Abstract
The infiltration of human myometrium and cervix with leukocytes and the formation of a pro-inflammatory environment within the uterus have been associated with the initiation of both term and preterm parturition. The mechanism regulating the onset of this pro-inflammatory cascade is not fully elucidated. We demonstrate that prokineticin 1 (PROK1) is up-regulated in human myometrium and placenta during labor. The expression of PROK1 receptor remains unchanged during labor and is abundantly expressed in the myometrium. Gene array analysis identified 65 genes up-regulated by PROK1 in human myometrium, mainly cytokines and chemokines, including IL-1β, chemokine C-C motif ligand 3, and colony-stimulating factor 3. In addition, we demonstrate that PROK1 increases the expression of chemokine C-C motif ligand 20, IL-6, IL-8, prostaglandin synthase 2, and prostaglandin E2 and F2α secretion. The treatment of myometrial explants with 100 ng/mL of lipopolysaccharide up-regulates the expression of PROK1, PROK1 receptor, and inflammatory mediators. The infection of myometrial explants with lentiviral microRNA targeting PROK1, preceding treatment with lipopolysaccharide, reduces the expression of inflammatory genes. We propose that PROK1 is a novel inflammatory mediator that can contribute to the onset of human parturition at term and partially mediate premature onset of inflammatory pathways during bacterial infection.
Human parturition is a pro-inflammatory event associated with the influx of leukocytes, such as neutrophils and macrophages, to the uterus.1, 2 Indeed, 40% to 60% of genes up-regulated in the myometrium and cervix at labor are involved in inflammation and chemotaxis.3 Recruited leukocytes secrete cytokines, including IL-1β, IL-6, and IL-8,4 and prostaglandins E2 (PGE2) and F2α (PGF2α). These cytokines and prostaglandins can stimulate production of matrix metalloproteinases that are involved in membrane rupture5 and can also promote cervical ripening.6 However, during labor, the greatest influx of leukocytes is observed in the myometrium.1 Cytokines, such as IL-1β, released by leukocytes, promote the synthesis of prostaglandin synthase 2 (PTGS2)–dependent prostaglandins (PGE2 and PGF2α), which are important regulators of myometrial contractility.7, 8 More important, independently of prostaglandins, IL-1β also regulates basal and store-operated calcium entry in myometrial smooth muscle cells, which is important for contractions of individual smooth muscle cells.9
The same pro-inflammatory events can be initiated before 37 weeks of gestation and lead to premature onset of parturition and preterm birth. Nearly 11% of all singleton deliveries are preterm, and this constitutes the biggest cause of neonatal morbidity and mortality.10, 11 Although several factors, such as smoking, alcohol, advanced maternal age, and structural abnormalities of the cervix,12, 13 can contribute to preterm initiation of labor, bacterial infection in the uteroplacental unit is believed to be a major cause. Such infections are detected in 79% of births at 23 weeks and in 11% of births at 31 to 34 weeks.14, 15 In most cases of preterm birth, even in the absence of infection, histological evidence of inflammation is apparent in the uteroplacental unit.16, 17 Intrauterine inflammation is associated with adverse perinatal outcome.18 However, therapies for preterm birth have predominantly focused on inhibition of the onset of labor. Therefore, there is increasing recognition that effective therapies should target myometrial contractility and inhibit/reduce intrauterine inflammation.
Prokineticins 1 and 2 (PROK1 and PROK2, respectively) are multifunctional secreted proteins that signal via two G protein–coupled receptors termed PROK receptors 1 and 2 (PROKR1 and PROKR2, respectively). PROKs induce smooth muscle contractility of the gut,19, 20 which is mediated via increased calcium influx into smooth muscle cells.19, 20 PROKs also have potent effects in multiple other processes, including neurogenesis, angiogenesis, hematopoiesis, and nociception.21 There is increasing evidence that PROK1, via PROKR1, can modulate immune responses. PROK1 can promote differentiation of bone marrow progenitors into macrophage-like adherent cells.22 In addition, PROK1 induces the expression of chemokines, including chemokine C-C motif ligand 4 (CCL4), CXCL1, and IL-8 (CXCL8) in human monocytes, which constitutes an important regulatory mechanism for leukocyte recruitment to the site of inflammation.23
PROKs may be important regulators of female reproductive functions, including regulation of uterine receptivity24 and placental function.25, 26, 27 Considering their well-described roles in mediating inflammatory pathways and smooth muscle contractility, we hypothesized that PROK1, via PROKR1, may be important in regulating events leading to the onset of parturition. This study was designed to determine the expression and potential role of PROK1 and PROKR1 in the human myometrium during labor.
Materials and Methods
Patients and Tissue Collection
Full-thickness lower-segment biopsy specimens of human myometrium, placenta, and fetal membranes were collected from women undergoing elective caesarean section at term (>37 weeks) before the onset of labor (NL) and women in spontaneous labor at term (>37 weeks) who required emergency lower-segment caesarean section (L). Women with medical complications, such as diabetes or hypertension, or with symptoms of infection (determined by temperature >38°C) were excluded from the study. Patients were also excluded if the pregnancy was multiple or if they received either prostaglandin for induction or oxytocin for augmentation of labor. Written consent was obtained from each patient before recruitment, in accordance with approval from the Lothian Local Research Ethics Committee (REC reference no. 07/S1103/25). Immediately after collection, tissue was placed in RNAlater (Applied Biosystems, Warrington, UK) for RNA extraction, fixed in 4% neutral-buffered formalin, and wax embedded for immunohistochemistry (IHC).
Tissue Culture and Treatments
Myometrial tissue explants from women at term and not in labor were collected for in vitro studies. Tissue was routinely maintained in RPMI 1640 medium (GIBCO, Invitrogen, Paisley, UK) supplemented with 100 IU penicillin and 100 μg/mL streptomycin (Sigma, Poole, UK) at 37°C and 5% CO2, as recommended. After collection, explants were finely chopped into equal portions with scissors and incubated overnight. Tissue was treated with vehicle (sterile double-distilled water), 100 ng/mL of lipopolysaccharide (LPS) from Salmonella abortus equi (Alexis Biochemicals, Axxora, Nottingham, UK), or 40 nmol/L PROK1 (PeproTech, London, UK) for 2, 4, 6, 8, and 24 hours. In previous studies, the PROK1 and LPS doses used in this study have given optimal responses.28, 29 Tissue and supernatants were collected at all time points. All experiments were performed in duplicate.
Gene Array and Data Analysis
Total RNA was extracted from myometrial explants treated with either vehicle or 40 nmol/L PROK1 for 6 and 24 hours (n = 6 for each time point). Sample preparation, array hybridization, and data analysis were performed by the Finnish Microarray and Sequencing Centre and the Turku Centre for Biotechnology (Turku, Finland). Briefly, total RNA was biotin labeled by a single in vitro transcription amplification step. Samples were hybridized to Illumina Human HT-12 v.3 Expression BeadChips (Illumina, San Diego, CA) and stained with streptavadin-Cy3. Signal intensities within data sets were normalized by applying quantile normalization. R package Limma30 (Bioconductor, Open Source Software for Bioinformatics) was used for performing the statistical testing between the groups. Functional grouping of the gene list into statistically significant overrepresented ontologies was performed using the protein analysis through evolutionary relationships database analysis tools (PANTHER).31
TaqMan Quantitative Real-Time PCR
Tissue was homogenized in QIAzol (Qiagen, Crawley, UK), followed by RNA extraction with on-column DNase digestion using the RNeasy Mini Kit, according to the manufacturer's instructions (Qiagen). RNA was reverse transcribed using the VILO SuperScript VILO cDNA Synthesis Kit (Invitrogen). TaqMan quantitative real-time PCR was performed using an ABI Prism 7500 System (Applied Biosystems). The sequences of all primers and FAM-labeled probes are listed in Table 1. The expression of target genes was normalized to RNA loading, measured with primers and VIC-labeled probe for ribosomal 18S RNA. Relative gene expression was calculated by using the comparative CT method. Results of in vitro experiments are presented relative to expression in vehicle-treated samples. A comparison of gene expression in tissues collected from women in labor versus not in labor is presented as relative to a calibrator endometrial sample included in all reactions.28
Table 1.
Sequences of Primers and Probes Used for Analysis of Gene Expression by TaqMan Quantitative Real-Time PCR
| Gene | Sequence or category number |
|---|---|
| PROK1 | |
| Forward | 5′-GTGCCACCCGGGCAG-3′ |
| Reverse | 5′-AGCAAGGACAGGTGTGGTGC-3′ |
| FAM | 5′-ACAAGGTCCCCTTGTTCAGGAAACGCA-3′ |
| CCL20 | |
| Forward | 5′-TCCTGGCTGCTTTGATGTCA-3′ |
| Reverse | 5′-CCAAGACAGCAGTCAAAGTTGCT-3′ |
| FAM | 5′-TGCTGCTACTCCACCTCTGCGGC-3′ |
| PROKR1 | |
| Forward | 5′-TCTTACAATGGCGGTAAGTCCA-3′ |
| Reverse | 5′-CTCTTCGGTGGCAGGCAT-3′ |
| FAM | 5′-TGCAGACCTGGACCTCAAGACAATTGG-3′ |
| IL-1β | |
| Forward | 5′-CGCATCCAGCTACGAAT-3′ |
| Reverse | 5′-CATGGCACAACAACTGA-3′ |
| FAM | 5′-CGACCACCACTACAGC-3′ |
| IL-6 | |
| Forward | 5′-GCCGCCCCACACAGACA-3′ |
| Reverse | 5′-CCGTCGAGGATGTACGGAAT-3′ |
| FAM | 5′-CCACTCACCTCTTCAGAACGAATTGACAAAC-3′ |
| IL-8 | |
| Forward | 5′-CTGGCCGTGGCTCTCTT-3′ |
| Reverse | 5′-TTAGCACTCCTTGGCAAAACTG-3′ |
| FAM | 5′-CCTTCCTGATTTCTGCAGCTCTGTGTGAA-3′ |
| COX-2 | |
| Forward | 5′-CCTTCCTCCTGTGCCTGATG-3′ |
| Reverse | 5′-ACAATCTCATTTGAATCAGGAAGCT-3′ |
| FAM | 5′-TGCCCGACTCCCTTGGGTGTCA-3′ |
| 18S | |
| Forward | 5′-CGGCTACCACATCCAAGGAA-3′ |
| Reverse | 5′-GCTGGAATTACCGCGGCT-3′ |
| VIC | 5′-TGCTGGCACCAGACTTGCCCTC-3′ |
| CCL3 | Hs00234142-m1 |
| CSF3 | Hs99999083-m1 |
| VCAM1 | Hs01003372-m1 |
| TACC2 | Hs00610617-m1 |
| CCL2 | Hs00234140-m1 |
| CCL8 | Hs00271615-m1 |
IHC Data
Paraffin-embedded sections were dewaxed in xylene and dehydrated in graded ethanol. Antigen retrieval was performed by boiling for 5 minutes in 0.01 mol/L citrate buffer (pH 6.0), followed by quenching the activity of endogenous peroxidase with 30% H2O2 in methanol. Sections were blocked in a solution of 20% normal goat serum and 5% bovine serum albumin at room temperature, followed by incubation with rabbit anti-human PROK1 at 1:500 dilution (Phoenix Pharmaceuticals, Belmont, CA) or PROKR1 at 1:250 dilution (Life Span Biosciences, Atlanta, GA) overnight at 4°C; negative controls were incubated with rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA). Subsequently, sections were incubated with biotinylated goat anti-rabbit antibody (Vector Laboratories, Peterborough, UK) (1:500), followed by incubation with streptavidin–horseradish peroxidase (GE Healthcare UK, Little Chalfont) (1:1000). Staining was detected with 3,3′-diaminobenzidine (Vector Laboratories, Peterborough, UK) as the chromogen.
Lentivirus miRNA Gene Silencing
Lentivirus microRNA (miRNA) targeting PROK1 mRNA was used to knock down PROK1 expression, as previously described.28, 32 Briefly, myometrial explants (n = 8) were finely chopped and subsequently infected with lentivirus containing scrambled sequence (plenti6/V5-EmGFP-miR-neg) as a negative control or PROK1 miRNA (pLenti6/V5-EmGFP-hum-PROK1-72-287) chained constructs for 72 hours. Subsequently, explants were incubated in the presence or absence of 100 ng/mL of LPS for 24 hours. Tissue and supernatants were then harvested, and RNA was extracted from tissues for PCR analysis. Results are presented relative to expression in vehicle-treated samples.
Measurement of Prostaglandins PGE2 and PGF2α
Myometrium explants (n = 5) were incubated in the presence or absence of 40 nmol/L PROK1 for 24 hours. Medium was collected and assayed by enzyme-linked immunosorbent assay for PGE2 and PGF2α, as previously described.33 Results are presented as pg/mg tissue.
Measurement of IL-1β, CCL3, and CCL20
Myometrial explants were infected with lentiviral constructs containing scrambled or PROK1 miRNA and subsequently treated with 100 ng/mL of LPS for 24 hours, as previously described. Conditioned media were collected and assayed for IL-1β, CCL3, and CCL20 by using appropriate DuoSet ELISA Development Systems, according to the manufacturer's protocols (R&D Systems, Abingdon, UK). Briefly, 96-well microplates were coated overnight with 100 μL of working concentration of appropriate Capture Antibody diluted in PBS (1:180). The following day, the plates were blocked with 300 μL of 1% bovine serum albumin in PBS for 1 hour at room temperature. Subsequently, 100 μL of samples and appropriate standards were added for 2 hours, followed by incubation with Detection Antibody diluted (1:180) for 2 hours and then incubation with streptavidin–horseradish peroxidase (1:200) for 20 minutes. After the addition of TMB Substrate Solution, optical density was determined at 450 nm using a microplate reader. Results are presented relative to expression in vehicle-treated samples.
Statistical Analysis
Data were analyzed using an unpaired t-test with Welch's correction or a paired t-test (GraphPad Prism 5.0; GraphPad Software, La Jolla, CA). The n values represent the number of independent experiments. Unless otherwise indicated, data are given as mean ± SEM.
Results
Expression of PROK1 Increases in Uteroplacental Tissues at Labor
PROK1 mRNA expression is significantly elevated during labor (L) versus not labor (NL) in the myometrium (16.78 ± 7.0-fold versus 1.15 ± 0.37-fold; P < 0.05) and placenta (1.97 ± 0.56-fold versus 0.9 ± 0.17-fold; P < 0.05) but not in fetal membranes (Figure 1A). PROKR1 expression does not change at term with the onset of labor in any of the uteroplacental tissues. However, expression is highest in the myometrium compared with other uteroplacental tissues (P < 0.0001), suggesting that myometrium may be the main target tissue for PROK1 signaling during labor (Figure 1B).
Figure 1.
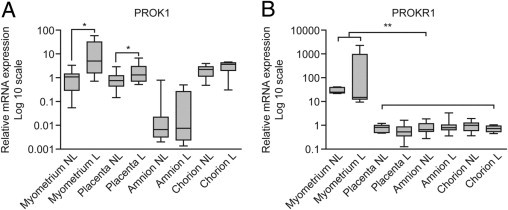
Expression of PROK1 and PROKR1 mRNA in human uteroplacental tissues at term. PROK1 mRNA expression is elevated in myometrium and placenta during labor at term compared with the tissues collected at term before the onset of labor (A). Levels of PROKR1 mRNA do not vary with the onset of labor; however, notably, PROKR1 levels are highest in myometrium compared with other uteroplacental tissues (B). Results are presented as mean ± SEM. *P < 0.05, **P < 0.0001 (n = 10 to 12).
We subsequently focused on the role of PROK1 and PROKR1 in human myometrium. During IHC, PROK1 was localized in the smooth muscle bundles in the myometrium of women at term before the onset of labor (Figure 2A) and during labor (Figure 2B). Similarly, PROKR1 was immunolocalized to the smooth muscle bundles in myometrial tissue collected from women at term before the onset of labor (Figure 2C) and during labor (Figure 2D). During labor, PROKR1 expression is additionally localized to the vasculature (Figure 2D).
Figure 2.
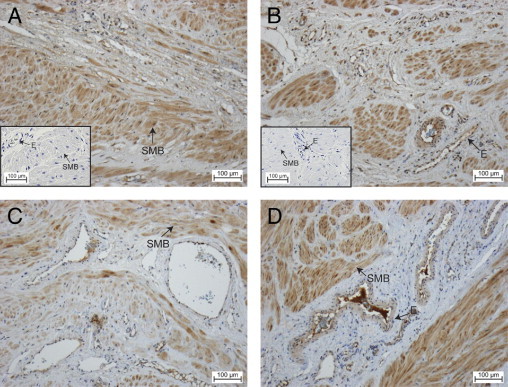
Immunolocalization of PROK1 and PROKR1 in myometrium collected from women at term before the onset of labor or during labor. PROK1 is localized to the smooth muscle bundles (A, not in labor; B, in labor). PROKR1 was also immunolocalized to the smooth muscle bundles in myometrial tissue collected from women not in labor (C) and in labor (D). During labor, PROKR1 expression is also localized to vasculature. Control sections were negative for immunoreactivity (insets). E, endothelium; SMB, smooth muscle bundles (n = 16: n = 8 for myometrium collected from women not in labor, and n = 8 for myometrium collected from women in labor).
PROK1/PROKR1 Regulates Inflammatory Mediators in Human Myometrium
After having established that PROK1 expression is elevated in the myometrium during labor and that PROKR1 transcript levels are highest in myometrial tissue, we sought to identify the downstream targets of PROK1/PROKR1 signaling in the myometrium. Gene array analysis was used to identify PROK1-regulated transcripts in myometrial explants at term (in six independent samples), treated with 40 nmol/L PROK1 for 6 or 24 hours and compared with vehicle treatment for each time point. The array analysis identified both up- and down-regulated transcripts at both time points (see Supplemental Figure S1, A and B, at http://ajp.amjpathol.org; http://www.ncbi.nlm.nih.gov/geo, Accession no. GSE28272), with an increase in the number of regulated transcripts from 6 to 24 hours (see Supplemental Figure S1C at http://ajp.amjpathol.org). The analysis shown there was a distinct response at 6 hours after PROK1 treatment compared with the response at 24 hours, although approximately 30% of the genes at 6 hours were common to 24-hour treatment, indicating a sustained regulation of a subset of genes. Genes were selected to verify the array analysis by real-time PCR. These genes were selected to represent high- and low-intensity signals and genes that were up- or down-regulated at either time point (Figure 3A). PCR verification confirmed the increased expression of CCL3 and CCL8 at 6 hours (Figure 3C) and IL-1β, CCL3, colony-stimulating factor 3 (CSF3), and vascular cell adhesion molecule 1 (VCAM1) at the 24-hour time point (Figure 3B). However, PCR did not validate regulation of transforming acidic coiled-coil–containing protein 2 and CCL2. The PCR validation permitted cutoff values to be established to generate a reliable list of 65 PROK1 regulated genes (Table 2). The gene list indicated that the response to PROK1 at 6 hours was mainly constituted of chemokine genes (7 of the 11 genes). Also, chemokines (ie, CCL3, CCL3L1, and CCL3L3) represented the genes that were regulated at both 6 and 24 hours after PROK1 treatment. The response to PROK1 treatment after 24 hours consisted mainly of chemokines and cytokines (ie, 22 of the 57 genes). A functional grouping of the gene list into statistically significant overrepresented ontologies revealed the immune response and related pathways to predominate, including macrophage activation and response to interferon-γ (Table 3).
Figure 3.
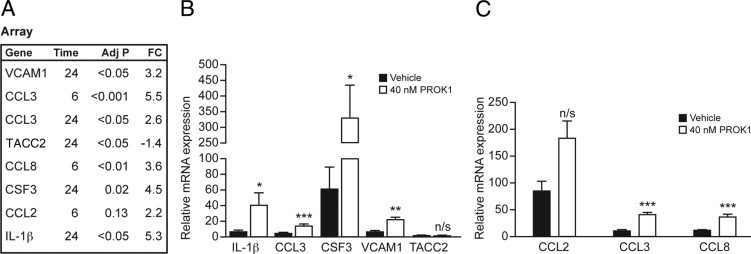
Validation of gene array analysis by quantitative real-time PCR. A: List of genes selected to verify the array analysis, including genes representing high- and low-intensity signals and genes that were up- or down-regulated at either the 6- or 24-hour time point. Adj, adjusted; FC, fold change. B: Treatment of myometrial explants with PROK1 for 24 hours up-regulates expression of IL-1β, CCL3, CSF3, and VCAM1, whereas levels of transforming acidic coiled-coil–containing protein 2 remained unaffected. C: Treatment of myometrial explants with 40 nmol/L PROK1 for 6 hours elevated expression of CCL3 and CCL8, whereas there was no difference in the expression of CCL2. *P < 0.05, **P < 0.005, and ***P < 0.0001. n/s, not significant (n = 6). Results are presented as mean ± SEM.
Table 2.
Differential Gene Expression of 65 Genes after PROK1 Treatment of Term Myometrial Explants
| Gene | Mean fold change | Adjusted P value | Time (hours) |
|---|---|---|---|
| ADAMDEC1 | 1.6 | 0.0741 | 24 |
| AMPD3 | 1.6 | 0.0861 | 24 |
| BDKRB1 | 1.9 | 0.0506 | 24 |
| C15orf48 | 3.2 | 0.0327 | 24 |
| C8orf4 | 1.6 | 0.0506 | 24 |
| CCL20 | 3.9 | 0.0709 | 24 |
| CCL3 | 5.5 | 0.0001 | 6 |
| CCL3 | 2.6 | 0.0296 | 24 |
| CCL3L1 | 3.6 | 0.0017 | 6 |
| CCL3L1 | 2.5 | 0.0327 | 24 |
| CCL3L3 | 4.9 | 0.0011 | 6 |
| CCL3L3 | 3.2 | 0.0249 | 24 |
| CCL4L1 | 2.6 | 0.0118 | 6 |
| CCL4L2 | 6.9 | 0.0000 | 6 |
| CCL8 | 3.6 | 0.0071 | 6 |
| CCR7 | 2.0 | 0.0969 | 24 |
| CD82 | 1.8 | 0.0420 | 24 |
| CSF3 | 4.5 | 0.0249 | 24 |
| CX3CL1 | 3.0 | 0.0100 | 6 |
| CXCL1 | 2.6 | 0.0249 | 24 |
| CXCL2 | 3.1 | 0.0474 | 24 |
| CXCL5 | 5.0 | 0.0000 | 24 |
| CXCL6 | 5.3 | 0.0062 | 24 |
| CYP27B1 | 3.0 | 0.0709 | 24 |
| ELL2 | 1.6 | 0.0085 | 24 |
| FBP1 | 1.8 | 0.0709 | 24 |
| FCGR2A | 1.6 | 0.0900 | 24 |
| G0S2 | 1.9 | 0.0841 | 24 |
| HCK | 1.8 | 0.0792 | 6 |
| IL1B | 5.3 | 0.0249 | 24 |
| IL1RN | 2.3 | 0.0861 | 24 |
| IL24 | 3.2 | 0.0581 | 24 |
| IL32 | 1.7 | 0.0280 | 24 |
| IL7R | 2.5 | 0.0177 | 24 |
| IRAK2 | 1.8 | 0.0474 | 24 |
| LILRB4 | 1.6 | 0.0177 | 24 |
| LTB | 2.7 | 0.0249 | 24 |
| MEOX1 | 2.6 | 0.0018 | 6 |
| MME | 1.6 | 0.0769 | 24 |
| MMP9 | 3.5 | 0.0313 | 24 |
| MT1H | 2.1 | 0.0275 | 24 |
| MUCL1 | 2.0 | 0.0799 | 24 |
| NAMPT | 1.7 | 0.0249 | 24 |
| NFKB2 | 1.5 | 0.0747 | 24 |
| NFKBIZ | 1.9 | 0.0707 | 24 |
| NRCAM | 1.5 | 0.0615 | 24 |
| OLR1 | 1.9 | 0.0387 | 24 |
| OSM | 1.9 | 0.0474 | 24 |
| PDE4B | 1.8 | 0.0327 | 24 |
| PPL | −1.6 | 0.0709 | 24 |
| PSME2 | 1.5 | 0.0480 | 24 |
| RCSD1 | 1.5 | 0.0383 | 24 |
| RND1 | 1.9 | 0.0172 | 6 |
| SELE | 6.0 | 0.0496 | 6 |
| SEMA4A | 1.7 | 0.0085 | 24 |
| SERPINB2 | 4.0 | 0.0085 | 24 |
| SERPINB7 | 3.5 | 0.0870 | 24 |
| SLC11A2 | 1.5 | 0.0730 | 24 |
| SLC39A14 | 1.6 | 0.0280 | 24 |
| SOCS1 | 1.5 | 0.0348 | 24 |
| SRGN | 1.7 | 0.0724 | 24 |
| TNFAIP8 | 1.6 | 0.0861 | 24 |
| TNFRSF6B | 1.7 | 0.0056 | 24 |
| TNIP1 | 1.6 | 0.0249 | 24 |
| UBD | 2.3 | 0.0709 | 24 |
| VCAM1 | 3.2 | 0.0474 | 24 |
| WNT5A | 1.6 | 0.0841 | 24 |
| ZC3H12A | 1.8 | 0.0673 | 24 |
The cutoff was established by quantitative PCR verification, a fold change >1.5, and an adjusted P value <0.1.
Table 3.
Over-represented PANTHER Ontologies
| Biological process | No. of genes in the category | No. of genes in the PROK1 list | No. of genes expected | P value |
|---|---|---|---|---|
| Immune response | 756 | 23 | 2.39 | 4.54 × 10-17 |
| Immune system process | 2628 | 36 | 8.32 | 2.63 × 10-16 |
| Response to stimulus | 1798 | 29 | 5.69 | 1.78 × 10-14 |
| Cell-cell signaling | 1331 | 25 | 4.21 | 8.34 × 10-14 |
| Cell surface receptor–linked signal transduction | 2235 | 30 | 7.07 | 6.25 × 10-13 |
| Signal transduction | 4191 | 39 | 13.26 | 2.58 × 10-12 |
| Cell communication | 4365 | 39 | 13.81 | 9.76 × 10-12 |
| Macrophage activation | 305 | 10 | 0.97 | 4.33 × 10-8 |
| Cellular process | 6258 | 40 | 19.80 | 1.67 × 10-7 |
| Cellular defense response | 457 | 10 | 1.45 | 1.71 × 10-6 |
| Intracellular signaling cascade | 1568 | 16 | 4.96 | 2.21 × 10-5 |
| Response to interferon-γ | 105 | 5 | 0.33 | 2.22 × 10-5 |
| Angiogenesis | 408 | 8 | 1.29 | 4.41 × 10-5 |
| B-cell–mediated immunity | 314 | 7 | 0.99 | 6.19 × 10-5 |
| Mesoderm development | 1528 | 14 | 4.83 | 2.50 × 10-4 |
| Response to external stimulus | 325 | 6 | 1.03 | 5.80 × 10-4 |
| Blood coagulation | 325 | 6 | 1.03 | 5.80 × 10-4 |
| Natural killer cell activation | 121 | 4 | 0.38 | 6.10 × 10-4 |
| Cell motion | 964 | 10 | 3.05 | 8.55 × 10-4 |
| Apoptosis | 966 | 9 | 3.06 | 3.27 × 10-3 |
| Cell-cell adhesion | 799 | 8 | 2.53 | 3.63 × 10-3 |
| System development | 2031 | 14 | 6.43 | 3.93 × 10-3 |
| Cell adhesion | 1333 | 10 | 4.22 | 8.77 × 10-3 |
PANTHER, protein analysis through evolutionary relationships.
Selected PROK1 up-regulated genes identified from the gene array list were analyzed for expression in term myometrium in L versus NL. The analysis demonstrated that the following cytokines are up-regulated during labor: IL-1β (L versus NL, 16.78 ± 7.0-fold versus 1.16 ± 0.37-fold; P < 0.05), CCL3 (L versus NL, 12.79 ± 5.23-fold versus 1.3 ± 0.33-fold; P < 0.05), and CSF3 (L versus NL, 66.7 ± 57.28-fold versus 0.06 ± 0.33-fold; P < 0.0001). There was no change in expression of VCAM1 (L versus NL, 10.36 ± 2.4-fold versus 13.04 ± 4.91-fold) or CCL8 (NL versus L, 13.45 ± 4.9-fold versus 27.82 ± 12.02-fold) (Figure 4). An elevation in expression of PROK1 at labor is included in the figure for comparison.
Figure 4.
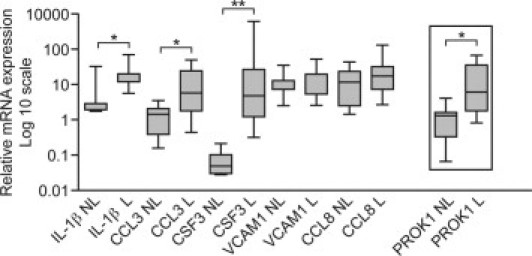
PROK1-regulated genes are elevated in myometrium during labor. The expression of genes that were demonstrated by gene array to be up-regulated in response to PROK1 was subsequently examined in term myometrium NL versus term myometrium L. Quantitative real-time PCR analysis demonstrated that IL-1β, CCL3, and CSF3 are up-regulated at labor, similar to PROK1, whereas there was no change in the level of VCAM1 and CCL8. *P < 0.05, **P < 0.0001 (n = 10). Results are presented as mean ± SEM.
There were several genes demonstrated by the gene array to be up-regulated in response to PROK1, such as PTGS2, IL-6, IL-8, and CCL20; however, they were less than cutoff values. Nevertheless, because they play an important role in the physiological characteristics of labor and have been previously demonstrated to be up-regulated in the myometrium during labor,3 we sought to confirm whether they are regulated by PROK1. We demonstrate that treatment with PROK1 resulted in up-regulation of mRNA levels for the following variables: PTGS2 (3.89 ± 1.38-fold over vehicle, P < 0.05), IL-6 (3.2 ± 0.76-fold over vehicle, P < 0.05), IL-8 (4.6 ± 1.4-fold over vehicle, P < 0.05), and CCL20 (6.27 ± 2.39-fold over vehicle, P < 0.05) (Figure 5A). We also demonstrate that the increase in PTGS2 transcript levels is reflected by an increase in the secretion of PTGS2-dependent prostaglandins: PGE2 (Figure 5B) [70.7 ± 17.1 versus 12.14 ± 2.28 pg/mL/mg tissue for PROK1 versus vehicle treatment; P < 0.05] and PGF2α (Figure 5C) (32.54 ± 10.9 versus 11.84 ± 4.22 pg/mL/mg tissue for PROK1 versus vehicle treatment; P < 0.05).
Figure 5.
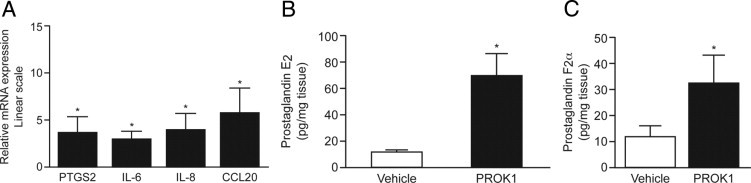
PROK1 up-regulates the expression of inflammatory mediators involved in term and preterm onset of parturition. Treatment of myometrial explants with 40 nmol/L PROK1 for 24 hours increased mRNA levels for PTGS2, IL-6, IL-8, and CCL20 (n = 8) (A). Treatment with 40 nmol/L PROK1 promoted secretion of PTGS2-dependent prostaglandins, PGE2 (B) and PGF2α (C), at 24 hours (n = 5). *P < 0.05. Results are presented as mean ± SEM.
LPS Increases Expression of PROK1 and PROKR1 in Term Myometrium
Subsequently, we examined whether treatment with a mimetic of infection, such as LPS, can regulate the expression of PROK1 and PROKR1. Treatment of term myometrial explants with 100 ng/mL LPS resulted in an increase in PROK1 mRNA at 2 hours (58.29 ± 30.25-fold over vehicle; P < 0.05) and PROKR1 mRNA at 6 hours (3.84 ± 1.6-fold over vehicle; P < 0.05) (Figure 6).
Figure 6.
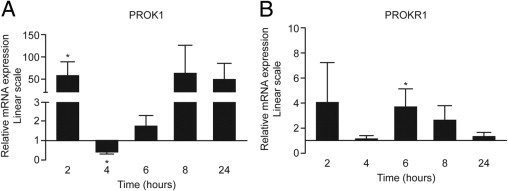
LPS promotes expression of PROK1 and PROKR1 in term myometrium. Myometrial explants collected from women at term, not in labor, were treated with 100 ng/mL LPS for 24 hours. Treatment with LPS up-regulated the mRNA level for PROK1 (A) and PROKR1 (B). *P < 0.05 (n = 8). Results are presented as mean ± SEM.
PROK1 Mediates LPS-Induced Inflammation in Term Myometrium
Several inflammatory mediators that are elevated during labor are also induced by bacterial infection.34, 35 After having demonstrated up-regulation of PROK1 and PROKR1 in response to LPS and PROK1-mediated regulation of inflammatory factors, we aimed to determine whether the pro-inflammatory effect of LPS is mediated via PROK1. Myometrial tissue was infected with lentiviral constructs containing a scrambled control miRNA sequence or a PROK1 chained miRNA sequence and subsequently treated with vehicle or 100 ng/mL of LPS. The expression of PROK1 was efficiently suppressed in myometrial tissue in response to infection with PROK1 miRNA versus scrambled miRNA (0.22 ± 0.07-fold versus 1.2 ± 0.39-fold; P < 0.05) (see Supplemental Figure S2 at http://ajp.amjpathol.org). The infection of myometrial tissue with PROK1 miRNA decreased the response to LPS: IL-1β (57.21 ± 17.87-fold after infection with scrambled miRNA versus 9.53 ± 4.23-fold after infection with PROK1 miRNA), CCL3 (16.6 ± 3.65-fold after infection with scrambled miRNA verus 5.9 ± 2.7-fold after infection with PROK1 miRNA), CSF3 (25.52 ± 18.81-fold after infection with scrambled miRNA versus 1.68 ± 0.53-fold after infection with PROK1 miRNA), and CCL20 (50.1 ± 3.65-fold after infection with scrambled miRNA versus 5.9 ± 2.4-fold after infection with PROK1 miRNA) (P < 0.05 for all genes) (Figure 7, A–D). However, there was no effect of PROK1 suppression on the level of PTGS2 (2.9 ± 0.74-fold after infection with scrambled miRNA versus 3.3 ± 1.84-fold after infection with PROK1 miRNA) (Figure 7E). As a negative control, we demonstrated that there was no effect of LPS on PTGS1 expression or when PROK1 was suppressed (Figure 7F). These data suggest that LPS-induced inflammation in term myometrium may be partially mediated via expression of PROK1.
Figure 7.
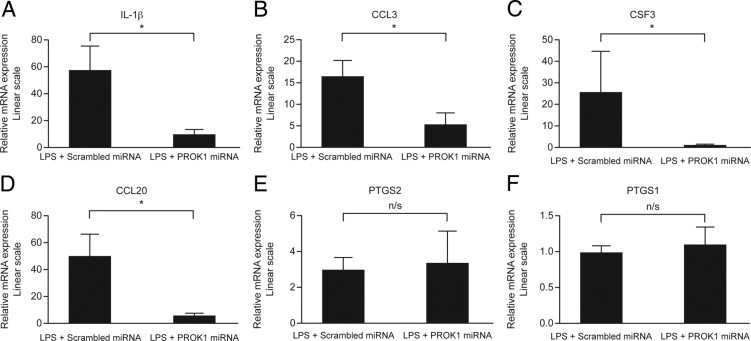
PROK1 mediates LPS-induced expression of inflammatory mediators in myometrium. Term myometrium was infected with lentivirus containing miRNA constructs targeting PROK1 or scrambled sequence. Infected explants were subsequently treated with 100 ng/mL of LPS for 24 hours. The expression of IL-1β (A), CCL3 (B), CSF3 (C), and CCL20 (D) in response to 100 ng/mL of LPS was significantly decreased by PROK1 miRNA constructs compared with the scrambled sequence. However, PROK1 suppression did not reduce the effect of LPS on PTGS2 expression (E). LPS treatment had no effect on PTGS1 expression, and this remained unaffected when PROK1 was suppressed (F). *P < 0.05. n/s, not significant (n = 8). Results are presented as mean ± SEM.
We subsequently investigated the effect of PROK1 suppression on secreted proteins in the supernatants of myometrial explant cultures. The treatment of myometrial explants with PROK1 miRNA reduced secretion of IL-β (13.92 ± 3.86-fold after infection with scrambled miRNA versus 0.58 ± 0.17-fold after infection with PROK1 miRNA), CCL3 (69.6 ± 26.23-fold after infection with scrambled miRNA versus 5.34 ± 1.76-fold after infection with PROK1 miRNA), and CCL20 (39.5 ± 22.25-fold after infection with scrambled miRNA versus 1.63 ± 0.97-fold after infection with PROK1 miRNA (P < 0.05 for all proteins) (Figure 8, A–C).
Figure 8.
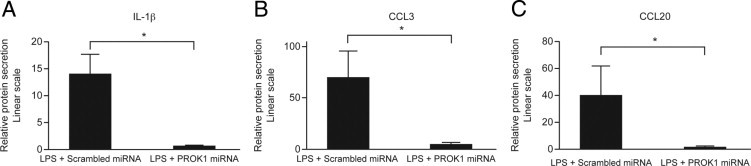
Targeting the PROK1 gene with miRNA reduces the secretion of inflammatory proteins in response to LPS. Term myometrium was infected with lentivirus containing miRNA constructs targeting PROK1 or a scrambled sequence. Infected explants were subsequently treated with either vehicle or 100 ng/mL of LPS for 24 hours. Results are presented relative to expression in vehicle-treated samples. IL-1β (A), CCL3 (B), and CCL20 (C) protein levels in culture supernatants after treatment with LPS were reduced in tissues infected with PROK1 miRNA compared with tissues infected with a scrambled sequence. *P < 0.05 (n = 5 to 7). Results are presented as mean ± SEM.
Discussion
Human parturition is well described as a pro-inflammatory event associated with recruitment of leukocytes, mainly neutrophils and macrophages, to the myometrium and cervix.1 Data indicate that 40% to 60% of genes up-regulated in these tissues at labor are involved in inflammation and chemotaxis.3 Recruited leukocytes secrete cytokines, such as IL-1β, IL-6, and IL-8, and prostaglandins PGE2 and PGF2α. These cytokines can stimulate production of matrix metalloproteinases that are involved in membrane rupture5 and can also promote cervical ripening.4 Although inflammatory events during parturition are well characterized, the underlying mechanism remains unknown.
Increasing evidence suggests that PROK1 can modulate immune responses. The expression of PROK1 and PROKR1 is increased at sites where inflammation occurs, and they are involved in mediating inflammatory pain.36, 37 PROK1 promoted differentiation of bone marrow progenitors into macrophage-like adherent cells in a mouse model and in human progenitor cells.22 PROK1 also induces expression of CCL4, CXCL1, and IL-8 in human monocytes, which is a part of an important regulatory mechanism for leukocyte recruitment to the site of inflammation.23 We have previously demonstrated that the expression of PROK1 and PROKR1 plays an important role during pregnancy.24 However, the expression of PROK1 and PROKR1 is also elevated during the third trimester in the placenta, where it regulates the expression of inflammatory mediators with established roles in the initiation of labor: IL-8 and PTGS2.25 This finding suggests that PROK1 may contribute to the induction of processes leading to the onset of parturition.
We demonstrate that the expression of PROK1 is significantly increased in term myometrium and placenta at labor compared with nonlaboring tissues. However, although not significantly affected by labor, the expression of PROKR1 is abundantly expressed in myometrium, suggesting that, within the uteroplacental unit, the myometrium is a major target tissue for PROK1 action.
Immunolocalization of PROKR1 demonstrated expression in term myometrium, predominantly in smooth muscle cells, confirming what we previously observed in nonpregnant myometrium.38 Because PROK1 was a potent inducer of contractility of guinea pig ileum,20 our results indicate that elevated expression of PROK1 in the myometrium, with the onset of labor, may regulate contractions of smooth muscle cells, resulting in uterine contractions during labor. This is further supported by the regulatory effect of PROK1 on expression of IL-1β demonstrated in this study. IL-1β plays a pivotal role in uterine contractility because it promotes an influx of calcium into uterine smooth muscle cells, enhancing contraction.9 In addition, IL-1β, via induction of PTGS2, stimulates production of prostaglandins that are well described to induce contractions of the uterus.7, 8 More important, our results demonstrate that PROK1 up-regulates expression of PTGS2 and secretion of PGE2 and PGF2α. It is possible that this occurs directly via PROK1 regulation of PTGS2 expression24 and/or indirectly via prior induction of IL-1β. These data warrant further research to confirm a role of PROK1/PROKR1 in myometrial contractility.
By using microarray gene expression analysis, we examined the effect of PROK1 on gene expression in myometrial tissue before the onset of labor at term. We identified 65 genes to be regulated by PROK1 in the myometrium, most of which are cytokines and chemokines. A functional grouping of these genes revealed that immune response and related pathways are predominantly regulated by PROK1, including IL-1β, CCL3, CSF3, CCL20, IL-6, IL-8, and PTGS2, which have been previously up-regulated in human myometrium and cervix during labor.3 Such a pronounced effect of PROK1 on immune pathways suggests that PROK1 may regulate recruitment of leukocytes to the myometrium at term and their activation and, thus, contributes to the initiation of parturition.
IL-1β, IL-8, and IL-6 may constitute an initial pro-inflammatory stimulus that then further establishes an inflammatory microenvironment in the uterus by activating local cells to secrete chemoattractants or effector molecules, such as prostaglandins or matrix metalloproteinases.39, 40 IL-8, which in gestational tissues is mainly stimulated by IL-1β, is a potent chemoattractant and activating factor, mainly for neutrophils.41, 42 Its concentrations correlate with the number of neutrophils and macrophages in the lower segment of the myometrium and cervix.2, 43 The main role of IL-8 during parturition may be to maintain neutrophil numbers in gestational tissues and disseminate a pro-inflammatory state.40 As labor progresses, the initial neutrophil influx may be followed by monocyte recruitment, which is believed to be regulated by IL-6.3, 44 Monocytes further promote inflammation by producing chemokines, such as CCL2, CCL3, CCL8, or CCL20, that are involved in further recruitment of leukocytes.40
During parturition, the absolute and percentage numbers of circulating neutrophils and monocytes are increased, indicating that proliferation of these cells is enhanced during labor.45 Our results suggest that PROK1 may also regulate this process. In addition to regulation of IL-8 and IL-6, we demonstrate that PROK1 up-regulates the expression of CSF3. The main function of CSF3 is to stimulate bone marrow to produce and mobilize neutrophils to the bloodstream and initiate their differentiation.46, 47 This is vital for the physiological features of labor, which is well associated with an influx of neutrophils to the myometrium and cervix.
In preterm birth, the pro-inflammatory environment is initiated prematurely. Although several factors, such as smoking, alcohol, advanced maternal age, and structural abnormalities of the cervix,12, 13 can contribute to preterm initiation of labor; bacterial infections account for nearly 25% of all incidents of preterm deliveries and they are detected in 79% of births at 23 weeks and in 11% of births at 31 to 34 weeks.14, 15 More important, antimicrobial therapies with antibiotics have had little or no benefit.15 In most cases, even though intrauterine infections are polymicrobial, Escherichia coli and LPS demonstrate the most robust effect on concentration of prostaglandins PGE2 and PGF2α and cytokines, such as IL-1β, IL-6, and IL-8.48 In most cases, evidence of intrauterine infection is not apparent or underlying infection is asymptomatic; nevertheless, histological evidence of inflammation is present in the decidua, fetal membranes, and umbilical cord.16, 17, 49, 50 Therefore, targeting downstream inflammation could constitute a novel and beneficial solution to prevent bacterially induced preterm births.
In this study, we demonstrate that several genes regulated by PROK1 increase during parturition both term and preterm and are associated with intrauterine bacterial infection.34, 35, 51 Considering the induction of PROK1 and PROKR1 by LPS, we suggest that the role of PROK1 in inducing a pro-inflammatory response may contribute to the activation of inflammatory events and preterm birth associated with bacterial infections.
Further evidence to support a role of PROK1 in mediating an inflammatory response was shown using a mimetic of infection, LPS. We selected three genes that were demonstrated by the gene array to be the most responsive to PROK1 (IL-1β, CCL3, and CFS) and also included CCL20 and PTGS2. The study demonstrated that LPS induction of pro-inflammatory genes CCL20, CCL3, CFS, and IL-1β is decreased by inhibiting PROK1 gene expression. LPS may regulate the expression of these genes in the human myometrium via toll-like receptor 2, which is similar to PROK1 and localizes to smooth muscle bundles.52
Our study demonstrates a novel role for PROK1 in regulating a wide range of inflammatory mediators in human myometrial tissue, which plays a pivotal role in the physiological characteristics of labor. We propose that PROK1 regulates inflammatory processes, in particular those involved in the recruitment of leukocytes to the myometrium, which, in turn, contribute to the onset of parturition at term. In addition, PROK1 may meditate the inflammatory cascade in myometrium during infection, resulting in premature initiation of labor. Therefore, targeting PROK1 signaling could provide novel and efficient therapy by reducing the inflammatory response during premature initiation of labor and, hence, improve neonatal survival.
Acknowledgments
We thank the research team, supported by Jennifer Brown and Tommy's charities, for tissue collection.
Footnotes
Supported by the Medical Research Council, Piggy Bank Kids.
M.R.G. and R.D.C. contributed equally to this work.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.08.029.
Supplementary data
Microarray data analysis of myometrial explants treated with PROK1 for 6 and 24 hours. Microarray plots showing the relationship between mean fold changes and average intensities in the compared groups. PROK1 versus vehicle at 6 hours (P6 versus V6) (A) and 24 hours (P24 versus V24) (B). The log2-transformed fold change is marked on the y axis (M), and the log2-transformed average intensity of the compared groups is marked on the x axis (A). The differentially expressed features are red, and the filtering threshold for the fold change (1.4-fold on a log2 scale) is marked with the dashed line. C: Venn diagram illustrating the number of differentially expressed genes between treatments and a summary of overlapping genes between treatments.
Analysis of efficiency of PROK1 suppression. Myometrial explants were infected with lentivirus containing scrambled miRNA control or chained PROK1 miRNA construct. Quantitative real-time PCR analysis of PROK1 mRNA demonstrated lower expression of PROK1 in explants infected with PROK1 miRNA compared with explants infected with a scrambled control sequence. *P < 0.05 (n = 8). Results are presented as relative to a calibrator sample.
References
- 1.Thomson A.J., Telfer J.F., Young A., Campbell S., Stewart C.J., Cameron I.T., Greer I.A., Norman J.E. Leukocytes infiltrate the myometrium during human parturition: further evidence that labor is an inflammatory process. Hum Reprod. 1999;14:229–236. [PubMed] [Google Scholar]
- 2.Osman I., Young A., Ledingham M.A., Thomson A.J., Jordan F., Greer I.A., Norman J.E. Leukocyte density and pro-inflammatory cytokine expression in human fetal membranes, decidua, cervix and myometrium before and during labor at term. Mol Hum Reprod. 2003;9:41–45. doi: 10.1093/molehr/gag001. [DOI] [PubMed] [Google Scholar]
- 3.Bollapragada S., Youssef R., Jordan F., Greer I., Norman J., Nelson S. Term labor is associated with a core inflammatory response in human fetal membranes, myometrium, and cervix. Am J Obstet Gynecol. 2009;200:104e101–104e111. doi: 10.1016/j.ajog.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 4.Young A., Thomson A.J., Ledingham M., Jordan F., Greer I.A., Norman J.E. Immunolocalization of proinflammatory cytokines in myometrium, cervix, and fetal membranes during human parturition at term. Biol Reprod. 2002;66:445–449. doi: 10.1095/biolreprod66.2.445. [DOI] [PubMed] [Google Scholar]
- 5.Vega-Sanchez R., Gomez-Lopez N., Flores-Pliego A., Clemente-Galvan S., Estrada-Gutierrez G., Zentella-Dehesa A., Maida-Claros R., Beltran-Montoya J., Vadillo-Ortega F. Placental blood leukocytes are functional and phenotypically different than peripheral leukocytes during human labor. J Reprod Immunol. 2010;84:100–110. doi: 10.1016/j.jri.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Stygar D., Wang H., Vladic Y.S., Ekman G., Eriksson H., Sahlin L. Increased level of matrix metalloproteinases 2 and 9 in the ripening process of the human cervix. Biol Reprod. 2002;67:889–894. doi: 10.1095/biolreprod.102.005116. [DOI] [PubMed] [Google Scholar]
- 7.Rauk P.N., Chiao J.P. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol. 2000;43:152–159. doi: 10.1111/j.8755-8920.2000.430304.x. [DOI] [PubMed] [Google Scholar]
- 8.Zaragoza D.B., Wilson R.R., Mitchell B.F., Olson D.M. The interleukin 1beta-induced expression of human prostaglandin F2alpha receptor messenger RNA in human myometrial-derived ULTR cells requires the transcription factor, NFkappaB. Biol Reprod. 2006;75:697–704. doi: 10.1095/biolreprod.106.053439. [DOI] [PubMed] [Google Scholar]
- 9.Tribe R.M., Moriarty P., Dalrymple A., Hassoni A.A., Poston L. Interleukin-1beta induces calcium transients and enhances basal and store operated calcium entry in human myometrial smooth muscle. Biol Reprod. 2003;68:1842–1849. doi: 10.1095/biolreprod.102.011403. [DOI] [PubMed] [Google Scholar]
- 10.Norman J.E., Morris C., Chalmers J. The effect of changing patterns of obstetric care in Scotland (1980–2004) on rates of preterm birth and its neonatal consequences: perinatal database study. PLoS Med. 2009;6:e1000153. doi: 10.1371/journal.pmed.1000153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heron M., Hoyert D.L., Murphy S.L., Xu J., Kochanek K.D., Tejada-Vera B. Deaths: final data for 2006. Natl Vital Stat Rep. 2009;57:1–134. [PubMed] [Google Scholar]
- 12.Goldenberg R.L., Culhane J.F., Iams J.D., Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Norman J.E. Preterm labor: cervical function and prematurity. Best Pract Res Clin Obstet Gynaecol. 2007;21:791–806. doi: 10.1016/j.bpobgyn.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 14.Onderdonk A.B., Hecht J.L., McElrath T.F., Delaney M.L., Allred E.N., Leviton A. Colonization of second-trimester placenta parenchyma. Am J Obstet Gynecol. 2008 doi: 10.1016/j.ajog.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]; 52e51-52e10199
- 15.Muglia L.J., Katz M. The enigma of spontaneous preterm birth. N Engl J Med. 2010;362:529–535. doi: 10.1056/NEJMra0904308. [DOI] [PubMed] [Google Scholar]
- 16.Goldenberg R.L., Hauth J.C., Andrews W.W. Intrauterine infection and preterm delivery. N Engl J Med. 2000;342:1500–1507. doi: 10.1056/NEJM200005183422007. [DOI] [PubMed] [Google Scholar]
- 17.Smith R. Parturition. N Engl J Med. 2007;356:271–283. doi: 10.1056/NEJMra061360. [DOI] [PubMed] [Google Scholar]
- 18.Lee S.E., Romero R., Jung H., Park C.W., Park J.S., Yoon B.H. The intensity of the fetal inflammatory response in intraamniotic inflammation with and without microbial invasion of the amniotic cavity. Am J Obstet Gynecol. 2007;197:294.e1–294.e6. doi: 10.1016/j.ajog.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Schweitz H., Pacaud P., Diochot S., Moinier D., Lazdunski M. MIT(1), a black mamba toxin with a new and highly potent activity on intestinal contraction. FEBS Lett. 1999;461:183–188. doi: 10.1016/s0014-5793(99)01459-3. [DOI] [PubMed] [Google Scholar]
- 20.Li M., Bullock C.M., Knauer D.J., Ehlert F.J., Zhou Q.Y. Identification of two prokineticin cDNAs: recombinant proteins potently contract gastrointestinal smooth muscle. Mol Pharmacol. 2001;59:692–698. doi: 10.1124/mol.59.4.692. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Q.Y., Meidan R. Biological function of prokineticins. Results Probl Cell Differ. 2008;46:181–199. doi: 10.1007/400_2007_053. [DOI] [PubMed] [Google Scholar]
- 22.Dorsch M., Qiu Y., Soler D., Frank N., Duong T., Goodearl A., O'Neil S., Lora J., Fraser C.C. PK1/EG-VEGF induces monocyte differentiation and activation. J Leukoc Biol. 2005;78:426–434. doi: 10.1189/jlb.0205061. [DOI] [PubMed] [Google Scholar]
- 23.Monnier J., Quillien V., Piquet-Pellorce C., Leberre C., Preisser L., Gascan H., Samson M. Prokineticin 1 induces CCL4, CXCL1 and CXCL8 in human monocytes but not in macrophages and dendritic cells. Eur Cytokine Netw. 2008;19:166–175. doi: 10.1684/ecn.2008.0138. [DOI] [PubMed] [Google Scholar]
- 24.Evans J., Catalano R.D., Morgan K., Critchley H.O., Millar R.P., Jabbour H.N. Prokineticin 1 signaling and gene regulation in early human pregnancy. Endocrinology. 2008;149:2877–2887. doi: 10.1210/en.2007-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denison F.C., Battersby S., King A.E., Szuber M., Jabbour H.N. Prokineticin-1: a novel mediator of the inflammatory response in third-trimester human placenta. Endocrinology. 2008;149:3470–3477. doi: 10.1210/en.2007-1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann P., Feige J.J., Alfaidy N. Expression and oxygen regulation of endocrine gland-derived vascular endothelial growth factor/prokineticin-1 and its receptors in human placenta during early pregnancy. Endocrinology. 2006;147:1675–1684. doi: 10.1210/en.2005-0912. [DOI] [PubMed] [Google Scholar]
- 27.Hoffmann P., Saoudi Y., Benharouga M., Graham C.H., Schaal J.P., Mazouni C., Feige J.J., Alfaidy N. Role of EG-VEGF in human placentation: physiological and pathological implications. J Cell Mol Med. 2009;13:2224–2235. doi: 10.1111/j.1582-4934.2008.00554.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evans J., Catalano R.D., Brown P., Sherwin R., Critchley H.O., Fazleabas A.T., Jabbour H.N. Prokineticin 1 mediates fetal-maternal dialogue regulating endometrial leukemia inhibitory factor. FASEB J. 2009;23:2165–2175. doi: 10.1096/fj.08-124495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maldonado-Pérez D., Golightly E., Denison F.C., Jabbour H.N., Norman J.E. A role for lipoxin A4 as anti-inflammatory and proresolution mediator in human parturition. FASEB J. 2011;25:569–575. doi: 10.1096/fj.10-170340. [DOI] [PubMed] [Google Scholar]
- 30.Gentleman R. Springer; New York, Cambridge: 2005. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. pp xix, 473. [Google Scholar]
- 31.Thomas P.D., Kejariwal A., Guo N., Mi H., Campbell M.J., Muruganujan A., Lazareva-Ulitsky B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic Acids Res. 2006;34:W645–W650. doi: 10.1093/nar/gkl229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook I.H., Evans J., Maldonado-Pérez D., Critchley H.O., Sales K.J., Jabbour H.N. Prokineticin-1 (PROK1) modulates interleukin (IL)-11 expression via prokineticin receptor 1 (PROKR1) and the calcineurin/NFAT signalling pathway. Mol Hum Reprod. 2010;16:158–169. doi: 10.1093/molehr/gap084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Denison F.C., Grant V.E., Calder A.A., Kelly R.W. Seminal plasma components stimulate interleukin-8 and interleukin-10 release. Mol Hum Reprod. 1999;5:220–226. doi: 10.1093/molehr/5.3.220. [DOI] [PubMed] [Google Scholar]
- 34.Calhoun D.A., Chegini N., Polliotti B.M., Gersting J.A., Miller R.K., Christensen R.D. Granulocyte colony-stimulating factor in preterm and term pregnancy, parturition, and intra-amniotic infection. Obstet Gynecol. 2001;97:229–234. doi: 10.1016/s0029-7844(00)01120-0. [DOI] [PubMed] [Google Scholar]
- 35.Hamill N., Romero R., Gotsch F., Kusanovic J.P., Edwin S., Erez O., Than N.G., Mittal P., Espinoza J., Friel L.A., Vaisbuch E., Mazaki-Tovi S., Hassan S.S. Exodus-1 (CCL20): evidence for the participation of this chemokine in spontaneous labor at term, preterm labor, and intrauterine infection. J Perinat Med. 2008;36:217–227. doi: 10.1515/JPM.2008.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Negri L., Lattanzi R., Giannini E., Metere A., Colucci M., Barra D., Kreil G., Melchiorri P. Nociceptive sensitization by the secretory protein Bv8. Br J Pharmacol. 2002;137:1147–1154. doi: 10.1038/sj.bjp.0704995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Negri L., Lattanzi R., Giannini E., Colucci M., Margheriti F., Melchiorri P., Vellani V., Tian H., De Felice M., Porreca F. Impaired nociception and inflammatory pain sensation in mice lacking the prokineticin receptor PKR1: focus on interaction between PKR1 and the capsaicin receptor TRPV1 in pain behavior. J Neurosci. 2006;26:6716–6727. doi: 10.1523/JNEUROSCI.5403-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Battersby S., Critchley H.O., Morgan K., Millar R.P., Jabbour H.N. Expression and regulation of the prokineticins (endocrine gland-derived vascular endothelial growth factor and Bv8) and their receptors in the human endometrium across the menstrual cycle. J Clin Endocrinol Metab. 2004;89:2463–2469. doi: 10.1210/jc.2003-032012. [DOI] [PubMed] [Google Scholar]
- 39.Arntzen K.J., Kjollesdal A.M., Halgunset J., Vatten L., Austgulen R. TNF, IL-1, IL-6, IL-8 and soluble TNF receptors in relation to chorioamnionitis and premature labor. J Perinat Med. 1998;26:17–26. doi: 10.1515/jpme.1998.26.1.17. [DOI] [PubMed] [Google Scholar]
- 40.Gomez-Lopez N., Laresgoiti-Servitje E., Olson D.M., Estrada-Gutierrez G., Vadillo-Ortega F. The role of chemokines in term and premature rupture of the fetal membranes: a review. Biol Reprod. 2010;82:809–814. doi: 10.1095/biolreprod.109.080432. [DOI] [PubMed] [Google Scholar]
- 41.Cherouny P.H., Pankuch G.A., Romero R., Botti J.J., Kuhn D.C., Demers L.M., Appelbaum P.C. Neutrophil attractant/activating peptide-1/interleukin-8: association with histologic chorioamnionitis, preterm delivery, and bioactive amniotic fluid leukoattractants. Am J Obstet Gynecol. 1993;169:1299–1303. doi: 10.1016/0002-9378(93)90297-v. [DOI] [PubMed] [Google Scholar]
- 42.Dudley D.J., Trautman M.S., Mitchell M.D. Inflammatory mediators regulate interleukin-8 production by cultured gestational tissues: evidence for a cytokine network at the chorio-decidual interface. J Clin Endocrinol Metab. 1993;76:404–410. doi: 10.1210/jcem.76.2.8432783. [DOI] [PubMed] [Google Scholar]
- 43.Elliott C.L., Slater D.M., Dennes W., Poston L., Bennett P.R. Interleukin 8 expression in human myometrium: changes in relation to labor onset and with gestational age. Am J Reprod Immunol. 2000;43:272–277. doi: 10.1111/j.8755-8920.2000.430505.x. [DOI] [PubMed] [Google Scholar]
- 44.Haddad R., Tromp G., Kuivaniemi H., Chaiworapongsa T., Kim Y.M., Mazor M., Romero R. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol. 2006;195:394.e1–394.e24. doi: 10.1016/j.ajog.2005.08.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yuan M., Jordan F., McInnes I.B., Harnett M.M., Norman J.E. Leukocytes are primed in peripheral blood for activation during term and preterm labor. Mol Hum Reprod. 2009;15:713–724. doi: 10.1093/molehr/gap054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nguyen Y.K. Granulocyte colony stimulating factor. J Fla Med Assoc. 1994;81:467–469. [PubMed] [Google Scholar]
- 47.Whalen M.J., Carlos T.M., Wisniewski S.R., Clark R.S., Mellick J.A., Marion D.W., Kochanek P.M. Effect of neutropenia and granulocyte colony stimulating factor-induced neutrophilia on blood-brain barrier permeability and brain edema after traumatic brain injury in rats. Crit Care Med. 2000;28:3710–3717. doi: 10.1097/00003246-200011000-00029. [DOI] [PubMed] [Google Scholar]
- 48.Menon R., Peltier M.R., Eckardt J., Fortunato S.J. Diversity in cytokine response to bacteria associated with preterm birth by fetal membranes. Am J Obstet Gynecol. 2009;201:306.e1–306.e6. doi: 10.1016/j.ajog.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 49.Romero R., Schaudinn C., Kusanovic J.P., Gorur A., Gotsch F., Webster P., Nhan-Chang C.L., Erez O., Kim C.J., Espinoza J., Gonçalves L.F., Vaisbuch E., Mazaki-Tovi S., Hassan S.S., Costerton J.W. Detection of a microbial biofilm in intraamniotic infection. Am J Obstet Gynecol. 2008;198:135.e1–135.e5. doi: 10.1016/j.ajog.2007.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Romero R., Kusanovic J.P., Espinoza J., Gotsch F., Nhan-Chang C.L., Erez O., Kim C.J., Khalek N., Mittal P., Goncalves L.F., Schaudinn C., Hassan S.S., Costerton J.W. What is amniotic fluid “sludge”? Ultrasound Obstet Gynecol. 2007;30:793–798. doi: 10.1002/uog.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Esplin M.S., Romero R., Chaiworapongsa T., Kim Y.M., Edwin S., Gomez R., Mazor M., Adashi E.Y. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med. 2005;17:365–373. doi: 10.1080/14767050500141329. [DOI] [PubMed] [Google Scholar]
- 52.Youssef R.E., Ledingham M.A., Bollapragada S.S., O'Gorman N., Jordan F., Young A., Norman J.E. The role of toll-like receptors (TLR-2 and -4) and triggering receptor expressed on myeloid cells 1 (TREM-1) in human term and preterm labor. Reprod Sci. 2009;16:843–856. doi: 10.1177/1933719109336621. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Microarray data analysis of myometrial explants treated with PROK1 for 6 and 24 hours. Microarray plots showing the relationship between mean fold changes and average intensities in the compared groups. PROK1 versus vehicle at 6 hours (P6 versus V6) (A) and 24 hours (P24 versus V24) (B). The log2-transformed fold change is marked on the y axis (M), and the log2-transformed average intensity of the compared groups is marked on the x axis (A). The differentially expressed features are red, and the filtering threshold for the fold change (1.4-fold on a log2 scale) is marked with the dashed line. C: Venn diagram illustrating the number of differentially expressed genes between treatments and a summary of overlapping genes between treatments.
Analysis of efficiency of PROK1 suppression. Myometrial explants were infected with lentivirus containing scrambled miRNA control or chained PROK1 miRNA construct. Quantitative real-time PCR analysis of PROK1 mRNA demonstrated lower expression of PROK1 in explants infected with PROK1 miRNA compared with explants infected with a scrambled control sequence. *P < 0.05 (n = 8). Results are presented as relative to a calibrator sample.


