds from Shenzhen Bureau of Science, Technology and Information (JC200903160367A, ED200609150042A, and ZD200806180051A), National Natural Science Foundation of China (NSFC30971616), and National Institutes of Health (R01CA989550) to H.Z.. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Shi Y. Histone lysine demethylases: emerging roles in development, physiology and disease. Nat Rev Genet. 2007;8:829–33. doi: 10.1038/nrg2218. [DOI] [PubMed] [Google Scholar]
- 2.Klose RJ, Zhang Y. Regulation of histone methylation by demethylimination and demethylation. Nat Rev Mol Cell Biol. 2007;8:307–18. doi: 10.1038/nrm2143. [DOI] [PubMed] [Google Scholar]
- 3.Agger K, Christensen J, Cloos PA, Helin K. The emerging functions of histone demethylases. Curr Opin Genet Dev. 2008;18:159–68. doi: 10.1016/j.gde.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Wysocka J, Swigut T, Milne TA, et al. WDR5 associates with histone H3 methylated at K4 and is essential for H3 K4 methylation and vertebrate development. Cell. 2005;121:859–72. doi: 10.1016/j.cell.2005.03.036. [DOI] [PubMed] [Google Scholar]
- 5.Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 6.Fang R, Barbera AJ, Xu Y, et al. Human LSD2/KDM1b/AOF1 regulates gene transcription by modulating intragenic H3K4me2 methylation. Mol Cell. 2010;39:222–33. doi: 10.1016/j.molcel.2010.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forneris F, Binda C, Battaglioli E, Mattevi A. LSD1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci. 2008;33:181–9. doi: 10.1016/j.tibs.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 8.Culhane JC, Cole PA. LSD1 and the chemistry of histone demethylation. Curr Opin Chem Biol. 2007;11:561–8. doi: 10.1016/j.cbpa.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Hevi S, Kurash JK, et al. The lysine demethylase LSD1 (KDM1) is required for maintenance of global DNA methylation. Nat Genet. 2009;41:125–9. doi: 10.1038/ng.268. [DOI] [PubMed] [Google Scholar]
- 10.Shilatifard A. Molecular implementation and physiological roles for histone H3 lysine 4 (H3K4) methylation. Curr Opin Cell Biol. 2008;20:341–8. doi: 10.1016/j.ceb.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte JH, Lim S, Schramm A, et al. Lysine-specific demethylase 1 is strongly expressed in poorly differentiated neuroblastoma: implications for therapy. Cancer Res. 2009;69:2065–71. doi: 10.1158/0008-5472.CAN-08-1735. [DOI] [PubMed] [Google Scholar]
- 12.Stavropoulos P, Hoelz A. Lysine-specific demethylase 1 as a potential therapeutic target. Expert Opin Ther Targets. 2007;11:809–20. doi: 10.1517/14728222.11.6.809. [DOI] [PubMed] [Google Scholar]
- 13.Szewczuk LM, Culhane JC, Yang M, Majumdar A, Yu H, Cole PA. Mechanistic analysis of a suicide inactivator of histone demethylase LSD1. Biochemistry. 2007;46:6892–902. doi: 10.1021/bi700414b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M, Culhane JC, Szewczuk LM, et al. Structural basis of histone demethylation by LSD1 revealed by suicide inactivation. Nat Struct Mol Biol. 2007;14:535–9. doi: 10.1038/nsmb1255. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Zhang H, Chen Y, et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–72. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 16.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–8. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 17.Singh SK, Hawkins C, Clarke ID, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- 18.Bapat SA, Mali AM, Koppikar CB, Kurrey NK. Stem and progenitor-like cells contribute to the aggressive behavior of human epithelial ovarian cancer. Cancer Res. 2005;65:3025–9. doi: 10.1158/0008-5472.CAN-04-3931. [DOI] [PubMed] [Google Scholar]
- 19.Maitland NJ, Collins A. A tumour stem cell hypothesis for the origins of prostate cancer. BJU Int. 2005;96:1219–23. doi: 10.1111/j.1464-410X.2005.05744.x. [DOI] [PubMed] [Google Scholar]
- 20.Bapat SA, Mishra GC. Stem cell pharmacogenomics: a reality check on stem cell therapy. Curr Opin Mol Ther. 2005;7:551–6. [PubMed] [Google Scholar]
- 21.Cheng L, Sung MT, Cossu-Rocca P, et al. OCT4: biological functions and clinical applications as a marker of germ cell neoplasia. J Pathol. 2007;211:1–9. doi: 10.1002/path.2105. [DOI] [PubMed] [Google Scholar]
- 22.Jones TD, Ulbright TM, Eble JN, Cheng L. OCT4: A sensitive and specific biomarker for intratubular germ cell neoplasia of the testis. Clin Cancer Res. 2004;10:8544–7. doi: 10.1158/1078-0432.CCR-04-0688. [DOI] [PubMed] [Google Scholar]
- 23.Jones TD, Ulbright TM, Eble JN, Baldridge LA, Cheng L. OCT4 staining in testicular tumors: a sensitive and specific marker for seminoma and embryonal carcinoma. Am J Surg Pathol. 2004;28:935–40. doi: 10.1097/00000478-200407000-00014. [DOI] [PubMed] [Google Scholar]
- 24.Strickland S, Smith KK, Marotti KR. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell. 1980;21:347–55. doi: 10.1016/0092-8674(80)90471-7. [DOI] [PubMed] [Google Scholar]
- 25.Mikkelsen TS, Hanna J, Zhang X, et al. Dissecting direct reprogramming through integrative genomic analysis. Nature. 2008;454:49–55. doi: 10.1038/nature07056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Takahashi K, Tanabe K, Ohnuki M, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–72. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 27.Forneris F, Binda C, Adamo A, Battaglioli E, Mattevi A. Structural basis of LSD1-CoREST selectivity in histone H3 recognition. J Biol Chem. 2007;282:20070–4. doi: 10.1074/jbc.C700100200. [DOI] [PubMed] [Google Scholar]
- 28.Morris GMea. Automated Docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–62. [Google Scholar]
- 29.DeLano WL. The PyMOL Molecular Graphics System. DeLano Scientific; Palo Alto, CA, USA: 2002. [Google Scholar]
- 30.Higa LA, Wu M, Ye T, Kobayashi R, Sun H, Zhang H. CUL4-DDB1 ubiquitin ligase interacts with multiple WD40-repeat proteins and regulates histone methylation. Nat Cell Biol. 2006;8:1277–83. doi: 10.1038/ncb1490. [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Gao C, Chai L, Ma Y. A novel SALL4/OCT4 transcriptional feedback network for pluripotency of embryonic stem cells. PLoS One. 2010;5:e10766. doi: 10.1371/journal.pone.0010766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karytinos A, Forneris F, Profumo A, et al. A novel mammalian flavin-dependent histone demethylase. J Biol Chem. 2009;284:17775–82. doi: 10.1074/jbc.M109.003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou X, Sun H, Chen H, et al. Hypoxia induces trimethylated H3 lysine 4 by inhibition of JARID1A demethylase. Cancer Res. 2010;70:4214–21. doi: 10.1158/0008-5472.CAN-09-2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motulsky HJ, Brown RE. Detecting outliers when fitting data with nonlinear regression - a new method based on robust nonlinear regression and the false discovery rate. BMC Bioinformatics. 2006;7:123. doi: 10.1186/1471-2105-7-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang M, Gocke CB, Luo X, et al. Structural basis for CoREST-dependent demethylation of nucleosomes by the human LSD1 histone demethylase. Mol Cell. 2006;23:377–87. doi: 10.1016/j.molcel.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Chen Y, Yang Y, Wang F, et al. Crystal structure of human histone lysine-specific demethylase 1 (LSD1) Proc Natl Acad Sci U S A. 2006;103:13956–61. doi: 10.1073/pnas.0606381103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nefzger CM, Haynes JM, Pouton CW. Directed expression of Gata2, Mash1, and Foxa2 synergize to induce the serotonergic neuron phenotype during in vitro differentiation of embryonic stem cells. Stem Cells. 29:928–39. doi: 10.1002/stem.640. [DOI] [PubMed] [Google Scholar]
- 38.Culhane JC, Szewczuk LM, Liu X, Da G, Marmorstein R, Cole PA. A mechanism-based inactivator for histone demethylase LSD1. J Am Chem Soc. 2006;128:4536–7. doi: 10.1021/ja0602748. [DOI] [PubMed] [Google Scholar]
- 39.Peng S, Maihle NJ, Huang Y. Pluripotency factors Lin28 and Oct4 identify a sub-population of stem cell-like cells in ovarian cancer. Oncogene. 2008;29:2153–9. doi: 10.1038/onc.2009.500. [DOI] [PubMed] [Google Scholar]
- 40.Hu T, Liu S, Breiter DR, Wang F, Tang Y, Sun S. Octamer 4 small interfering RNA results in cancer stem cell-like cell apoptosis. Cancer Res. 2008;68:6533–40. doi: 10.1158/0008-5472.CAN-07-6642. [DOI] [PubMed] [Google Scholar]
- 41.Karoubi G, Gugger M, Schmid R, Dutly A. OCT4 expression in human non-small cell lung cancer: implications for therapeutic intervention. Interact Cardiovasc Thorac Surg. 2009 doi: 10.1510/icvts.2008.193995. [DOI] [PubMed] [Google Scholar]
- 42.Yamanaka S. A fresh look at iPS cells. Cell. 2009;137:13–7. doi: 10.1016/j.cell.2009.03.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.