Abstract
We measured daily intracage ammonia levels and performed weekly assessments of CD1 male, female, and breeder mice housed within disposable, ventilated cages that remained unchanged for 28 d. We tested housing groups comprising 1, 3, or 5 sex-matched mice per cage and breeder pairs with litters. Mice housed in cages with higher concentrations of ammonia developed degeneration and inflammatory lesions in the nasal passages. Mean ammonia exposure levels that caused rhinitis were 181 ppm for 18 d. Ammonia exposures of 93 ppm for 16 d caused necrosis of the olfactory epithelium, whereas 52 ppm for 13 d caused epithelial degeneration. Observers could not detect visible signs of rhinitis or identify cages with elevated ammonia levels, nor did they identify any sick or distressed mice. Observers consistently assigned poorer welfare scores as cages became dirtier. We conclude that we can extend the cage-change interval to at least 28 d for disposable, ventilated caging housing a single CD1 mouse. Cages containing 3 CD1 mice of either sex should be changed biweekly, and cages containing 5 CD1 mice or breeder pairs should be changed at least once weekly.
Abbreviation: IVC, individually ventilated caging; TWA, The 8-h time-weighted threshold limit value of 25 ppm of ammonia
One of the primary goals of an adequate husbandry program for research mice is to provide them with living conditions that support their welfare without compromising the quality of the research data that is collected from them. An important development in the maintenance of large research colonies of mice has been the introduction of individually ventilated caging (IVC) systems, which offer advantages in protection against transmission of infectious diseases between rodents, minimize exposure of personnel to mouse allergens, permit increased stocking density, and reduce the costs of labor that are incurred by changing cages. In addition, IVC have been shown to provide an environment with low amounts of moisture, because the air is supplied in quantities that keep the bedding dry. This feature minimizes the generation of ammonia, which is formed when urease-positive bacteria metabolize urea excreted in the animal's urine and feces.5 At our institution, a biweekly cage-change interval was selected almost 10 y ago as the standard schedule for mice in our IVC, mainly because this practice was common among users of IVC systems. This 14-d cage changing interval was used as the baseline schedule for changing cages regardless of the number of mice within the cage, with the caveat that cages containing 5 adult mice or breeding mice with large litters were ‘spot-changed’ once each week to keep the mice acceptably clean and dry.
At our facility, the biweekly schedule was retained when a new ventilated rack housing system that used disposable polyethylene terepthalate cages was implemented. However, published data suggested that air quality in IVC remained good and ammonia levels remained safely below established threshold levels for human exposure when cages were not changed for 17 or 21 d.18,19 In addition, the disposable caging system in our facility had been shown to provide excellent air quality and good living conditions for mice.21 In particular, ammonia levels never exceeded 3.2 ppm within cages that contained 5 retired breeder female CD1 mice, and the mice remained healthy for the 9-d duration of the study.21 An additional report described improved breeding performance in several different genetically engineered and inbred strains that were maintained by using the disposable caging system.13 None of these previous studies were sufficiently prolonged to permit the identification of the time point at which air quality deteriorated. Furthermore, additional research suggested that changing cages at intervals shorter than 14 d might actually be detrimental: breeding mice that had their cages changed once every 7 d raised fewer pups than did mice whose cages were changed on either a 14- or 21-d cycle.16 A behavioral research study showed that providing a clean cage disrupted odor cues, and this situation caused stress and increased aggression in group-housed mice.3 Given this information, we thought that extending the cage-change frequency beyond our standard interval of once every 14 d might be reasonable.
The eighth edition of the Guide for the Care and Use of Laboratory Animals recommends that the IACUC use a performance-based approach to decide appropriate husbandry practices.10 Furthermore, these guidelines suggest that a decreased sanitation frequency might be appropriate in IVC if the microenvironment in the cage provides sufficient air quality and if the bedding within the cage is reasonably clean and dry.10 The current study was undertaken to collect data that would permit us to use a performance-based approach to identify the appropriate interval for changing cages in our facility. We evaluated the effects of extending the cage-change interval for disposable IVC to 28 d. We measured intracage ammonia levels daily for 28 d and subjectively scored the appearance of the cage and assessed the mice at weekly intervals. Body weights were measured. At the conclusion of the study, the respiratory tracts of the mice were examined for pathology. We selected CD1 mice as animal subjects because they are large, robust mice that produce large litters of pups. Other studies13,21 that used the same disposable ventilated caging system found that mice that are difficult to breed and produce small litters have acceptable breeding performance in this system. However, we tested the housing system under conditions in which the biomass in the cage was larger than that tested previously.
Materials and Methods
Humane care and use of animals.
Mice were housed in an AAALAC-accredited facility and in compliance with the Guide for the Care and Use of Laboratory Animals.10 All experimental procedures were approved by the IACUC of the Stowers Institute for Medical Research.
Animals.
The CD1 male and female mice that were used in this study originated from our core breeding colony. Prior to study assignment, mice were housed in disposable polyethylene terepthalate cages (Innovive, San Diego, CA) and received 68 air changes hourly. These cages contained 1/8-in. corncob bedding and were changed biweekly. At the time of the study, mice were 2 to 5 mo old and weighed between 32 and 59 g. They were maintained throughout on a 14:10-h light:dark cycle. Mice were free of infection with the following rodent pathogens: mouse hepatitis virus, minute virus, mouse parvoviruses (types 1, 2, and 3), murine norovirus, Sendai, Mycoplasma pulmonis, Theiler encephalomyelitis virus, epizootic diarrhea of infant mice virus, reovirus 3, lymphocytic choriomeningitis virus, ectromelia virus, mouse adenoviruses (types 1 and 2), polyoma virus, Encephalitozoon cuniculi, cilia-associated respiratory bacillus, Clostridium piliforme, Helicobacter spp., murine cytomegalovirus, all species of fur mites, and all species of rodent pinworms. Mice were fed Purina Diet 5053 (PMI Nutrition International, St Louis, MO) ad libitum. Acidified water was provided in polyethylene terepthalate bottles that rested in a depression in the cage lid. In the breeder group, each cage housed 1 male and 1 female mouse and a litter of pups.
Equipment and housing.
Mice were housed within disposable, prebedded polyethylene terepthalate cages (Innovive) that were maintained on a single, positive-pressure, ventilated rack. Each cage contained irradiated 1/8-in. corncob bedding and was weighed prior to the study. The weight of an empty cage bottom was approximately 130.5 g, and the amount of bedding was adjusted so that every cage weighed 329.5 g at the start of the study. A single cotton nesting pad was placed in each cage for environmental enrichment. Each cage received HEPA-filtered air that was delivered at 68 air changes hourly, according to the manufacturer's recommendations (Innovive). Air flow to each cage was verified. The study was conducted in Kansas City, MO, which is 740 ft. above sea level. During the study, the temperature within the macroenvironment ranged from 74 to 77 °F (22.3 to 25.0 °C), and the humidity ranged between 52% and 55%. Ammonia levels in the macroenvironment measured less than 10 ppm throughout the study.
General study design.
We exposed 7 groups of mice to an extended cage-change interval of 28 d. Mice were sorted by sex, and then each was assigned randomly to 1 of the following 6 groups (no. of mice per cage; 6 cages per group): 1 male; 1 female; 3 male; 3 female; 5 male; and 5 female. The remaining group comprised 24 cages of breeder pairs. During the study, mice were checked daily, and feeders were replenished as needed, but cage bottoms were not disturbed. In breeder cages, litters were weaned and pups were counted when they were 21 d of age. Male mice and nonpregnant female mice were weighed at the initiation of the study and at necropsy. Because pregnant female mice were at different stages of gestation at their time of assignment to the study, they were not weighed. The concentration of ammonia within each cage was measured once each morning between 0700 and 1000 for 28 d.
Subjective scoring of cages.
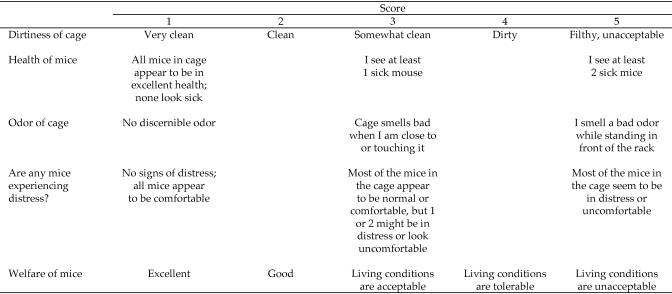
The suitability of the housing environment within the cage was assessed subjectively by 10 observers who each scored a subgroup of 3 cages from each of the 7 groups. The 10 evaluators were IACUC members, animal research technicians, animal husbandry technicians, animal facility managers, and the attending veterinarian. Each evaluator independently scored the same 3 cages in each group at 4 defined evaluation periods. Evaluation 1 occurred on study days 6 through 8, evaluation 2 occurred on study days 13 through 15, evaluation 3 occurred on study days 20 through 22, and evaluation 4 was done on day 27 or 28. A modified Likert scoring scale (range, 1 through 5) was developed and assigned a value between 1 and 5 for the following parameters: dirtiness of the cage, health of the mice in the cage, odor, signs of distress, impression of the welfare of the mice in the cage (Figure 1). Cage dirtiness reflected the amount of gross fecal and urine soiling the inside of the cage. Animal welfare was defined as the health and general condition of the animals on study. The total score for each cage was the sum of the individual parameters. The scale was designed so that a cage that was clean, had no odor, contained healthy mice with no evidence of distress, and excellent animal welfare received a low total score. Conversely, a cage that was excessively dirty, had an offensive odor, contained sick mice or mice that appeared to be in distress, and in which animal welfare was perceived to be poor received a high score. In addition, evaluators documented specific abnormal behaviors that they observed, such as barbering, fight wounds, stereotypies, and hyperactivity.
Figure 1.
Evaluator assessment of living conditions within cages: subjective cage-scoring scale. The total score for each cage was derived by summing the scores assigned for dirtiness, health, odor, distress, and welfare.
Measurement of intracage ammonia levels.
Intracage ammonia levels were measured (CMS Chip Management Analyzer System, Draeger Safety, Pittsburgh, PA) daily. This device combines chemical-specific chips and an electronic analyzer to record gas and vapor measurements. Ammonia-specific chips measuring from 10 to 150 ppm or 100 to 2000 ppm were used. Measurements were taken by inserting the attached rubber tip of the monitor into the air supply hole on the cage lid. The monitor was self-calibrating, and it performed a self-test when it was switched on each day. Cages were numbered, and ammonia was measured in the same cage order each day. Ammonia readings were recorded on a log sheet immediately after each reading, after which mice were checked for overall health, and the feeder and water bottle were replenished or replaced, as needed. Each removal and replacement of the cage lid was noted on the log sheet.
Necropsy and histopathology of the nasal cavity and lungs.
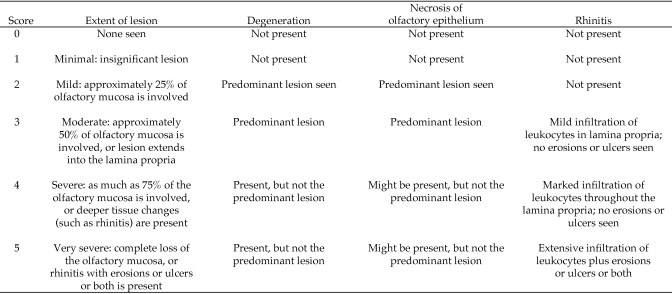
After the measurement of ammonia levels on day 28, mice were injected intraperitoneally with 2 mL 2.5% tribromoethanol and a bilateral pneumothorax was created to ensure death. Mice were weighed, and the major thoracic and abdominal organs were examined grossly. Lungs were inflated with 10% neutral buffered formalin by passing a 20-gauge needle attached to a 3-mL syringe into the trachea. Lungs were removed and immersed in fixative. Nasal passages were infiltrated with fixative by passing the needle retrograde into the nasopharynx and injecting 10% buffered formalin until the solution was observed to drip from the nares. The head was removed; the mandible was dissected free from the skull; and the remaining skin, muscle, and soft tissues were removed. The skull then was immersed in fixative for at least 24 h, decalcified (Cal-EX, Fisher Scientific, Pittsburgh, PA, or Immunocal, Decal Chemical, Suffern, NY) for 48 h, paraffin-embedded, cut into 5-µm sections, and stained with hematoxylin and eosin. Four different section levels of the nasal cavity were prepared, stained, and examined for lesions, as previously described.9,15,23 The scoring method for each section is summarized in Figure 2. Lesions were identified and categorized by using recommended diagnostic criteria and standardized nomenclature.8,9,17 Each nasal section was scored by using a standardized grading system that assigned a numerical score based on the recognition of specific degenerative changes in the nasal epithelium, presence of inflammation, occurrence of erosions or ulcerations, and extent and depth of the lesions.7,12 Standardized nomenclature for nonproliferative lesions of the respiratory tract was used to assign a primary diagnosis for each section.17 Briefly, a diagnosis of degeneration was assigned when epithelial cells were swollen with vacuolated cytoplasm or contained eosinophilic inclusion droplets. Necrosis was defined by exfoliation or loss of cells or the presence of cells with eosinophilic cytoplasm and evidence of nuclear degradation. When severe, necrosis culminated in erosions or ulcerations of the nasal mucosal surface. Rhinitis was defined as the loss of epithelium that was accompanied by the accumulation of leukocytes on the mucosal surface or within the lamina propria. The duration of rhinitis was acute when neutrophils were the predominant infiltrating leukocyte, subacute when lymphocytes were predominant, and chronic–active when both neutrophils and macrophages were present. The extent of the lesions in each of the 4 sections was graded by using a previously described method.7 Each section was scored from 0 to 5 based on the type and extent of lesions present (Figure 2). The nasal histology score for each mouse was the sum of the scores for each of the 4 section levels. In addition, the locations of lesions were mapped as previously described.11 Lungs were sectioned longitudinally, stained with hematoxylin and eosin, and examined for lesions. Histology sections from 65 mice (42% of total samples) were examined.
Figure 2.
Scoring method for nasal histology sections. Degeneration means that mucosal epithelial cells were swollen or that they contained amorphous eosinophilic cytoplasmic droplets.
Statistical analyses.
For comparison of intracage ammonia levels among the 7 housing groups, data at each evaluation point were analyzed by using ANOVA. Differences were considered significant when the P value was 0.05 or less. P values were adjusted by using the Bonforonni method to ensure a family-wise error rate of 0.05 or less. F tests were used to test for differences between groups.
Slides from sections of the nasal cavity and the lungs were examined by a pathologist (CMV) who had no knowledge of the ammonia level to which each mouse had been exposed. The summary diagnoses that resulted from the evaluations were rhinitis, necrosis of the olfactory epithelium, degeneration of the respiratory or transitional epithelium (or both), and no lesions seen. After all mice had been examined, the concentration of ammonia to which each mouse was exposed was compared to ascertain whether histology score, the diagnosis assigned to the mouse, and intracage concentration of ammonia were related. Exposure to potentially harmful levels of ammonia was defined as any day on which the intracage ammonia level was greater than or equal to 25 ppm. Total ammonia level (in ppm) was calculated by summing intracage ammonia levels beginning on the day on which ammonia was greater than or equal to 25 ppm until the end of the study on day 28. Analysis methods used to determine the relationships between diagnosis, histology score, and intracage ammonia levels were the Kruskal–Wallis test, Wilcoxon scores test, and ANOVA.
To evaluate the relationship between the intracage concentration of ammonia and the subjective scoring components, Pearson correlation coefficients were determined. If the correlation coefficient was between 0.7 and 1.0, a strong positive association existed between the variables. If the correlation coefficient was between 0.3 and 0.7, the association between the variables was weak. A correlation coefficient between −0.3 and 0.3 indicated no association between the variables. All statistical analyses were performed by using SAS software version 9.2 (SAS Institute, Cary, NC).
Results
Intracage ammonia levels.
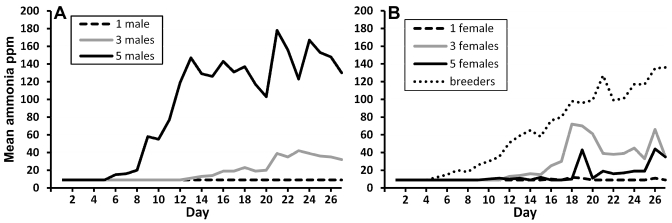
Mean intracage ammonia levels throughout the 28-d study period (Figure 3, Table 1.) varied greatly, depending on the housing density and sex of the mice within the cage. In particular, cages containing 5 male mice had significantly (P < 0.05) higher levels of ammonia (119 ppm) than did all other groups, beginning on day 12 and continuing for the duration of the study, with the exception of days 18 to 20. During these days, ammonia levels did not differ between cages containing 5 male mice or breeder pairs. At this time, the ammonia level of cages containing 5 male mice dropped, coincident with removal of the cage lid to replenish the feed supply. Throughout the duration of the study, intracage ammonia levels did not differ between the 3 housing groups of female mice, between male mice housed at 1 or 3 mice per cage, or between female and male mice housed at the same density. Ammonia levels were significantly (P < 0.05) higher for 5 male mice housed per cage than for 5 female mice, 1 male mouse, or 3 male mice per cage.
Figure 3.
Intracage ammonia concentrations. (A) Male mice. (B) Female mice and breeders.
Table 1.
Intracage ammonia levels (in ppm; mean ± 1 SD)
| Housing group | Ammonia level over entire 28 d | Ammonia level on day 12 | Ammonia level on day 18 | Day on which ammonia level (in parentheses) exceeded TWA |
| 1 male mouse | <10 | <10 ± 0 | <10 ± 0 | Never |
| 1 female mouse | <10 | <10 ± 0 | 12.3 ± 8.0 | Never |
| 3 male mice | 19 | <10 ± 0 | 22.8 ± 26.4 | day 21 (39) |
| 3 female mice | 29 | 13 ± 9.6 | 72 ± 124.4 | day 17 (30) |
| 5 male mice | 95 | 119 ± 144a | 137.3 ± 82 | day 9 (58) |
| 5 female mice | 32 | 9.5 ± 1.1 | 10.3 ± 2.95 | day 12 (27) |
| Breeder pair | 63 | 51.4 ± 72.4 | 98 ± 115.9 | day 9 (26) |
TWA, 8-h time-weighted threshold limit value for human exposure (that is, 25 ppm).
Ammonia level was significantly (P ≤ 0.05) greater than that of all other groups.
The 8-h time-weighted threshold limit value (TWA) for ammonia for humans is 25 ppm,18 and this level has been proposed as a safe level of exposure for mice,18 as well. In our study, ammonia concentrations exceeded the TWA in all of the higher housing density groups but not in mice housed individually (Table 1).
Histopathology of the lungs and nasal passages.
Mice that were exposed to increasing concentrations of ammonia had progressively more severe lesions in their nasal cavities, and their histology scores were increased significantly (P < 0.05) compared with those of groups of mice that had no exposure to ammonia. Lesions were most severe in the most rostral section of the nasal cavity, decreased in severity and extent as one moved caudally toward the nasopharynx, and were absent in the lungs. Lesions in the nasal cavity tended to occur at specific anatomic locations. The respiratory epithelium that lined the nasal septum, the transitional epithelium that covered the nasoturbinates, maxilloturbinates and lateral wall of the nasal cavity, and the olfactory epithelium lining the dorsal medial meatus of the nasal cavity were most likely to show degenerative changes, necrosis, or rhinitis.
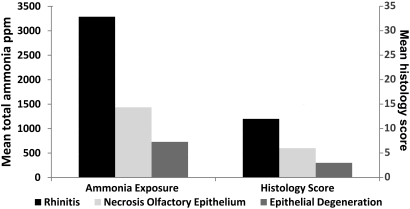
The histopathology data are summarized in Table 2 and Figure 4. Compared with those exposed to less ammonia, mice that were exposed to higher concentrations of ammonia had significantly (P < 0.05) higher histology scores. Mice with a primary diagnosis of rhinitis had been exposed to significantly (P < 0.05) higher concentrations of ammonia than had mice with necrosis of the olfactory epithelium, degeneration of the transitional and respiratory epithelium, and groups with no lesions. Mice with rhinitis had erosions and ulcerations of the nasal epithelium that were accompanied by extensive infiltration of leukocytes (predominantly neutrophils) in the lamina propria (Figure 5), These lesions led to this group's mean histology score of 12, indicating that lesions were severe and present at multiple levels.
Table 2.
Primary diagnoses, histology scores (mean ± 1 SD), and ammonia exposure (ppm; mean ± 1 SD)
| Primary diagnosis | n | Histology score | Total ammonia exposure | Daily ammonia exposure | No. of days on which ammonia levels were ≥ 25 ppm |
| Rhinitis | 19 | 12.0 ± 2.79a | 3288 ± 1409b | 181 ± 67b | 18 ± 2.79 |
| Necrosis of olfactory epithelium | 9 | 6.0 ± 1.58a | 1436 ± 768b | 93 ± 66b | 16 ± 3.46 |
| Degeneration of respiratory or transitional epithelium (or both) | 11 | 3.0 ± 1.0a | 730 ± 510 | 52 ± 28b | 13 ± 4.1 |
| Ammonia exposure and no lesions | 9 | 0.22 ± 0.44 | 182 ± 57 | 32 ± 18 | 6 ± 3.26 |
| Exposure to 0 to 10 ppm ammonia and no lesions | 17 | 0.88 ± 1.05 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
Score significantly (P ≤ 0.05) increased compared with those for ammonia exposure and no lesions and exposure to 1 to 10 ppm ammonia and no lesions.
Exposure level significantly (P ≤ 0.05) increased compared with those for ammonia exposure and no lesions and exposure to 1 to 10 ppm ammonia and no lesions.
Figure 4.
Diagnoses, mean total ammonia exposures, and histology scores.
Figure 5.
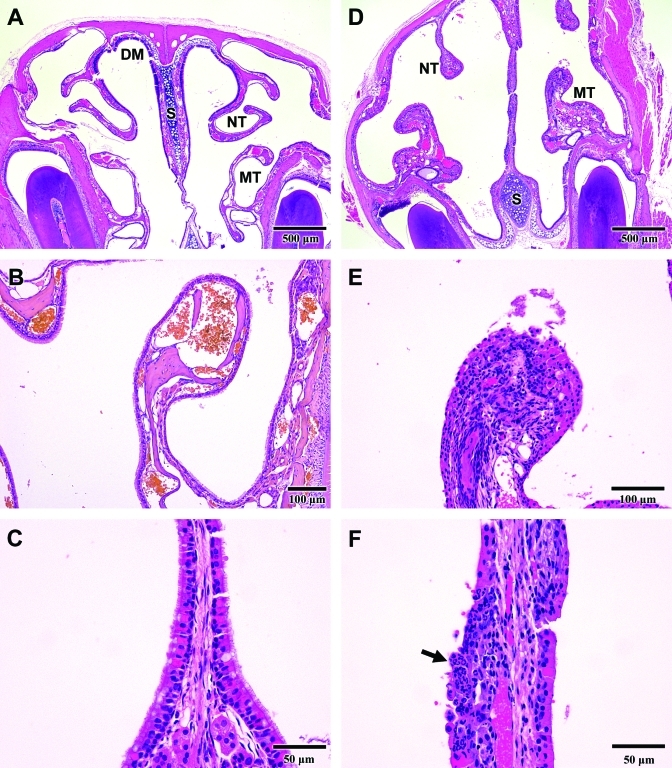
Rhinitis. (A) Cross-section of a normal nasal cavity. Anatomic structures shown include the dorsal medial meatus (DM), nasoturbinate (NT), maxilloturbinate (MT), and nasal septum (S). (B) Normal maxilloturbinate. The surface is covered by a single layer of intact cuboidal or ciliated epithelial cells. (C) Normal respiratory epithelium lining the nasal septum is comprised of a uniform layer of pseudostratified, ciliated cells. (D) Nasal cavity from a mouse with rhinitis that was exposed to 120 ppm ammonia for 23 d. The nasoturbinates (NT) are blunted, and the maxilloturbinates (MT) and nasal septum (S) are thickened compared with normal structures (panel A). (E) A maxilloturbinate shows necrosis and sloughing of the surface epithelium with cellular debris in the lumen of the nasal cavity. The lamina propria contains a dense infiltration of inflammatory cells (compare with panel B). (F) Nasal septum from a mouse with rhinitis shows ulceration of the surface epithelium and dense infiltration with neutrophils (arrow).
Mice with a primary diagnosis of necrosis of the olfactory epithelium had been exposed to higher (P < 0.05) concentrations of ammonia than were mice with degeneration of the respiratory or transitional epithelium or no lesions (Table 2). Histology scores and ammonia concentrations of mice with olfactory necrosis both were significantly (P < 0.05) increased compared with those from other groups, excluding mice with rhinitis. Olfactory necrosis was characterized by loss of neuroepithelial cells predominantly from the dorsal medial meatus of the nasal cavity (Figure 6).
Figure 6.
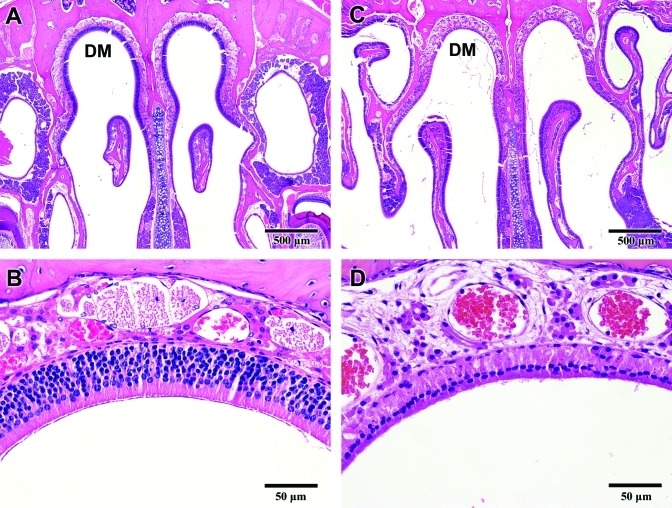
Necrosis of the olfactory epithelium. (A) Cross-section of a normal nasal cavity. The olfactory epithelium forms a dense, inner uniform cellular layer in the dorsal medial meatus (DM). (B) Normal olfactory epithelium comprises neuroepithelial and supporting cells that are tightly opposed and are at least 5 to 6 cell-layers thick. (C) Necrosis of the olfactory epithelium in the dorsal medial meatus (DM) in a mouse exposed to 72 ppm ammonia for 17 d. The uniform cell layer that normally lines the dorsal medial meatus (DM) is largely absent. (D) Increased magnification of the region shown in panel C reveals the absence of neuroepithelial cells and the presence of fine granular debris from necrotic cells (compare with panel B).
Mice with a primary diagnosis of degeneration of respiratory or transitional epithelium experienced higher (P < 0.05) histology scores and concentrations of ammonia than did mice with no lesions (Table 2). Degenerative changes in the epithelium lining the nasal passages (Figure 7) included swollen epithelial cells with eosinophilic cytoplasm, the presence of eosinophilic inclusions within epithelial cells, and cystic dilation of submucosal glands. No lesions were seen in 2 different groups of mice (Table 2): one group of 9 mice with exposure to 32 ppm ammonia for 6 d, no lesions in the nasal cavity, and a mean histology score of 0.22 and a group of 17 mice with exposure to 0 to 10 ppm ammonia for the duration of the study, no remarkable lesions in the nasal cavity, and a mean histology score of 0.88.
Figure 7.
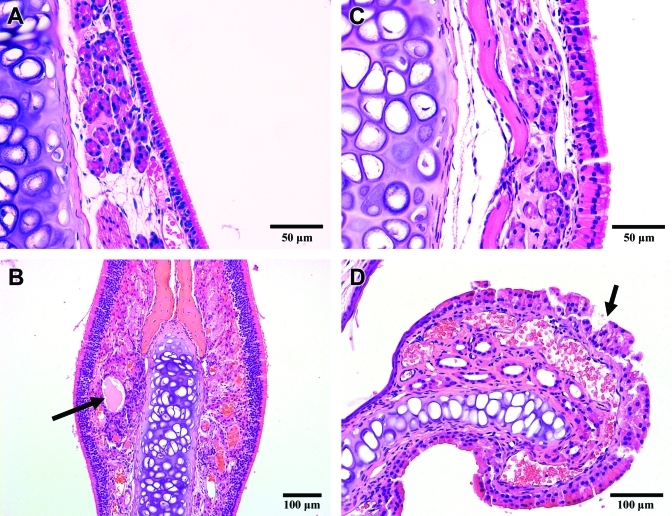
Degeneration in the nasal cavity. Mice were exposed to a mean level of 52 ppm ammonia for 13 d. (A) Normal respiratory epithelium is pseudostratified, ciliated epithelium. (B) A submucosal cyst (arrow) located beneath the olfactory epithelium. C) The majority of the respiratory epithelial cells are swollen and contain eosinophilic, cytoplasmic droplets. D) The epithelium lining the surface of this nasoturbinate is effaced (arrow) and will be sloughed.
We calculated the amount of weight change (weight lost or gained as a percentage of initial body weight) for each mouse (Table 3 ) to reveal any potential relationship between the pathologic diagnosis (rhinitis, necrosis of the olfactory epithelium, or degeneration) and loss of physical condition during the course of the study. There was no statistically significant interaction between body weight and diagnosis.
Table 3.
Mean body weight (g) of mice and proportion of mice that lost weight (% of group; mean ± 1 SD)
| Diagnosis | Starting weight | Weight at necropsy | % of mice in group that lost weight |
| Rhinitis | 48 | 47.3 | 6.90 ± 4.86 |
| Necrosis of olfactory epithelium | 47.8 | 48.4 | 2.80 ± 2.32 |
| Degeneration of respiratory or transitional epithelium (or both) | 45.6 | 47.1 | 2.70 ± 2.68 |
| Ammonia exposure but no lesions | 42.6 | 47.1 | 5.60 ± 2.91 |
| No exposure and no lesions | 45.3 | 47.0 | 4.4 ± 2.91 |
Fisher's exact test showed that there was no significant association between diagnosis and loss of body weight.
Living conditions as evaluated by subjective cage scoring.
In addition to the criteria used to score living conditions within the cage (Figure 1), evaluators were also asked to document the presence of barbering, fighting, stereotypies, and hyperactivity. These behaviors were either absent or observed only rarely and therefore are not addressed further.
Observer perceptions of the living conditions within the cages were dependent on housing density, sex of mice within the cage, and the time between cage changes. The results of the subjective cage scoring procedure are summarized in Table 4. In cages containing one male or female mouse, the mean scores never exceeded 2.0, suggesting that evaluators judged welfare to be good to excellent in these cages over all evaluation periods. Cages containing 3 mice of either sex, 5 female mice, or breeder pairs were scored as good to excellent until evaluation 2 (between study days 13 to 15), when mean scores exceeded 2.0. Mean scores for groups of 5 male mice were 2.0 at evaluation 1. Mean total score at subsequent evaluations were significantly higher (P < 0.05) for all but individually housed male mice. The increase in mean total cage score was largely due to increased dirtiness of the cage rather than to observation of distressed or sick mice, with a clear tendency to judge welfare as poorer as the cage became dirtier. Strong positive correlations emerged between total score and cleanliness (correlation coefficient, 0.8859) and cleanliness and welfare (correlation coefficient, 0.8523). In contrast, cages with high levels of ammonia did not have higher total (correlation coefficient, 0.50385) or odor (correlation coefficient = 0.35482) scores. There was no correlation between the level of ammonia in the cage and the evaluators’ scores for welfare (correlation coefficient, 0.482966), distress (0.384159), and health (0.299882) of the mice.
Table 4.
Results of subjective cage scoring (mean; see Figure 1) to evaluate living conditions of mice
| Mice per cage |
||||||||
| Evaluation | 1 Male | 1 Female | 3 Male | 3 Female | 5 Male | 5 Female | Breeder pair | |
| Total score | 1 2 3 4 | 6.4 6.8 7.7 7.9 | 5.6 6.3 7.5a 8.3a | 7.4 9.4 11.0a 12.5a | 7.8 8.9 12.4a 14.0a | 9.4 12.0 15.0a 16.0a | 7.8 11.4a 13.2a 13.4a | 8.3 12.0a 13.3a 14.3a |
| Welfareb | 1 2 3 4 | 1.3 1.4 1.7 1.8 | 1.2 1.4 1.8 1.9 | 1.5 2.1 3.0 3.4 | 1.7 2.0 3.6 4.0 | 2.0 2.9 3.9 4.2 | 1.7 2.8 3.5 3.6 | 1.9 3.0 3.8 3.9 |
| Dirtinessb | 1 2 3 4 | 1.7 2.0 2.5 2.6 | 1.4 1.9 2.3 2.6 | 2.4 3.2 3.6 4.0 | 2.5 3.1 3.9 4.2 | 3.1 4.0 4.2 4.5 | 2.6 3.7 3.9 4.1 | 2.9 4.0 4.2 4.3 |
| Healthb | 1 2 3 4 | 1.0 1.0 1.0 1.0 | 1.0 1.0 1.0 1.2 | 1.0 1.0 1.3 1.5 | 1.0 1.0 1.4 1.7 | 1.0 1.1 1.9 2.1 | 1.0 1.2 1.6 1.7 | 1.0 1.2 1.4 1.7 |
| Distressb | 1 2 3 4 | 1.1 1.0 1.1 1.0 | 1.0 1.0 1.0 1.2 | 1.0 1.1 1.5 1.6 | 1.2 1.3 1.6 2.0 | 1.3 1.7 2.2 2.5 | 1.1 1.5 1.7 2.0 | 1.1 1.5 1.6 2.1 |
| Odorb | 1 2 3 4 | 1.2 1.3 1.4 1.4 | 1.0 1.0 1.4 1.3 | 1.5 1.9 1.9 2.0 | 1.4 1.4 1.9 1.8 | 2.0 2.4 2.7 2.7 | 1.4 2.1 2.6 2.1 | 1.4 2.2 2.3 2.3 |
Evaluation periods for which the mean total score was significantly (P ≤ 0.05) greater than that at evaluation 1 for that cage group.
These subcomponents were summed to determine the mean total cage score.
Discussion
The optimal interval for cage changing must balance ensuring welfare of the mice, maintaining an acceptably clean and healthy living space for them, and implementing management practices that are both energy and cost efficient. We used equipment already in use in our facility in an effort to determine the optimal time point at which to change cages. Our performance-based criteria were measurement of intracage ammonia concentration, determination of effects of exposure on the nasal passages and lungs, and subjective assessment of animal welfare and living conditions in the cage. Our goal was to explore the feasibility of extending our standard cage change interval to greater than 14 d. We found no single optimal time point at which to change a cage but rather that the sex of the mice, housing density, and length of time between cage changes were all important factors for the mice living in that cage.
Our data show that ammonia levels within a cage can vary greatly, depending on both the sex of the mice and the housing density. Overall, cages containing 5 male mice had greater concentrations of ammonia than did all other groups. Ammonia levels for this group began to increase on day 6, with a mean of 58 ppm on day 9, and reached 140 ppm by day 12. This sex-influenced increase in ammonia in cages containing 5 male mice probably occurred because male CD1 mice produced more urine than did female mice and because the behavior of the male mice enabled ammonia to accumulate within the cage. If densely housed male mice both excreted more urine and minimally disturbed urine-wet bedding, the urine-soaked bedding could serve as a substrate for the generation of ammonia by urease-positive gut flora.5,16 In one report,4 urine output was nearly equal between male and female CD1 mice, but an earlier study of Mus domesticus mice noted that urine output in group-housed male mice increased both with age and dominant social status.2 Dominant male mice produced greater volumes of urine, which they used for territorial marking, than did subordinate male mice.2 Another study reported variable urine production between the sexes of several inbred strains, with C3H male mice producing more urine than female mice.22 We postulate that dominant male CD1 mice established and maintained a social hierarchy within their cage, and they produced increased amounts of urine to enable this behavior. Cages containing 3 male mice did not show this effect because the dominant male mouse had fewer subordinates in the cage over which to establish dominance.
Cages containing breeder pairs had higher concentrations of ammonia when compared with the other groups. Ammonia levels in these cages increased to 26 ppm on day 9, reached 51 ppm on day 12, and achieved a mean level of 63 ppm for the duration of the study. The accumulation of ammonia in breeder cages was reasonable because of the increased biomass in the cage when litters were present.
The levels of ammonia in the cages containing 5 female mice in our study were unexpectedly higher than previously reported data.18,19 In our study, ammonia levels first increased to 12 ppm on day 10, and the mean level was 32 ppm for the duration of the study. Earlier studies indicated that ammonia levels remained below 5 ppm for as long as 17 d in cages containing 5 female CD1 mice.18,19 The type of IVC caging system differed between the earlier studies, in which the cage lid contained a large filter, and ours, in which a solid polyethylene terepthalate top with 2 small filters snapped firmly onto the cage. Other factors that might explain differences in intracage ammonia levels among IVC racks include the number of air changes hourly, temperature and humidity levels within cages, type and volume of bedding, and the devices used to measure the ammonia levels. We conclude that rack design is another important variable that influences the amount of ammonia that accumulates within IVC cages and that data regarding ammonia accumulation probably should not be extrapolated between different IVC caging systems.
The 8-h TWA of 25 ppm ammonia for human exposure has generally been accepted as a safe, low exposure level for ammonia in mice housed in IVC. However, this value seems to have been selected arbitrarily, and other authors6,20,21 have reported that exposure of mice to much higher levels of ammonia did not appear to be harmful. In one study,21 exposure levels between 50 and 150 ppm during a 9-d study led to no overt clinical signs of illness. In a second study, exposures between 500 and 700 ppm over 7 d were reported to cause no overt clinical signs in mice.20 Furthermore, mice did not find exposure to 110 ppm ammonia aversive during a preference testing experiment.6 Our data show that the nasal epithelium was affected adversely by exposure to inhaled ammonia and that the development of lesions depended on both the concentration of ammonia that was inhaled and the duration of the exposure. Mice in our study that were exposed to concentrations of 181 ppm ammonia for 18 d developed rhinitis as their primary lesion. These results are similar to those of other studies1 that reported rhinitis in rats that were exposed to 300 ppm ammonia for 6 h daily for either 5 or 10 d. The lesions that we observed in the nasal cavities of our mice and the absence of lesions in their lungs agree with the findings of several published studies in which rodents inhaled ammonia or were exposed to pollutants from soiled bedding.8,9,12,14 We saw an anterior-to-posterior gradient in lesion severity, with the most severe lesions occurring in the most rostral section of the nose. The degeneration and necrosis of epithelial cells occurred at specific sites—the lateral wall of the proximal nasal cavity and the surfaces of the nasoturbinates and maxilloturbinates. The lesions were present in specific sites at which ammonia is known to cause pathologic changes.1,8 Mice with significantly elevated histology scores and rhinitis did not have a parallel decline in body weight.
Our data do not support absolute numbers regarding safe levels for ammonia exposure in mice, but we can make some inferences from our histology data. We saw rhinitis when the exposure level was 181 ppm for 18 d, necrosis of the olfactory epithelium when exposure was 93 ppm for 16 d, and degeneration of epithelium when exposure was 52 ppm for 13 d. We did not observe lesions in the nasal passages when the mean ammonia level was 32 ppm for only 7 d or 10 ppm or less for 28 d. Therefore, our data suggest that for mice housed in IVC, which have the potential for 24-h exposure to ammonia for days or weeks at a time, intracage ammonia levels should remain below 25 ppm.
We included a subjective evaluation of mice and living conditions in the cage as a possible criterion upon which to base cage change-out during the planning phase of this study. If intracage ammonia accumulations were negligible, and there were no lesions in the respiratory tract, observers’ perceptions about welfare of the mice within the cage would have been the defining criterion for when to change a cage. As the study progressed, the mean total cage scores increased significantly for all groups except singly housed males. We recommend cage changing when the score equals or exceeds 2.0. Human perceptions of favorable animal welfare were strongly associated with cleanliness of the cage. Perceptions of deteriorating welfare were not associated with observations of increased numbers of sick or distressed mice. Observers did not reliably identify mice with severe rhinitis. Subtle clinical signs, such as squinting and rubbing of the eyes, might have been present in mice with severe rhinitis but such signs likely were missed because the mice were examined during the light cycle, when they were aroused from sleep. More importantly, observers did not reliably identify cages that had high concentrations of ammonia; the statistical association between increased mean total cage score and increased concentration of ammonia was weak.
We initiated this study hoping to find a single time point and a simple method to use when deciding cage changing frequency. Our data analyses yielded a complex answer to our simple query. Clearly, the sex of the cage occupants and the density at which they are housed greatly influenced the accumulation of ammonia within the cage. Accumulation of ammonia was highest in cages containing 5 male mice. We also found that ammonia accumulation had deleterious effects on the mice in the cages. Mice that were exposed to concentrations of ammonia that exceeded 52 ppm for approximately 2 wk developed inflammatory and degenerative lesions in the nasal passages. However, these lesions were clinically silent. Mice with severe rhinitis did not develop significant weight loss, and they showed no obvious signs of discomfort or illness.
Our data also show that human perceptions about cage cleanliness are not a reliable predictor of air quality within the cage. Humans tended to equate poor animal welfare with increased soiling of the cage, even though mice did not appear to be either sick or distressed. Mice with rhinitis were not detected reliably by visual observation, even when conducted by experienced, well-trained personnel and a laboratory animal veterinarian. Observers could not distinguish cages with unsafe levels of ammonia by either sight or smell, whereas they consistently judged the welfare of mice in dirty cages to be unacceptably poor. Another interesting observation is that evaluators did not score welfare as poor in cages containing a single female mouse, despite the social isolation.
We determined that the change-out interval can be extended to at least 28 d in cages containing 1 mouse of either sex. Both intracage ammonia levels and observer impressions of good to excellent welfare suggest that this practice is reasonable. For cages containing 3 mice, we suggest a biweekly cage changing schedule because observers graded welfare greater than 2 at the 14-d evaluation and because intracage ammonia levels exceeded 25 ppm between days 14 and 19. We recommend changing cages containing 5 female mice once weekly because evaluators scored welfare greater than 2 at day 14, even though ammonia levels remained low until day 17. According to our data, cages containing 5 male mice or breeder pairs should be changed at least once weekly to keep intracage ammonia levels below the threshold noted to cause lesions and to maintain perceptions of animal welfare as good to excellent.
Acknowledgments
Funding was provided by the Stowers Institute for Medical Research. We thank Kieran Pemberton, Diana Baumann, Jennifer Richey, Patsy Thompson, Stephanie Green, Jessica Fabro, Rachael Prater, Heidi Monin, and Andrea Moran for assistance with the subjective evaluation of the mice and the cages; Teri Johnson for preparation of the histology slides; and Howard Wilson for his assistance with the preparation of the tables and figures.
References
- 1.Bolon B, Bonnefoi MS, Roberts KC, Marshall MW, Morgan KT. 1991. Toxic interactions in the rat nose: pollutants from soiled bedding and methyl bromide. Toxicol Pathol 19:571–579 [DOI] [PubMed] [Google Scholar]
- 2.Drickamer LC. 1995. Rates of urine excretion by house mouse (Mus domesticus): differences by age, sex, social status, and reproductive condition. J Chem Ecol 21:1481–1493 [DOI] [PubMed] [Google Scholar]
- 3.Fitchett AE, Barnard CJ, Cassaday HJ. 2006. There's no place like home: cage odours and place preference in subordinate CD1 male mice. Physiol Behav 87:955–962 [DOI] [PubMed] [Google Scholar]
- 4.Fox JG, Anderson LC, Lowe FM, Quimby FW, 2002. Biology and diseases of mice, chapter 3. : Laboratory animal medicine, 2nd ed San Diego (CA): Academic Press [Google Scholar]
- 5.Gamble MR, Clough G. 1976. Ammonia build-up in animal boxes and its effect of rat tracheal epithelium. Lab Anim 10:93–104 [DOI] [PubMed] [Google Scholar]
- 6.Green AR, Wathes CM, Demmers TGM, MacArthur Clark J, Xin H. 2008. Development and application of a novel environmental preference chamber for assessing responses of laboratory mice to atmospheric ammonia. J Am Assoc Lab Anim Sci 47:49–56 [PMC free article] [PubMed] [Google Scholar]
- 7.Hardisty JF, Garman RH, Harkema JR, Lomax LG, Morgan KT. 1999. Histopathology of nasal olfactory mucosa from selected inhalation toxicology studies conducted with volatile chemicals. Toxicol Pathol 27:618–627 [DOI] [PubMed] [Google Scholar]
- 8.Harkema JR. 1990. Comparative pathology of the nasal mucosa in laboratory animals exposed to inhaled irritants. Environ Health Perspect 85:231–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Harkema JR, Carey SA, Wagner JG. 2006. The nose revisited: a brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol Pathol 34:252–269 [DOI] [PubMed] [Google Scholar]
- 10.Institute for Laboratory Animal Research 2010. Guide for the care and use of laboratory animals, 8th ed Chapter 3: environment, housing, and management, p 41–77 Washington (DC): National Academies Press [Google Scholar]
- 11.Mery S, Gross EA, Joyner DR, Godo M, Morgan KT. 1994. Nasal diagrams: a tool for recording the distribution of nasal lesions in rats and mice. Toxicol Pathol 22:353–372 [DOI] [PubMed] [Google Scholar]
- 12.Morgan KT. 1991. Approaches to the identification and recording of nasal lesions in toxicology studies. Toxicol Pathol 19:337–351 [DOI] [PubMed] [Google Scholar]
- 13.Piechowiak M. [Internet]. 2010. Adopting disposable caging: case study of breeding results. [Cited June 2010]. Available at: http://www.alnmag.com/article/adopting-disposable-caging-case-study-breeding-results.
- 14.Pinson DM, Schoeb TR, Lindsey JR, Davis JK. 1986. Evaluation by scoring and computerized morphometry of lesions of early Mycoplasma pulmonis infection and ammonia exposure in F344/N rats. Vet Pathol 23:550–555 [DOI] [PubMed] [Google Scholar]
- 15.Popp JA, Monteiro-Riviere NA. 1985. Macroscopic, microscopic, and ultrastructural anatomy of the nasal cavity, rat, p 3–10 : Jones TC, Mohr U, Hunt RD. Monographs on pathology of laboratory animals, respiratory system. New York (NY): Springer-Verlag [Google Scholar]
- 16.Reeb-Whitaker CK, Paigen B, Beamer WG, Bronson RT, Churchill GA, Schweitzer IB, Myers DD. 2001. The impact of reduced frequency of cage changes on the health of mice housed in ventilated cages. Lab Anim 35:58–73 [DOI] [PubMed] [Google Scholar]
- 17.Renne RA, Dungworth DL, Keenan CM, Morgan KT, Hahn FF, Schwartz LW. 2003. Nonproliferative lesions of the respiratory tract in rats, R1, p 1–26 : Guides for toxicologic pathology. Society of Toxicologic Pathologists/Armed Forces Institute of Pathology. Washington (DC): Society of Toxicologic Pathologists [Google Scholar]
- 18.Rosenbaum MD, VandeWoude S, Johnson TE. 2009. Effects of cage-change frequency and bedding volume on mice and their microenvironment. J Am Assoc Lab Anim Sci 48:763–773 [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenbaum MD, VandeWoude S, Volckens J, Johnson TE. 2010. Disparities in ammonia, temperature, humidity, and airborne particulate matter between micro- and macroenvironments of mice in individually ventilated caging. J Am Assoc Lab Anim Sci 49:177–183 [PMC free article] [PubMed] [Google Scholar]
- 20.Silverman J, Bays DW, Baker SP. 2009. Ammonia and carbon dioxide concentrations in disposable and reusable static mouse cages. Lab Anim (NY) 38:16–23 [DOI] [PubMed] [Google Scholar]
- 21.Silverman J, Bays DW, Cooper SF, Baker SP. 2008. Ammonia and carbon dioxide concentrations in disposable and reusable ventilated mouse cages. J Am Assoc Lab Anim Sci 47:57–62 [PMC free article] [PubMed] [Google Scholar]
- 22.Stechman MJ, Ahmad BN, Loh NY, Reed AA, Stewart M, Wells S, Hough T, Bentley L, Cox RD, Brown SDM, Thakker RJ. 2010. Establishing normal plasma and 24-hour urinary biochemistry ranges in C3H, BALB/c and C57BL/6J mice following acclimatization in metabolic cages. Lab Anim 44:218–225 [DOI] [PubMed] [Google Scholar]
- 23.Uraih LC, Maronpot RR. 1990. Normal histology of the nasal cavity and application of special techniques. Environ Health Perspect 85:187–208 [DOI] [PMC free article] [PubMed] [Google Scholar]