Abstract
Pinworm detection in laboratory rodents typically is accomplished by using the tape test or various modifications of fecal flotation test to detect eggs. Direct examination of intestinal contents remains the ‘gold standard’ for pinworm detection, with the limitation of euthanasia of animals. Here, we compare traditional and real-time PCR methodologies during screening for and confirming the presence of Aspiculuris tetraptera. Two sets of pooled fecal samples collected from each of 521 microisolation cages in a mouse facility suspected to be pinworm-positive were tested by PCR and fecal flotation methods. The number of PCR-positive cages was 48 (9.2%) compared with 5 (0.96%) by the fecal flotation method. All of the cages determined to be positive by fecal flotation were positive by PCR. We evaluated 8 positive cages containing 26 mice from the screening group 5 wk later to confirm the initial findings; for 7 of these cages, PCR results from the initial screening were confirmed by fecal centrifugation concentration (FCC) or direct worm detection. Among the 26 mice, 4 were pinworm-positive by FCC, 5 by maceration, and 16 by PCR. All 4 mice positive by FCC were positive by PCR; PCR was positive for 7 of the 9 mice in which pinworms were detected by FCC or maceration. Our study demonstrates that real-time PCR for survival testing of mice for A. tetraptera effectively augments current detection methods for quarantine and routine health monitoring.
Abbreviation: FCC, fecal centrifugation concentration
The common rodent pinworms Aspiculuris tetraptera, Syphacia obvelata, and S. muris belong to the superfamily Oxyuroidea. A. tetraptera and S. obvelata are found more commonly in mice in North America, with a prevalence of 0.19% for A. tetraptera and 0. 11% for S. obvelata, as determined by direct detection of worms in the intestinal contents (maceration).23 Young animals and male mice reportedly are particularly susceptible to pinworm infection.3 Although pinworm infections are typically asymptomatic in immunocompetent mice, many studies describe induction of a Th2- associated immune response8 that could affect studies on infectious diseases, immunology, autoimmune diseases, and others. In addition, pinworm infection may affect behavior and growth.25
Pinworm infection is diagnosed by finding the eggs in fecal material or the worms in intestinal contents. A. tetraptera and S. obvelata can be differentiated through the characteristic features of male and female worms of the 2 species, their preferred anatomic sites (A. tetraptera primarily is found in the ascending colon, S. obvelata resides in the cecum), and morphology of their eggs (A. tetraptera eggs are symmetrical, oval, and approximately 86 × 37 μm; eggs of S. obvelata are pointed, oval, and measure approximately 75 × 29 μm).21 Coinfections with multiple pinworm species have been reported to occur in mice.2
The prepatent period for A. tetraptera is 21 to 25 d; the larvae hatch in the colon, where they remain for 3 to 5 d, before the adult worms migrate to the distal colon. An adult female A. tetraptera pinworm lays an average of 17 eggs daily, which are excreted in the feces, where they can be detected by fecal flotation methods. The released eggs are not infective for 5 to 8 d. Female A. tetraptera pinworms have a lifespan of 45 to 50 d.20 In contrast, Syphacia female worms lay about 350 eggs before dying.21 Because the eggs of Syphacia spp. frequently stick to the perianal hair, diagnostic perianal tape testing is recommended.5,10 In addition, anal swabbing is effective for detection of A. tetraptera and S. obvelata.7
Differences in egg laying frequency and number of eggs for different pinworm species should be considered when designing diagnostic protocols based on the screening of fecal material. Evaluation of cecal and colonic contents for pinworms is considered to be most accurate for diagnosis of both Aspiculuris6 and Syphacia;14 however, these methods require euthanasia of the animals.
PCR is a more recent method for detection of pinworms.6,19 PCR in general is a sensitive technique. Fluorogenic real-time PCR is more sensitive and specific than is gel-based PCR; fewer than 10 copies of DNA can reliably be detected by using real-time PCR,15 giving it an increased chance of success if only a few eggs are present in the sample. Using PCR assays for detection of A. tetraptera, S. obvelata, and S. muris could support earlier diagnosis, which could limit dissemination of pinworms in a facility.
An opportunity to evaluate PCR for pinworm detection arose when routine health monitoring of soiled-bedding sentinels by fecal flotation identified an A. tetraptera outbreak in a mouse housing room at facility A. Mice from this room had been relocated to several rooms in facility B just prior to detection of initial pinworm-positive results. To determine the potential spread of infection to facility B, we screened all of the 521 cages from 5 rooms of facility B with the objective of comparing the diagnostic sensitivity of fluorogenic real-time PCR with fecal flotation. PCR, fecal centrifugation concentration (FCC), and direct worm detection methods were used to examine all mice from selected positive and negative cages for further confirmation of PCR-based results. Our results demonstrate the usefulness of PCR for detection of pinworms in mouse feces and highlight the need for using multiple screening methods for increasing diagnostic efficacy.
Materials and Methods
Animal husbandry and sentinel monitoring.
The pinworm-positive colony animals included various strains of genetically modified mice, all on C57BL/6 backgrounds. All mice were housed in individually ventilated racks under barrier conditions. All supplies, feed, water, cages, and so forth were autoclaved prior to use. Animal husbandry technicians used full personal protective equipment (gown, mask, shoe covers, head cover, and gloves), and all handling of animals was done under a vertical laminar flow cabinet.
Outbred female Crl:CD1 (Charles River Labs, Wilmington, MA; age, 4 to 6 wk) mice were used as sentinels. One sentinel was placed per side of a cage rack (maximum, 65 cages), and dirty bedding was transferred into sentinels’ cages at each cage change. All sentinels were tested (Division of Comparative Medicine, University of Miami, FL) quarterly for viral, microbial, and endo- and ectoparasitic infection. Serology tests were negative for mouse hepatitis virus, Sendai virus, Mycoplasma spp., pneumonia virus of mice, minute virus of mice, mouse parvovirus, Theiler murine encephalomyelitis virus, mouse rotavirus, lymphocytic choriomeningitis virus, ectromelia virus, K virus, polyoma virus, mouse adenovirus, reovirus3, mouse cytomegalovirus, hantavirus, mouse thymic virus, Clostridium piliforme, cilia-associated respiratory bacillus, and Encephalitozoon cuniculi. The only parasite found by fecal flotation testing was A. tetraptera.
Collection of fecal sample.
For the initial screen, 2 sets of 4 fecal pellets were collected from each cage and pooled for fecal flotation and PCR assays. At 5 wk after the initial screen, 2 fecal pellets were collected directly from the anus or rectum of individual mice for subsequent confirmation of initial findings using FCC and PCR techniques. All the procedures on animals were approved by the IACUC of the University of Miami or Charles River Labs, as appropriate.
Fecal flotation.
Pooled fecal pellets were evaluated (Division of Comparative Medicine) by using the fecal flotation method. Fecal pellets were placed in a 3-mL test tube containing sodium nitrate solution (Fecasol, Vetoquinol USA, Fort Worth, TX). Once the pellets were softened, they were triturated to break up the pellets so as to facilitate release of the eggs. The test tube then was filled with sodium nitrate solution and covered with a coverslip for at least 15 min, which then was evaluated at a magnification of at least ×100 for the presence of eggs.
FCC.
Fecal pellets for FCC were collected directly from the anus or rectum of mice submitted to the Health Monitoring Department (Research Animal Diagnostic Services, Charles River Labs). The fecal samples were mixed by vortexing in ZnSO4 (specific gravity, 1.18; Sigma–Aldrich, St Louis, MO) in a centrifuge tube. After dispersion of the sample, additional ZnSO4 solution was added to each tube to form a slight positive meniscus, a coverslip was placed on top, and the sample tube was centrifuged at 820 × g for 10 min. After centrifugation, the coverslip was placed on a glass slide and evaluated (magnification, 100× or greater) for eggs.
Worm detection.
Mice were submitted to the Health Monitoring Department (Research Animal Diagnostic Services, Charles River Labs) for direct detection of worms in intestinal contents. After euthanasia, the entire gastrointestinal tract was excised, placed in a culture dish, and macerated by submersion in warm (35 to 40 °C) water for at least 15 min, allowing the pinworms to migrate out from the fecal contents. The sample solution was evaluated under a dissecting microscope. Any pinworms detected were collected and mounted for microscopic identification (magnification, 100×).
Real-time PCR for pinworm detection.
Pooled or individual fecal pellets were submitted to the Molecular Diagnostics department (Research Animal Diagnostic Services, Charles River Labs) for PCR testing. Fecal slurry was prepared by adding 0.3 mL PBS to an individual fecal pellet or 0.6 mL PBS to a pool of 4 fecal pellets; only 0.1 mL of each sample was used for the extraction of nucleic acid. Total nucleic acid was extracted (RNeasy, Qiagen, Valencia, CA) from fecal slurries according to the manufacturer's instructions. All nucleic acid preparations were tested for possible PCR inhibitors by adding 100 genomic copies of an exogenous nonhomologous ‘spike’ DNA template cloned from the algae Chlamydomonas reinhardtii to each sample and running separate ‘spike-DNA’ TaqMan PCR reactions. DNA samples demonstrating PCR inhibition were diluted as needed to pass the spike-DNA test, after which pinworm PCR was performed.
A fluorogenic real-time PCR assay was designed to target the 18S rRNA gene of A. tetraptera. The thermodynamic properties of the primers (Sigma-Aldrich) and MGB probe (Applied Biosystems, Carlsbad, CA) with FAM detector and BHQ quencher (Sigma-Aldrich) were evaluated by using NetPrimer Software (Premier BioSoft International, Palo Alto, CA). Primer and probe sequences are proprietary and therefore not available for publication. Calf thymus DNA was used as a negative control, and 100 copies of the cloned target region from A. tetraptera was used as the positive control. The analytical sensitivity of the assays was determined to be 1 to 10 copies per sample (data not shown). The assays were performed on the ABI 7300 thermocycler (Applied Biosystems) with 55 cycles of denaturation at 95 °C for 15 s and primer annealing and extension at 58 °C for 1 min. Results of real-time PCR assays were analyzed by using 7300 System SDS software (Applied Biosystems). Endpoint fluorescence values were obtained by using a plate-reading fluorometer (Fluoroskan Accent FL, ThermoLabsystems, Thermo Fisher Scientific, Waltham, MA). The copy number of the target gene in a sample was estimated based on signal comparison with that from 100 copies of positive control template, assuming a 10-fold increase in PCR amplicon with every 3.32 cycles during the exponential phase of amplification in real-time PCR.1
Statistics.
The McNemar test was used to calculate statistical significance by using the Mcnemar.exact function from the extact2 × 2 library (http://www.r-project.org/). A P value of less than 0.05 was used to define statistical significance.
Results
Initial screening of rooms suspected of being pinworm-positive.
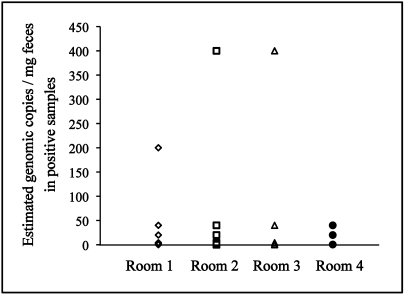
Two sets of pooled fecal pellets (4 each for fecal flotation and PCR) were collected concurrently from each of the 521 cages among 5 rooms of facility B. Rooms 1 through 4 were suspected to be pinworm-positive due to transfer of mice from facility A, which sentinel monitoring had identified to be pinworm-infested; room 5 was included on a precautionary basis due to its proximity to rooms 1 through 4. The 2 sets of pooled pellets collected were analyzed in parallel by independent technicians using fecal flotation and PCR assays. The results demonstrated that 9.2% (n = 48) of the 521 cages were pinworm-positive by PCR but 0.96% (n = 5) were positive by fecal flotation. All 5 samples that were positive by fecal flotation were positive by PCR (Table 1). The estimated copy number of the genomic templates detected by PCR varied from less than 10 to approximately 400 copies per milligram of fecal material (Figure 1). None of the cages in room 5, which had no direct transfer of mice from facility A, were pinworm-positive, thereby providing support for specificity of PCR.
Table 1.
Comparison of 2 noninvasive methods during initial screening
| No. of cages | No. of pinworm-positive cages |
||
| Room | Fecal flotation | PCR | |
| 1 | 189 | 2 | 19 |
| 2 | 167 | 1 | 19 |
| 3 | 80 | 0 | 7 |
| 4 | 26 | 2 | 3 |
| 5 | 59 | 0 | 0 |
| Total | 521 | 5 | 48 |
Figure 1.
Results of real-time PCR analyses (estimated no. of copies of genomic DNA per milligram feces) for cages that tested positive during the initial screening. A 100-copy positive control was used as a reference to estimate copy number in the test samples. Real-time PCR analysis was performed on pooled fecal samples (4 pellets per cage) obtained from all positive cages in rooms 1 (19 cages positive), 2 (19), 3 (7), and 4 (3); room 5 had no cages positive for pinworms.
Confirmation of pinworm status of all mice in selected cages.
To confirm our initial results, we compared PCR with the intestinal maceration technique, which is considered the ‘gold standard’ for detection of pinworms,6 and FCC, which has been more sensitive than fecal flotation method in our experience. Because the intestinal maceration method requires euthanasia of animals, only a limited number of mouse cages were available for confirmatory analysis. For this experiment, we selected 8 cages containing a total of 26 mice representing the following results: positive by both fecal flotation and PCR; negative by fecal flotation but PCR-positive at greater than 10 genomic copies per milligram of feces; negative by fecal flotation but positive by PCR at fewer than 10 copies per milligram of feces; PCR-negative but from which a potential pinworm egg was obtained. All mice from selected cages were submitted to Research Animal Diagnostic Services 5 wk after the samples for the initial screening were collected.
All of the PCR-positive results that were obtained during the initial screen were confirmed by at least 1 of the 3 methods (Table 2). Cage 8 contained a single mouse that was PCR negative during the initial testing but that yielded an object that potentially was a pinworm egg after fecal flotation. This cage was negative for pinworms as determined by all 3 confirmatory methods, thereby affirming the initial negative PCR result. Traditional diagnostic methods (that is, FCC and direct worm detection) validated PCR results from initial screening for all but cage 7. In cage 7, low levels of pinworm DNA were detected during both the initial and confirmatory screens.
Table 2.
Pinworm-positive results during initial screen on pooled fecal samples and confirmatory testing of all mice from a selected group of cages
| Initial screening |
Confirmatory testing |
|||||
| Cage | Fecal flotation | PCR (no. of genomic copies) | Mouse no. | Worm detection | FCC | PCR (no. of genomic copiesa) |
| 1 | + | + (40) | 1 | + | + (<10) | |
| 2 | ||||||
| 3 | + | + (40) | ||||
| 4 | + (200) | |||||
| 2 | + | + (40) | 5 | + (40) | ||
| 6 | + | + (4000) | ||||
| 3 | + (<10) | 7 (adult) | + (40) | |||
| 8 (adult) | + (40) | |||||
| 9 (adult) | + (<10) | |||||
| 10 | ||||||
| 11 | ||||||
| 12 | ||||||
| 13 | ||||||
| 14 | + | + (<10) | ||||
| 15 | + | |||||
| 16 | + | + (400) | ||||
| 4 | + (400) | 17 | + (20) | |||
| 18 | + | + (4000) | ||||
| 19 | + (40) | |||||
| 5 | + (400) | 20 | + (40) | |||
| 21 | + | + (<10) | ||||
| 6 | + (<10) | 22 | ||||
| 23 | + | |||||
| 7 | +(<10) | 24 | ||||
| 25 | + (<10) | |||||
| 8 | Ovumb | 26 | ||||
| Total no. of mice positive for pinworms | 5 | 4 | 16 | |||
| % of mice positive for pinworms | 19.2% | 15.4% | 61.5% | |||
Fecal samples for which the estimated copy number was less than 10 per milligram of fecal sample and that yielded a normal sigmoidal amplification curve and a positive endpoint fluorescence value are indicated as <10.
Possible egg with indistinct msorphology.
Comparison of the 3 methods used for confirmation of initial results identified 4 mice that were both FCC- and PCR-positive for pinworms. A total of 16 mice were positive by PCR, indicating that real-time PCR is diagnostically more sensitive (P < 0.01) than is FCC for antemortem detection of A. tetraptera. Diagnostic sensitivity did not differ between direct worm detection and FCC. Of the 5 mice positive by intestinal maceration, 3 were positive by PCR but negative by FCC. Overall, PCR was more sensitive (P < 0.01) than was direct worm detection. Although PCR was not positive for all mice in which pinworms were detected, it was always positive when eggs were detected in the feces (Table 2).
Discussion
Our study demonstrates the usefulness of real-time PCR for noninvasive testing of mice for pinworms, specifically A. tetraptera. Real-time PCR was a more sensitive technique than were traditional fecal diagnostic methods. In addition, because fecal samples can be screened by PCR, this technology can be used for health monitoring of quarantined and study mice.
A preliminary study by our laboratory indicated that FCC confirmed 18% (n = 56) of A. tetraptera infections detected by direct examination during screening of sentinel mice for routine health monitoring. The current study found that PCR confirmed 60% (n = 5) of cases where worms were detected by direct examination (Table 2). Furthermore, PCR results were always positive when eggs were detected in fecal samples by using either fecal flotation (initial screen) or FCC (confirmation). PCR was approximately 10 times more sensitive than was fecal floatation (Table 1) and 4 times more sensitive than was FCC (Table 2). Our previous experience with these techniques led us to expect a discrepancy between results obtained with fecal floatation and FCC: the additional centrifugation step in the FCC method likely assists the eggs of rodent pinworms to float to the top of the meniscus.
The increased sensitivity of real-time PCR over traditional methods of fecal examination for detection of A. tetraptera infection can be attributed to the ability of PCR to detect pinworm DNA originating from any type of pinworm cell, including eggs, sloughed cells, those of dead worms. Similarly, PCR for detection of Syphacia species likely will prove to be more sensitive than traditional methods, although Syphacia eggs are sticky, and a comparison study should include anal swabs or tapes as additional sample types for PCR besides fecal material.
We did not expect absolute agreement between the results obtained by using various detection methods. Considering that the life cycle of A. tetraptera is 45 to 50 d20 and that the estimated time between introduction of pinworm infection and the initial screen was as long as 6 mo, the mice in the current study likely were at different stages of infection, leading to discrepancies in diagnosis between postmortem and antemortem tests. Possible explanations for mice that were FCC- or PCR-positive but not worm-positive (Table 2) include ingestion and elimination of unembryonated eggs, recent expulsion of worms, and failure to detect an infection. The absence of worms could also be attributed to activation of immune response. The role of a Th2 immune response in providing protective immunity has been described for S. obvelata,16 and similar findings have been reported for other intestinal helminths.18 A protective immune response may similarly be triggered on A. tetraptera infection and may have contributed to the absence of pinworms in some mice from positive cages (Table 2). Possible explanations for the 2 mice that were egg- and PCR-negative but worm-positive include: the worm detected was male and therefore unable to lay eggs; the worm was female but in the prepatent period and had not yet begun to lay eggs; and FCC and PCR failed to detect eggs or other worm components.
Pinworms likely have multiple copies of the 18S rRNA gene in their genome, but the exact copy number of this gene is unknown. Therefore, the copy number information provided in Figure 1 and Table 2 is only a reference for expected PCR copies in fecal samples and may not necessarily represent the number of eggs or worm cells in samples. However, if A. tetraptera does have multiple copies of the 18S rRNA gene, this feature could contribute to the increased sensitivity of PCR assays based on this gene.
DNA copy number estimates in fecal samples by real-time PCR indicated that many samples had fewer than 10 copies of target DNA per milligram of fecal sample. Low-level infection, minimal amounts of pinworm DNA, and PCR inhibitors in feces can all negatively affect correlation among various detection methods. These disadvantages emphasize the need for, when feasible, concurrent use of multiple methods for pinworm detection.
None of these speculations alone can explain the discordant results among various methods in the current study. Whether soiled-bedding sentinels demonstrate increased correlation between FCC, PCR, and direct worm detection methods for pinworm diagnosis should be investigated. A 3-mo sentinel-monitoring period would accommodate multiple generations of pinworms, and the probability of finding all lifestages of pinworms in an animal would increase with the duration of exposure.
The current study demonstrates the advantage of PCR for detection of A. tetraptera in research mice on study. In working with different animal facilities beyond our laboratory, we have experienced several cases in which evaluating soiled-bedding sentinels failed to reveal pinworm infestation but worms were found in research animals. Pinworm detection through sentinel monitoring can be complicated by several factors, including age3 and strain4 of sentinels. Several additional factors, such as variation in the frequency and amount of bedding transferred to the sentinel cages, can affect pinworm detection. For example, weekly bedding transfer to sentinels has been shown to be effective for detection of S. obvelata,4 but there are no reports for A. tetraptera. The eggs of A. tetraptera are not infective until day 5 to 8, so the sentinel monitoring program should ensure that, if bedding is transferred more often than once weekly, only a partial bedding change is performed so that the eggs have time to embryonate and become infective. Only a few studies describe the persistence of various pinworm eggs in the environment; A. tetraptera11 and S. muris24 eggs can embryonate in water, a characteristic that may help them remain viable outside of the host. However, more studies are required to understand the persistence and spread of pinworm infection.
In the current study, despite the high prevalence of A. tetraptera among cages in the colony (9.2% as determined by real-time PCR), a substantial portion of individual mice (28%) in pinworm-positive cages tested negative for A. tetraptera infection by all methods used during the confirmatory analysis. Therefore, according to our data, when possible, fecal pellets from every mouse in a cage or a comprehensive set of pooled fecal material likely representing each mouse in a cage should be evaluated for effective diagnosis. When screening mice in quarantine, we advocate screening fecal material from individual animals by PCR to maximize chances of detection.
In the endemically infected colony in the current study, more samples were positive by PCR than direct worm detection, yet PCR was not positive for all mice in which worms were found. Therefore, we suggest that if performing both assays is possible (for example, sentinels that will be euthanized), using both PCR and direct worm detection is the most effective scheme for pinworm screening. However, PCR alone can be effective for antemortem screening of suspect colonies and quarantined animals.
Fenbendazole13,17 and ivermectin9,14 are popular and successful drugs for pinworm treatment; however, complete eradication of pinworms is difficult.12,22 The effect of these treatments on PCR testing is unknown, and caution should be exercised when using PCR for screening of treated mice, because even dead (noninfectious) worm cells would yield positive PCR results.
In conclusion, our current study demonstrates greater sensitivity of antemortem real-time PCR over the traditional methods for detection of A. tetraptera. Therefore, pinworm PCR can be used effectively to augment routine health monitoring methods for screening sentinels, monitoring rack cages in case of an outbreak, and direct screening of quarantined rodents.
Acknowledgments
We acknowledge the Molecular Diagnostics and Health Monitoring groups at Charles River Labs (Wilmington, MA) and the Veterinary group at the University of Miami (Miami, FL) for their excellent technical assistance. We thank Meredith Simon (Charles River Labs) for reviewing the manuscript. Funding for this study was provided in part by the Rodent Health Surveillance Program (University of Miami).
The authors state that 4 of them are employees of a company that has a direct commercial interest in the subject matter of this manuscript. The experimental study was performed as a scientific evaluation of the effectiveness of a commercially available product with costs being shared by both institutions. The manuscript should not be viewed as an endorsement of that product.
References
- 1.Applied Biosystems [Internet] 2008. Guide to performing relative quantitation of gene expression using real-time quantitative PCR. [Cited 04 January 2011]. Available at: http://www3.appliedbiosystems.com/cms/groups/mcb_support/documents/generaldocuments/cms_042380.pdf
- 2.Bazzano T, Restel TI, Pinto RM, Gomes DC. 2002. Patterns of infection with the nematodes Syphacia obvelata and Aspiculuris tetraptera in conventionally maintained laboratory mice. Mem Inst Oswaldo Cruz 97:847–853 [DOI] [PubMed] [Google Scholar]
- 3.Behnke JM. 1976. Aspiculuris tetraptera in wild Mus musculus. Age resistance and acquired immunity. J Helminthol 50:197–202 [PubMed] [Google Scholar]
- 4.Clarke CL, Perdue KA. 2004. Detection and clearance of Syphacia obvelata infection in Swiss Webster and athymic nude mice. Contemp Top Lab Anim Sci 43:9–13 [PubMed] [Google Scholar]
- 5.Eguiluz C, Viguera E, Perez J. 2001. Modification of the anal tape method for detection of pinworms in rodents. Lab Anim (NY) 30:54–55 [DOI] [PubMed] [Google Scholar]
- 6.Feldman SH, Bowman SG. 2007. Molecular phylogeny of the pinworms of mice, rats, and rabbits, and its use to develop molecular beacon assays for the detection of pinworms in mice. Lab Anim (NY) 36:43–50 [DOI] [PubMed] [Google Scholar]
- 7.Goncalves L, Pinto RM, Vicente JJ, Noronha D, Gomes DC. 1998. Helminth parasites of conventionally maintained laboratory mice. II. Inbred strains, with an adaptation of the anal swab technique. Mem Inst Oswaldo Cruz 93:121–126 [DOI] [PubMed] [Google Scholar]
- 8.Grencis RK. 1997. Th2-mediated host protective immunity to intestinal nematode infections. Philos Trans R Soc Lond B Biol Sci 352:1377–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hickman D, Swan M, Hartman GP. 2008. A cost-effective and efficacious method of pinworm treatment for large colonies of mice. Lab Anim (NY) 37:308–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill WA, Randolph MM, Mandrell TD. 2009. Sensitivity of perianal tape impressions to diagnose pinworm (Syphacia spp.) infections in rats (Rattus norvegicus) and mice (Mus musculus). J Am Assoc Lab Anim Sci 48:378–380 [PMC free article] [PubMed] [Google Scholar]
- 11.Hsieh KY. 1952. The effect of the standard pinworm chemotherapeutic agents on the mouse pinworm Aspiculuris tetraptera. Am J Hyg 56:287–293 [PubMed] [Google Scholar]
- 12.Huerkamp MJ, Benjamin KA, Webb SK, Pullium JK. 2004. Long-term results of dietary fenbendazole to eradicate Syphacia muris from rat colonies. Contemp Top Lab Anim Sci 43:35–36 [PubMed] [Google Scholar]
- 13.Huerkamp MJ, Benjamin KA, Zitzow LA, Pullium JK, Lloyd JA, Thompson WD, Webb SK, Lehner ND. 2000. Fenbendazole treatment without environmental decontamination eradicates Syphacia muris from all rats in a large, complex research institution. Contemp Top Lab Anim Sci 39:9–12 [PubMed] [Google Scholar]
- 14.Klement P, Augustine JM, Delaney KH, Klement G, Weitz JI. 1996. An oral ivermectin regimen that eradicates pinworms (Syphacia spp.) in laboratory rats and mice. Lab Anim Sci 46:286–290 [PubMed] [Google Scholar]
- 15.Leutenegger CM. 2001. The real-time TaqMan PCR and applications in veterinary medicine. Vet Sci Tomorrow 1:1–15 [Google Scholar]
- 16.Michels C, Goyal P, Nieuwenhuizen N, Brombacher F. 2006. Infection with Syphacia obvelata (pinworm) induces protective Th2 immune responses and influences ovalbumin-induced allergic reactions. Infect Immun 74:5926–5932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mohn G, Philipp EM. 1981. Effects of Syphacia muris and the anthelmintic fenbendazole on the microsomal monooxygenase system in mouse liver. Lab Anim 15:89–95 [DOI] [PubMed] [Google Scholar]
- 18.Ohnmacht C, Schwartz C, Panzer M, Schiedewitz I, Naumann R, Voehringer D. 2010. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity 33:364–374 [DOI] [PubMed] [Google Scholar]
- 19.Parel JD, Galula JU, Ooi HK. 2008. Characterization of rDNA sequences from Syphacia obvelata, Syphacia muris, and Aspiculuris tetraptera and development of a PCR-based method for identification. Vet Parasitol 153:379–383 [DOI] [PubMed] [Google Scholar]
- 20.Phillipson RF. 1974. Intermittent egg release by Aspiculuris tetraptera in mice. Parasitology 69:207–213 [DOI] [PubMed] [Google Scholar]
- 21.Pritchett KR. 2007. Helminth parasites of laboratory mice, p 551–564 :, Fox JG, Davisson MT, Quimby FW, Barthold SW, Newcomer CE, Smith AL. The mouse in biomedical research, 2nd ed New York (NY): Academic Press [Google Scholar]
- 22.Pritchett KR, Johnston NA. 2002. A review of treatments for the eradication of pinworm infections from laboratory rodent colonies. Contemp Top Lab Anim Sci 41:36–46 [PubMed] [Google Scholar]
- 23.Pritchett-Corning KR, Cosentino J, Clifford CB. 2009. Contemporary prevalence of infectious agents in laboratory mice and rats. Lab Anim 43:165–173 [DOI] [PubMed] [Google Scholar]
- 24.Van der Gulden WJ, van Aspert-van Erp AJ. 1976. Syphacia muris: water permeability of eggs and its effect on hatching—2. Exp Parasitol 39:40–44 [DOI] [PubMed] [Google Scholar]
- 25.Wagner M. 1988. The effect of infection with the pinworm (Syphacia muris) on rat growth. Lab Anim Sci 38:476–478 [PubMed] [Google Scholar]