Abstract
Rodents are often anesthetized by using ketamine and medetomidine, with reversal by atipamezole. Methods vary for times of administration of the atipamezole, and literature is lacking regarding appropriate reversal time. We investigated the recovery of mice reversed with atipamezole 10 min (early) or 40 min (late) after induction of anesthesia. Time to regain pinch-reflex or righting reflex did not differ between the 2 reversal points, but time to walking was significantly greater in mice that underwent early reversal with atipamezole. This delay was not mitigated by administration of atropine as part of the anesthetic regimen. Inclusion of acetylpromazine in the anesthetic regimen shortened the time needed to reach a surgical plane of anesthesia but also prolonged recovery times as determined by righting reflex and time to walking.
The combination of ketamine and medetomidine is used widely for injectable anesthesia of sheep, primates, cats, dogs, pigs, rabbits, and small rodents.3,6,9,24 Atipamezole is used in conjunction with these to reverse medetomidine-induced anesthesia, analgesia, and muscle relaxation, resulting in much shorter recovery times for animals than can be achieved with barbiturate-based anesthesia. The manufacturer's protocol is unclear regarding the optimal time at which to administer atipamezole to achieve reversal, and little is known about how the timing of reversal may affect anesthetic outcome. One study noted prolonged recovery times with early reversal in reindeer, which showed an elimination half-life of 76 min for medetomidine and 60 min for atipamezole,20 and suggested that residual medetomidine may lead to a ‘resedation effect.’ In a recent survey, approximately 5% of veterinarians reported observing resedation effects after atipamezole administration.14 An interval of 15 to 40 min between administration of medetomidine and reversal with atipamezole was recommended for use in rabbits, but the reasoning behind this recommendation was not given.10
Ketamine acts as an antagonist of the N-methyl-D-aspartate receptor by blocking the effects of the excitatory neurotransmitter glutamate at this receptor and perhaps preventing central sensitization.3 In addition, ketamine is a sympathetic nervous system stimulant of cardiovascular activity.22 Medetomidine is an α2-adrenoceptor agonist that acts at central receptors to cause sedative and analgesic effects. In addition, medetomidine is a depressant of the sympathetic nervous system, lowering heart rate and blood pressure, and has diuretic effects.15,17 In humans, medetomidine has the benefit of reducing ketamine-induced postanesthetic delirium, but a similar benefit in small animals has not been shown.17 The stimulatory effects of ketamine on the heart and circulation counterbalance the depressant effects of medetomidine,16,18 and together these agents achieve adequate anesthesia and analgesia for surgical procedures.3,6,12,15,22,24,25
Atropine, an anticholinergic agent,4,16,19 is sometimes used in conjunction with ketamine–medetomidine, because it prevents medetomidine-induced bradycardia.5,21,27 In addition, atropine causes bronchodilation and decreased bronchial secretions.4,5,19,21 Acetylpromazine is a dopamine receptor antagonist with tranquilizer effects and can be used with ketamine–medetomidine to induce a surgical plane of anesthesia.18 Atipamezole is an α2-adrenoceptor antagonist that rapidly reverses the effects of medetomidine on central and peripheral receptors to restore cardiovascular, respiratory, and gastrointestinal function, thereby markedly decreasing recovery time.12 Because medetomidine-induced anesthesia can result in hypothermia, hypotension, and bradycardia,1,5,16,21,23,27 the use of atipamezole may benefit anesthetic recovery in rodents, by speeding recovery to walking, drinking, and eating and reducing the chances of anesthesia-induced hypothermia.
We completed an observational study to investigate recovery parameters for mice treated with ketamine–medetomidine and reversed with atipamezole at 10 min (early) or 40 min (late) after initiation of anesthesia. Both early and late reversal times were assessed, because early reversal might be used after short-term immobilization, as for minimally or noninvasive procedures (such as radiography or bioimaging), whereas later reversal typically would follow surgery. In addition, because little information is available on the effect of atropine and acetylpromazine on induction time to surgical plane of anesthesia and time to recovery, we investigated the effect of the addition of these 2 drugs to the medetomidine–ketamine regimen.
Materials and Methods
Mice and anesthetic protocols.
Protocol approval was obtained from the local Animal Ethics Committee. Mice were obtained from Jackson Laboratory (Bar Harbor, ME) and bred under SPF conditions at the University of Otago, maintained at 20 ± 2 °C under a 12:12-h light:dark cycle, housed on corncob bedding, and tested for specific pathogens in accordance with international guidelines.11 All mice were maximally bright, alert, and responsive and were within the weight range of 17 to 23 g. All mice were female, inbred BALB/cJ mice of similar age (9 to 12 wk), from the same breeding facility. Groups of 6 BALB/cJ mice were weighed, treated with mineral oil to prevent corneal drying, and injected (subcutaneously in the right flank) with ketamine (75 mg/kg; Parnell NZ, Auckland, NZ) and medetomidine (1 mg/kg; Pfizer Animal Health, Auckland, NZ) with or without atropine (0.05 mg/kg) or acetylpromazine (1mg/kg; both from Phoenix Pharm, Auckland, NZ, and both administered subcutaneously in the left flank). Anesthesia was reversed with atipamezole (5 mg/kg SC left flank; Pfizer Animal Heath) at 10 or 40 min after injection of ketamine–medetomidine. All drugs were diluted in saline and administered in a final volume of 100 µL. Saline was given in the place of omitted drugs to maintain the injection volume among mice. Dosing was staggered, with mice being anesthetized 5 to 10 min after one another. Mice were placed supine in separate plastic containers on a prewarmed 37 °C heated pad. In all experiments, mice were monitored until normal walking ability had returned, after which they were monitored for general condition (bright, alert, responsive) and food and water intake for a further 48 h.
Reflex testing.
For each mouse, a forelimb, a hindlimb, and the tail were pinched with blunt, plastic forceps for as long as 2 s every 5 min after administration of drugs until complete loss or gain of reflexes was recorded at 2 successive time points. Reflex scoring was performed by a single operator, based on the strength of the reaction on a scale of 0 to 3: 0, no reaction; 1, slow and weak reaction; 2, strong but slow reaction; 3, quick and strong reaction (consistent with the normal preanesthetic state). Summing the scores for each of these reflexes yielded a possible maximal combined reflex score (CRS) of 9. The time taken to regain the righting reflex (turning supine to prone) was recorded. Regarding quantitation of ‘recovery of walking,’ we observed in a preliminary study that recovery of walking begins with movement of the forefeet alone, followed by dragging of the hindquarters, and eventual involvement of the hindfeet to allow sustained walking. Therefore, time to recovery of walking is defined in the current study as the time to sustained walking that involves the hind feet. One or more instances of audible vocalisation26 (defined here as a high-pitched noise occurring with respiration) were recorded by the operator as a positive value for vocalization. Mice were anesthetized at the same time of day to control for circadian (diurnal) variation. The data first were assessed for normality, and selected data sets assigned a priori were analyzed for significance by parametric, 2-tailed t tests by using Graph Pad Prism 5 (GraphPad Software, La Jolla, CA). A P of less than 0.05 was defined as the level of statistical significance.
Results
Reflex monitoring.
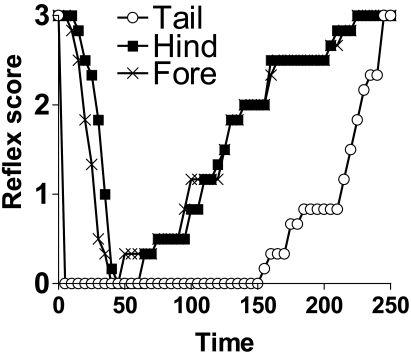
We initially investigated the reflex response at different anatomic sites in mice after ketamine–medetomidine anesthesia, but the time to loss of hindfoot, forefoot, and tail reflexes varied greatly (Figure 1). Due to these differences, we monitored the combined reflex score (CRS) in the current study. Therefore a CRS of 0 reflects complete loss of reflex response after pinch stimulation at the forelimb, hindlimb, and tail sites.
Figure 1.
Individual scores (mean, n = 6) for hindfoot, forefoot, and tail reflexes of mice anesthetized with ketamine–medetomidine only.
Effect of acetylpromazine and atropine on reflex loss and recovery.
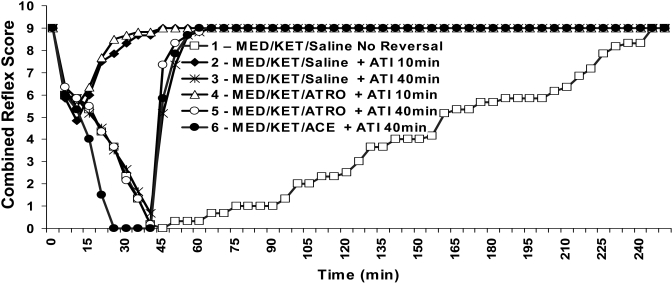
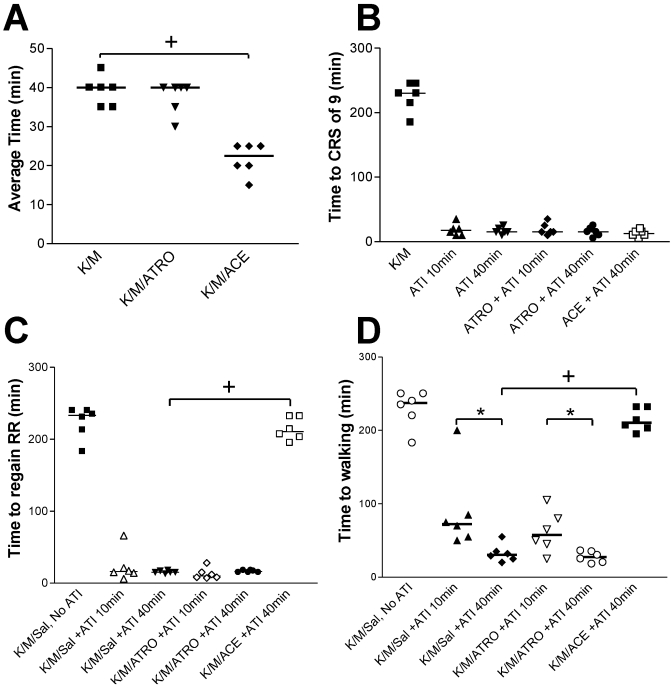
The average time between administration of ketamine–medetomidine and reaching a surgical plane of anesthesia (CRS = 0) was significantly (P < 0.05) shorter when acetylpromazine was included (Figures 2 and 3 A). However, acetylpromazine significantly (P < 0.05) increased recovery time, as determined by righting reflex and time to walking (Figure 3 C and D). In contrast, coadministration of atropine did not significantly alter the time to complete reflex loss in ketamine–medetomidine-anesthetized mice (Figures 2 and 3 A), nor did it alter recovery time (Figures 2 and 3).
Figure 2.
Time course showing the combined reflex scores (mean) of mice (n = 6) treated with different regimens. Anesthesia was initiated at time 0. ACE, acetylpromazine; ATI, atipamezole; ATRO, atropine; KET, sketamine; MED, medetomidine.
Figure 3.
Time to (A) achieve a combined reflex score (CRS) of 0, (B) achieve a CRS of 9, (C) regain righting reflex (RR), and to (D) resume walking well according to treatment group. Times to recovery for each parameter for ketamine-medetomidine-only mice are plotted (from t = 0) in panels B through D and are shown for descriptive comparison only. Values for atipamezole-reversed mice are plotted as times starting from the point of atipamezole reversal (t = 10 or t = 40). Unless indicated, there was no significant difference between atipamezole-reversed groups. +, P < 0.0001; *, P < 0.05; ACE, acetylpromazine; ATI, atipamezole; ATRO, atropine; K/M, ketamine-medetomidine; Sal, saline.
Reflex response after early and late reversal with atipamezole.
Groups that received atipamezole at 10 min after initiation of anesthesia did not differ from the those that received the reversal agent at 40 min in the time needed to regain a CRS of 9 (Figure 3 B) or the righting reflex (Figure 3 C). However, the time to walking was significantly (P < 0.05) delayed in the groups undergoing early (10 min) as compared with late (40 min) atipamezole reversal (Figure 3 D). Vocalization was recorded in 4 of the 6 mice that underwent atipamezole reversal at 10 min. This vocalization was not mitigated by atropine (5 of 6 mice) and was not observed in mice reversed at 40 min. Regardless of group, there were no mortalities and no weight loss or reduced water intake at 24 and 48 h after anesthesia.
Discussion
In the current study, we have shown that early administration of atipamezole to reverse ketamine–medetomidine-induced anesthesia (that is, at 10 min after anesthesia induction) can prolong the recovery time of mice. This effect was exemplified by the delayed time to walking in the early atipamezole reversal group. The lack of a significant difference in the regaining of full CRS and righting reflex between the 10 min and 40 min atipamezole reversal groups suggests that the prolonged time to walking was not related to pedal withdrawal reflex activity, because the mice had full reflexes at similar times in both of these groups. The time to normal locomotion is a biologically important parameter because the more rapid the return to normal water and food intake, the faster the animal will regain homeostasis. In addition, prompt regaining of locomotion may reduce anesthesia-induced hypothermia. Vocalization was noted in groups that underwent early atipamezole reversal. Vocalization may occur during painful or stressful procedures.26 Although we did not try to determine the cause of vocalization in the current study, it may have been indicative of animal disorientation, nausea, or pain. Anecdotal evidence at our facility, supported by reports in the literature,14,20 alerted us to the possibility that early atipamezole reversal of ketamine–medetomidine-induced anesthesia might lead to delayed recovery or even death. Because atipamezole administration may antagonize the antinocociceptive effects of opioids,13 we urge consideration of inclusion of appropriate analgesia to accompany atipamezole use.
Because the addition of atropine to the anesthetic regimen did not alter the variables measured in this experiment, atropine does not seem to alter the extended recovery time associated with early atipamezole reversal of ketamine–medetomidine anesthesia. The effectiveness of atropine in preventing medetomidine-induced bradycardia or reducing lung secretions4,19 was not investigated in the current study. Atropine has the potential to induce hypertension and tachycardia in dogs2 and humans.19 The effect of atropine on blood pressure and heart rate in mice has not been investigated extensively and may be minimal.4,7,8 However, the use of atropine for anesthetic protocols remains controversial.2,19 Although no mice died during the current study, further trials on larger groups, using both sexes and other strains of mice, may be necessary to uncover adverse effects of either atropine administration2 or early atipamezole reversal.14,20
Our additional finding was significant differences in the time to loss of forelimb, hindlimb, and tail reflexes (Figure 1). This finding emphasizes the need to test all of these reflexes before beginning a major surgical procedure. The tail reflex appears to give a false-negative test result, and neither forelimb nor hindlimb should be used alone. This dilemma presents an animal welfare concern if personnel performing surgery rely on a single reflex test to judge the depth of surgical anesthesia. In mice anesthetized with medetomidine–ketamine, considerable delay in the loss of pedal withdrawal after induction (as determined by sedation and loss of righting reflex) has been reported.6,9 Although the pedal withdrawal test is likely to be a conservative indicator of the surgical plane of anesthesia, it is a useful benchmark to include in the CRS for major surgery, particularly when access to more sophisticated monitoring equipment is not available.
The time to achieve a CRS of 0 was shortened significantly when acetylpromazine was added, providing a useful regimen for those wishing to begin procedures more promptly. The use of acetylpromazine, however, did significantly prolong recovery times, as determined by the righting reflex and time to walking. Therefore, acetylpromazine-mediated delays in recovery from ketamine–medetomidine anesthesia may delay homeostatic recovery and increase the probability of hypothermia. The decision to add this drug to the regimen should be made based on the specific requirements of the intended procedure.
In light of the current study, we make the following recommendations to optimize anesthetic protocols to avoid prolonged recovery in rodents: anesthesia should be administered 40 min before surgery when medetomidine–ketamine is used or 25 min beforehand when acetylpromazine is added; both forelimb and hindlimb reflexes should be absent before commencement of surgery; and atipamezole should be administered no sooner than 40 min after administration of ketamine–medetomidine.
Acknowledgments
This study was supported by a Summer Research Scholarship from the Division of Health Sciences, University of Otago. We thank Lesley Schofield for assistance.
References
- 1.Albarran-Juarez J, Gilsbach R, Piekorz RP, Pexa K, Beetz N, Schneider J, Nurnberg B, Birnbaumer L, Hein L. 2009. Modulation of α2-adrenoceptor functions by heterotrimeric Galphai protein isoforms. J Pharmacol Exp Ther 331:35–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alibhai HI, Clarke KW, Lee YH, Thompson J. 1996. Cardiopulmonary effects of combinations of medetomidine hydrochloride and atropine sulphate in dogs. Vet Rec 138:11–13 [DOI] [PubMed] [Google Scholar]
- 3.Bennett GJ. 2000. Update on the neurophysiology of pain transmission and modulation: focus on the NMDA receptor. J Pain Symptom Manage 19:S2–S6 [DOI] [PubMed] [Google Scholar]
- 4.Brock KA. 2001. Preanaesthetic use of atropine in small animals. Aust Vet J 79:24–25 [DOI] [PubMed] [Google Scholar]
- 5.Carrol GL. 2008. Small animal anaesthesia and analgesia. Hoboken (NJ): Wiley-Blackwell [Google Scholar]
- 6.Cruz JI, Loste JM, Burzaco OH. 1998. Observations on the use of medetomidine–ketamine and its reversal with atipamezole for chemical restraint in the mouse. Lab Anim 32:18–22 [DOI] [PubMed] [Google Scholar]
- 7.Fox JG. 2007. The mouse in biomedical research. Amsterdam (the Netherlands): Academic Press [Google Scholar]
- 8.Gehrmann J, Hammer PE, Maguire CT, Wakimoto H, Triedman JK, Berul CI. 2000. Phenotypic screening for heart rate variability in the mouse. Am J Physiol Heart Circ Physiol 279:H733–H740 [DOI] [PubMed] [Google Scholar]
- 9.Hahn N, Eisen RJ, Eisen L, Lane RS. 2005. Ketamine–medetomidine anesthesia with atipamezole reversal: practical anesthesia for rodents under field conditions. Lab Anim (NY) 34:48–51 [DOI] [PubMed] [Google Scholar]
- 10.Harcourt-Brown F. 2004. Textbook of rabbit medicine. London (UK): Reed Educational and Professional Publishing [Google Scholar]
- 11.Howard B, van Herck H, Guillen J, Bacon B, Joffe R, Ritskes- Hoitinga M. 2004. Report of the FELASA Working Group on evaluation of quality systems for animal units. Lab Anim 38:103–118 [DOI] [PubMed] [Google Scholar]
- 12.Jang HS, Choi HS, Lee SH, Jang KH, Lee MG. 2009. Evaluation of the anaesthetic effects of medetomidine and ketamine in rats and their reversal with atipamezole. Vet Anaesth Analg 36:319–327 [DOI] [PubMed] [Google Scholar]
- 13.Jang HS, Lee MG. 2009. Atipamezole changes the antinociceptive effects of butorphanol after medetomidine–ketamine anaesthesia in rats. Vet Anaesth Analg 36:591–596 [DOI] [PubMed] [Google Scholar]
- 14.Kaartinen MJ, Cuvelliez S, Brouillard L, Rondenay Y, Kona-Boun JJ, Troncy E. 2007. Survey of utilization of medetomidine and atipamezole in private veterinary practice in Quebec in 2002. Can Vet J 48:725–730 [PMC free article] [PubMed] [Google Scholar]
- 15.Ko JC, Fox SM, Mandsager RE. 2000. Sedative and cardiorespiratory effects of medetomidine, medetomidine–butorphanol, and medetomidine–ketamine in dogs. J Am Vet Med Assoc 216:1578–1583 [DOI] [PubMed] [Google Scholar]
- 16.Kodama Y, Iino S, Shigemasa Y, Suzuki H. 2010. Properties of acetylcholine-induced relaxation of smooth muscle isolated from the proximal colon of the guinea pig. J Smooth Muscle Res 46:185–200 [DOI] [PubMed] [Google Scholar]
- 17.Levanen J, Makela ML, Scheinin H. 1995. Dexmedetomidine premedication attenuates ketamine-induced cardiostimulatory effects and postanesthetic delirium. Anesthesiology 82:1117–1125 [DOI] [PubMed] [Google Scholar]
- 18.Maddison JE, Page SW, Church DB. 2002. Small animal clinical pharmacology. London (UK): Harcourt Publishers [Google Scholar]
- 19.Micromedex T. 2008. Drugdex evaluations: atropine. Ann Arbor (MI): Thomson Reuters [Google Scholar]
- 20.Ranheim B, Horsberg TE, Nymoen U, Soli NE, Tyler NJ, Arnemo JM. 1997. Reversal of medetomidine-induced sedation in reindeer (Rangifer tarandus tarandus) with atipamezole increases the medetomidine concentration in plasma. J Vet Pharmacol Ther 20:350–354 [DOI] [PubMed] [Google Scholar]
- 21.Seymour C, Gleed R. 1999. Manual of small animal anaesthesia and analgesia. Quedgeley (UK): British Small Animal Veterinary Association [Google Scholar]
- 22.Sinner B, Graf BM. 2008. Ketamine. Handb Exp Pharmacol (128):313–333 [DOI] [PubMed] [Google Scholar]
- 23.Tan CM, Wilson MH, MacMillan LB, Kobilka BK, Limbird LE. 2002. Heterozygous α2A-adrenergic receptor mice unveil unique therapeutic benefits of partial agonists. Proc Natl Acad Sci USA 99:12471–12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taylor R, Hayes KE, Toth LA. 2000. Evaluation of an anesthetic regimen for retroorbital blood collection from mice. Contemp Top Lab Anim Sci 39:14–17 [PubMed] [Google Scholar]
- 25.Williams AM, Wyatt JD. 2007. Comparison of subcutaneous and intramuscular ketamine–medetomidine with and without reversal by atipamezole in Dutch belted rabbits (Oryctolagus cuniculus). J Am Assoc Lab Anim Sci 46:16–20 [PubMed] [Google Scholar]
- 26.Williams WO, Riskin DK, Mott AK. 2008. Ultrasonic sound as an indicator of acute pain in laboratory mice. J Am Assoc Lab Anim Sci 47:8–10 [PMC free article] [PubMed] [Google Scholar]
- 27.Zuurbier CJ, Emons VM, Ince C. 2002. Hemodynamics of anesthetized ventilated mouse models: aspects of anesthetics, fluid support, and strain. Am J Physiol Heart Circ Physiol 282:H2099–H2105 [DOI] [PubMed] [Google Scholar]