Abstract
The chronically catheterized fetal sheep is a widely used model for fetal physiologic and pathophysiologic investigations. Catheterization involves opening the amniochorion to gain access to the fetus. In the current study, we explored the role of the amnion and amniochorion in maintaining normal amniotic fluid volume (AFV) and composition and fetal blood-gas status after surgery. Fetal sheep were catheterized at 119.6 ± 0.3 (mean ± SE, n = 25) d gestation (term, approximately 147 d). An opening equal to approximately 5% of total membrane surface area was created by resecting a portion of the amnion or amniochorion during surgery. The uterine wall was closed in all animals. Compared with control sheep (AFV = 992 ± 153 mL, n = 11), resection of the amnion had no significant effect on AFV (745 ± 156 mL, n = 7) measured 5 d after surgery, whereas resection of the amniochorion resulted in extensive loss of amniotic fluid (AFV = 131 ± 38 mL, n = 7). This loss resulted from extensive entry of amniotic fluid into the space between the chorion and uterine wall. Amniotic fluid, fetal plasma, and urinary solute concentrations; arterial pH; oxygen tension; and carbon dioxide tension were unchanged. A small opening in the amnion has minimal effects on ovine AFV, whereas a small opening in the amniochorion results in oligohydramnios. In addition, the amnion appears to be the primary site that limits the rate of amniotic fluid absorption by the chorionic vasculature.
Abbreviation: AF, amniotic fluid; AFV, amniotic fluid volume; ANCOVA, analysis of covariance
The chronically catheterized fetal sheep was developed as an experimental animal model approximately 40 y ago3,9,19,20,22 and continues to be widely used to explore many aspects of fetal physiology and pathophysiology. A wide variety of surgical methodologies have been described and these involve surgical opening of the amniochorion to gain access to the fetus. However, the effects of incomplete closure of the ovine fetal membranes on the fetus during the postsurgical period have not been described, and whether an opening in the amnion or amniochorion will result in the loss of amniotic fluid (AF) in fetal sheep is unknown.
During our studies of AF dynamics in fetal sheep, we noted anecdotally that incomplete membrane closure resulted in near complete loss of AF. Because the failure to achieve membrane closure occurred rarely, whether the loss of AF resulted from preexisting abnormalities in the membranes or from the moderately sized opening in the membranes after surgery has been unclear. To determine the role of the fetal membranes in regulating amniotic fluid volume (AFV), we performed experiments to evaluate whether an opening in the amnion or amniochorion altered AFV or composition. In humans, a loss of AF is associated with fetal acidosis and hypoxia due to umbilical cord compression.16,23 Therefore, we explored whether an opening in the amnion or amniochorion would alter fetal blood-gas status in sheep.
Materials and Methods
These studies were approved by our IACUC, and we followed the National Research Council's Guide for the Care and Use of Laboratory Animals.14
A total of 25 late-gestation pregnant sheep (mixed western breed; Nebeker Ranch, Lancaster, CA) each carrying a single fetus were studied. Ewes were anesthetized with intravenously administered sodium pentobarbital (25 mg/kg). Anesthesia was maintained with halothane (0.5% to 2%) in oxygen through an endotracheal tube. This regimen also anesthetizes the fetus. At the time of surgery, the sheep were at 119.6 ± 0.3 d of gestation (term, 145 to 150 d).
The sheep underwent surgical placement of catheters in the fetal femoral arteries, urinary bladder, AF by attachment to the fetal skin, and maternal femoral artery; the fetal urachus was ligated at the base of the umbilical cord as previously described to prevent urine entry into the allantoic sac.1,7,8 Aseptic technique was used throughout the surgical procedure. In 11 control animals, the amniochorion was closed carefully by tying the membranes around the catheters to ensure no leakage of AF prior to uterine closure. In 7 ewes, a portion of the amnion was peeled off the underlying chorion and resected prior to closing the chorion and uterus. In another 7 sheep, a portion of the amniochorion was resected prior to uterine closure.
Prophylactic antibiotics were given to the ewe (1,500,000 U penicillin G IM) at the time of surgery and to the fetus through the AF (500 mg ampicillin) on the day of surgery and on the 4 subsequent days. Fetal and maternal vascular catheters were flushed daily with a heparinized saline solution (100 U/mL). Each ewe was given buprenorphine (0.6 mg SC) for analgesia for 2 d.
Five days after surgery, osmolality (Advanced Instruments, model 3D3, Norwood, MA) and solute concentrations (sodium, potassium, chloride, calcium, glucose, lactate, and bicarbonate; model 725, Radiometer ABL analyzer, Danaher, Washington, DC) of fetal blood, maternal blood, AF, and fetal urine were measured. Fetal arterial pH, carbon dioxide tension, oxygen tension, hematocrit, and oxygen saturation were measured by using the analyzer (Radiometer, Danaher). Fetal urinary flow rate was measured by gravity drainage. In the 11 control sheep, AFV was measured by indicator dilution.1,5,8,18 In the resected ewes, AFV was measured by direct collection (drainage) at autopsy on postsurgical day 5. To compare the indicator dilution and drainage methods for measuring AFV, we measured AFV in 26 fetuses with complete membrane closure but used for other studies first by indicator dilution and then by drainage at autopsy 5 to 6 h later. The delay occurred because 5 to 6 h were required to complete the indicator dilution measurement.
After completion of the experiments, the sheep were euthanized by using IACUC-approved euthanasia procedures (130 mg/kg sodium pentobarbital IV). Thirty minutes prior to euthanasia, indigo carmine blue dye was injected into the amniotic sac to mark tissues exposed to AF. During autopsy, the area of the opening in the amnion or amniochorion was estimated by measuring the rough edges of the opening to the nearest centimeter in 2 perpendicular dimensions and taking their product.
Data are expressed as mean ± SE. Unpaired t tests, Mann–Whitney nonparametric test, bivariate linear regression analysis, one-factor ANOVA, and one-factor analysis of covariance (ANCOVA) as provided through inhouse software were used for statistical testing. Statistical significance was accepted as P ≤ 0.05.
Results
Five days after surgery of pregnant ewes, resection of a portion of the amnion resulted in an opening ranging from 50 to 500 cm2 (181 ± 71 cm2), whereas amniochorionic resection created an opening ranging from 25 to 144 cm2 (79 ± 20 cm2). The sizes of the openings were not significantly different (P = 0.19). There was no significant relationship between the area of the opening and the AFV for either the amnion-resected (P = 0.30) or amniochorion-resected (P = 0.57) groups or for all 3 groups combined (P = 0.18), as determined by linear bivariate regression. There were no membrane openings in the control fetuses.
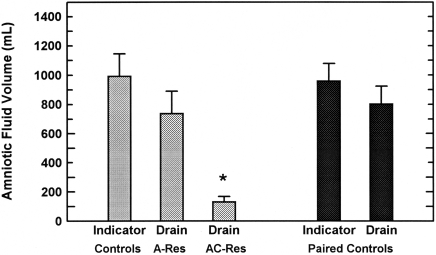
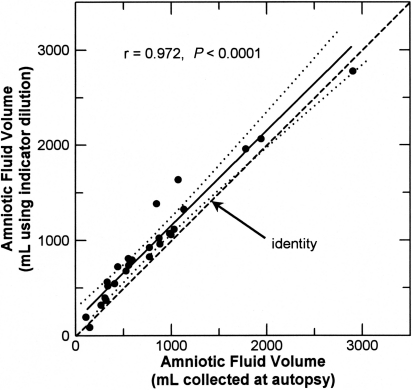
According to indicator dilution, AFV in the control fetuses was 992 ± 153 mL, whereas AFV determined by drainage was 745 ± 156 mL in amnion-resected fetuses (P = 0.28 compared with control) and 131 ± 38 mL in amniochorion-resected fetuses (P < 0.001 compared with control). In 26 fetuses with complete membrane closure, AFV determined by indicator dilution was 957 ± 121 mL and that determined on the same day by drainage was 801 ± 122 mL (P < 0.0001, paired t test). AFV in amniochorion-resected fetuses differed significantly from that in all other groups (Figure 1). The paired values for AFV determined by indicator dilution and drainage were highly correlated (r = 0.972, n = 26), and the bivariate regression slope was not different from unity (0.996 ± 0.052). However, the intercept was significantly (P < 0.01) greater than 0, indicating that the dilutional volume was 159 ± 57 mL greater that the drainage volume (Figure 2).
Figure 1.
Amniotic fluid volume (mean ± SE) determined by using indicator dilution techniques (Indicator) or direct collection at autopsy (Drain). The 3 bars on the left were determined in 3 separate groups of fetuses, and the 2 bars on the right were paired measurements in a different group of fetuses. According to one-factor ANOVA, volume in amniochorion-resected (AC-Res) fetuses was significantly (*, P < 0.01) different from that of all other groups, whereas that in amnion-resected (A-Res) fetuses differed only from that in amniochorion-resected fetuses.
Figure 2.
Comparison of amniotic fluid volumes measured by indicator dilution techniques with those measured in the same animal by direct collection at autopsy 5 to 6 h later. Each dot represents a different animal. The solid line is the regression line; dotted lines indicate the 95% confidence interval about the regression line; dashed line is the line of identity.
There were no significant differences in AF solute concentrations or osmolality between the 11 control, 7 amnion-resected, and 7 amniochorion-resected fetuses (Table 1). Further, the concentration gradients between fetal blood and AF were not different between the 3 groups. The transplacental concentration gradients between maternal and fetal blood for potassium, chloride, calcium, glucose, lactate, and bicarbonate were unchanged with resection of the amnion or amniochorion. Neither fetal urinary concentrations (Table 1) nor the fetal urinary flow rate (0.46 ± 0.04 mL/min; n = 25) differed between the 3 groups. However, the transplacental sodium gradient was reduced after amniochorionic resection compared with that in control sheep (5.2 ± 0.5 mmol/L compared with 8.0 ± 0.5 mmol/L, ANOVA P = 0.0097) but not after amnionic resection (6.8 ± 0.8 mmol/L). A significant positive relation (r = 0.70, P = 0.024) existed between AFV and urinary flow rate in the control and amnion-resected fetuses but was no longer significant after amniochorionic resection (ANCOVA P = 0.004 compared with control and amnion-resected).
Table 1.
Solute concentrations and osmolalities (mean ± SE) 5 d after fetal catheterization
| Maternal plasma | Fetal plasma | Amniotic fluid | Fetal urine | |
| Sodium (mmol/L) | 144.8 ± 0.5 | 137.9 ± 0.6 | 111.9 ± 2.5 | 45.8 ± 3.6 |
| Potassium (mmol/L) | 4.13 ± 0.05 | 4.27 ± 0.09 | 8.29 ± 0.79 | 9.14 ± 1.27 |
| Chloride (mmol/L) | 110.8 ± 0.6 | 103.0 ± 0.7 | 87.8 ± 2.1 | 17.0 ± 2.2 |
| Calcium (mmol/L) | 0.95 ± 0.08 | 1.43 ± 0.02 | 1.01 ± 0.12 | 0.29 ± 0.04 |
| Bicarbonate (mmol/L) | 25.9 ± 0.5 | 28.0 ± 0.4 | 13.8 ± 1.0 | 6.5 ± 0.9 |
| Glucose (mmol/L) | 3.9 ± 0.1 | 1.17 ± 0.05 | 0.92 ± 0.21 | 0.28 ± 0.03 |
| Lactate (mmol/L) | 1.0 ± 0.1 | 1.35 ± 0.05 | 1.35 ± 0.21 | 0.33 ± 0.02 |
| Osmolality (mOsm/kg water) | 299.2 ± 1.3 | 297.0 ± 1.0 | 268.1 ± 2.9 | 159.4 ± 7.4 |
Because there were no statistically significant differences in fetal or amniotic fluid composition between control, amnion-resected, and amniochorion-resected fetuses, data were combined (n = 25).
Fetal arterial (descending aorta) pH (7.35 ± 0.01, n = 25), carbon dioxide tension (54.5 ± 0.8 mm Hg), oxygen tension (20.4 ± 0.5 mm Hg), hematocrit (30.3% ± 0.7%), and oxygen saturation (53.0% ± 1.4%) were similar in all 3 groups; therefore the groups were combined. Further, none of these variables were correlated with AFV.
In control fetuses, the indigo carmine blue dye was contained within the amniotic space. With amniotic resection, only the portion of the chorion below the opening in the amnion was stained by the dye. In contrast, with amniochorionic resection, the entire space between the chorion and uterine wall was stained. In no sheep was blue dye outside the uterus, and there was no vaginal leakage of dye.
Discussion
In chronically catheterized fetal sheep, a normal AFV should contribute to the fetus being physiologically normal. To understand the maintenance of normal AFV, it is necessary to understand the contribution of the individual components of the amniochorion to the maintenance of AFV. In the current study, we found that a small opening in the amnion that remains after fetal surgery has little effect on AFV. This outcome is in contrast to the extensive loss of AF that occurs with a similarly sized opening in the amniochorion. Past studies in fetal sheep with intact membranes have shown that intraamniotic administration of exogenous fluids results in increased fluid absorption across the amnion into the fetal circulation, with subsequent transfer to the maternal circulation.5,7,8,10-13,18,21,24 Presumably, the fluid lost from the amniotic compartment after resection of the amniochorion in the present study similarly was absorbed into the fetal circulation and transferred across the placenta into the maternal circulation.
In fetal sheep, AFV is regulated primarily by modulating the rate of intramembranous absorption of AF across the amnion and into the underlying fetal vasculature in the chorion and fetal surface of the placenta.5,6,8,10,11,13,18,21 The present study begins to address the separate roles of the amnion and chorion in this process. First, the amniochorion plays a critical role, in that a relatively small hole in the amniochorion exposed the entire outer surface of the chorion to AF and resulted in an extensive loss of the AF. Because the absorption of amniotic urea and water into maternal blood within the uterine wall in sheep is undetectably low,1,2 it appears that the reduced AFV after amniochorionic resection is due to leakage of AF into the choriouterine space and subsequent absorption by the fetal blood vessels within the chorion. This conclusion is supported by several observations. First, fetal urinary production was not reduced in amniochorion-resected fetuses. Further, it has been previously shown that fetal swallowing of AF decreases when AFV is reduced,15 and fetal lung liquid secretion changes little as AFV is altered.21 Therefore, changes in fetal urinary production, swallowing of AF, and altered lung liquid secretion are not responsible for the reduction in AFV after amniochorionic resection. Because there are only 4 major amniotic inflows (fetal urine and lung liquid) and outflows (fetal swallowing and intramembranous absorption across the amnion in to the underlying fetal vasculature), the reduction in AFV appears to be due to an increased absorption by the chorionic portion of the intramembranous pathway.
In contrast to the extensive loss of AF caused by a small opening in the amniochorion, a similarly sized hole in the amnion has little, if any, effect on AFV. The difference in mean AFV between the control and amnion-resected groups was 247 mL when different methods were used to measure AF volumes. When corrected for the 159-mL difference in volumes determined with paired measurements by using the 2 methods, the difference between the control and amnion-resected volumes is reduced to only 88 mL. This potentially small reduction in AFV in the amnion-resected group suggests that the remaining amnion continues to function as an important but incomplete barrier for retaining AF and maintenance of AFV. However, the 188-cm2 opening in the amnion is less than 10% of the surface area of the amnion and amniochorion.1,6 In view of the extensive fluid loss with exposure of the entire outer surface of the chorion to AF combined with the observation that the chorion contributes little to the diffusive barrier of the amniochorion,17 exposure of the entire inner surface of the chorion to AF by complete removal of the amnion (if it were surgically possible while maintaining a viable preparation) likely would result in a large reduction in AFV similar to that with resection of the amniochorion. Therefore, the amnion appears to be the primary structure that limits the rate of intramembranous absorption. Further, the mechanisms that mediate the extensive loss of AF with amniochorionic resection appear very different from those that occur in humans after rupture of the amniochorion, because our sheep had no vaginal or extrauterine leakage whereas vaginal fluid leakage is a primary sign of membrane rupture in humans.4,23
The failure of AF solute concentrations to change strongly suggests that the chorionic absorption process primarily involved bulk transfer of AF water together with dissolved solutes. This notion is consistent with our previous studies in ovine fetuses with intact membranes, in which intramembranous absorption of solutes was highly correlated with the rate of water absorption and in which clearances of most AF solutes were similar.7,12 Collectively, these observations suggest that the bulk absorptive process that mediates intramembranous absorption may not be located within the amnion but rather is located in the endothelial cells of blood vessels lining the fetal surface of the placenta and, in sheep, those perfusing the chorion and the connective tissue layer between the amnion and chorion.6,7,12 This hypothesis implies that a primary role of the amnion is to limit the rate of intramembranous absorption by limiting the rate at which AF water and solutes move across the amniotic membrane. The factors that change the transport characteristics of the amnion to allow very large volumes of fluid to cross the amnion during administration of exogenous fluids5,7,8,10-13,18,21,24 have yet to be explored.
We anticipated that very low AFV would be associated with fetal acidosis, hypercarbia, and hypoxia because these conditions are a common concern during oligohydramnios in human pregnancies and because we have observed hypoxia in response to complete drainage of AF in previous studies.7 However, reduced AFV was not associated statistically with deteriorated fetal pH or blood gas status in the current study. Perhaps the amniochorion-resected fetuses would have become acidotic, hypercarbic, or hypoxia if they were maintained beyond 5 d after surgery or if the loss of AF had been more extensive.
The observed significant positive correlation between the rate of fetal urinary production and AFV is consistent with the fact that fetal urine is the primary source of AF and is consistent with our previous observations.24 Resection of a small portion of the amnion did not alter this relationship, suggesting that the mechanisms that maintain AFV were functioning at a near-normal level in the amnion-resected fetuses. In contrast, AFV was not related to the urinary production rate after amniochorionic resection. This observation suggests that any fluid entering the amniotic sac rapidly passed through the hole in the amniochorion and was absorbed.
The small but statistically significant decrease in the transplacental sodium gradient in the amniochorion-resected fetuses is difficult to explain. Multiple previous studies have shown that large volumes (maximum, 4 L/d) of physiologic fluids that are infused into either the fetal circulation or AF rapidly cross the placenta into the maternal circulation with little or no change in the transplacental concentration gradients.5,8,10,11,13,21,24 With amniochorionic resection and a fluid loss of approximately 600 mL over 5 d (that is, 120 mL/d), little change in the transplacental gradient would be expected, so the observed decrease is unexplained.
In summary, the present study suggests that a modest-sized opening in the ovine amnion has little effect on AFV after 5 d, whereas a similarly sized opening in the amniochorion results in an extensive loss of AF. This difference appears to be due to the exposure of only a small portion of the highly vascularized ovine chorion to AF after resection of the amnion, whereas the entire chorion is exposed to AF after resection of the amniochorion. This finding is consistent with the notion that the amnion normally limits the rate of AF movement through the intramembranous pathway, whereas the chorion primarily is involved in absorption of AF through the chorionic blood vessels. Therefore, to promote conditions for optimal fetal condition after fetal catheterization surgery, it is essential to ensure that the fetal membranes are intact and successfully closed. Further, even though AFV changed with amniochorionic resection, the AF concentrations of the major solutes changed little. The mechanism responsible for maintenance of solute concentrations in the presence of such large changes in AFV deserves further investigation.
Acknowledgments
Supported in part by grants HD064541 and HD035890 from the National Institute of Child Health and Human Development, NIH.
References
- 1.Adams EA, Choi HM, Cheung CY, Brace RA. 2005. Comparison of amniotic and intramembranous unidirectional permeabilities in late-gestation sheep. Am J Obstet Gynecol 193:247–255 [DOI] [PubMed] [Google Scholar]
- 2.Anderson DF, Faber JJ, Parks CM. 1988. Extraplacental transfer of water in the sheep. J Physiol 406:75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassett JM, Thorburn GD. 1969. Foetal plasma corticosteroids and the initiation of parturition in sheep. J Endocrinol 44:285–286 [DOI] [PubMed] [Google Scholar]
- 4.Bornstein J, Ohel G, Sorokin Y, Reape KZ, Shnaider O, Kessary-Shoham H, Ophir E. 2009. Effectiveness of a novel home-based testing device for the detection of rupture of membranes. Am J Perinatol 26:45–50 [DOI] [PubMed] [Google Scholar]
- 5.Brace RA, Cheung CY. 2004. Amniotic fluid volume responses to amnio-infusion of amniotic fluid versus lactated Ringer's solution in sheep. J Soc Gynecol Investig 11:363–368 [DOI] [PubMed] [Google Scholar]
- 6.Brace RA, Gilbert WM, Thornburg KL. 1992. Vascularization of the ovine amnion and chorion: a morphometric characterization of the surface area of the intramembranous pathway. Am J Obstet Gynecol 167:1747–1755 [DOI] [PubMed] [Google Scholar]
- 7.Brace RA, Vermin W, Huijssoon E. 2004. Regulation of amniotic fluid volume: intramembranous volume and solute fluxes in late-gestation sheep. Am J Obstet Gynecol 191:837–846 [DOI] [PubMed] [Google Scholar]
- 8.Daneshmand SS, Cheung CY, Brace RA. 2003. Regulation of amniotic fluid volume by intramembranous absorption in sheep: role of passive permeability and vascular endothelial growth factor. Am J Obstet Gynecol 188:786–793 [DOI] [PubMed] [Google Scholar]
- 9.Dawes GS, Fox HE, Leduc BM, Liggins GC, Richards RT. 1972. Respiratory movements and rapid eye movement sleep in the foetal lamb. J Physiol 220:119–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Faber JJ, Anderson DF. 1999. Regulatory response of intramembranous absorption of amniotic fluid to exogenous fluid in sheep. Am J Physiol 277:R236–R242 [DOI] [PubMed] [Google Scholar]
- 11.Faber JJ, Anderson DF. 2002. Absorption of amniotic fluid by amniochorion in sheep. Am J Physiol Heart Circ Physiol 282:H850–H854 [DOI] [PubMed] [Google Scholar]
- 12.Gesteland KM, Anderson DF, Davis LE, Robertson P, Faber JJ, Brace RA. 2009. Intramembranous solute and water fluxes during high intramembranous absorption rates in sheep with and without lung liquid diversion. Am J Obstet Gynecol 201: 85.e1–85.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert WM, Brace RA. 1989. The missing link in amniotic fluid volume regulation: intramembranous absorption. Obstet Gynecol 74:748–754 [PubMed] [Google Scholar]
- 14.Institute for Laboratory Animal Research 2010. Guide for the care and use of laboratory animals, 8th ed Washington (DC): National Academies Press [Google Scholar]
- 15.Kullama LK, Agnew CL, Day L, Ervin MG, Ross MG. 1994. Ovine fetal swallowing and renal responses to oligohydramnios. Am J Physiol 266:R972–R978 [DOI] [PubMed] [Google Scholar]
- 16.Künzel W. 1981. Umbilical circulation—physiology and pathology. J Perinat Med 9 Suppl 1:68–71 [DOI] [PubMed] [Google Scholar]
- 17.Lingwood BE, Wintour EM. 1983. Permiability of ovine amnion and amniochorion to urea and water. Obstet Gynecol 61:227–232 [PubMed] [Google Scholar]
- 18.Matsumoto LC, Cheung CY, Brace RA. 2001. Increased urinary flow without development of polyhydramnios in response to prolonged hypoxia in the ovine fetus. Am J Obstet Gynecol 184:1008–1014 [DOI] [PubMed] [Google Scholar]
- 19.Mellor DJ, Williams JT, Matheson IC. 1972. A technique for chronic catheterization of the bladder of the foetal sheep. Res Vet Sci 13:87–88 [PubMed] [Google Scholar]
- 20.Meschia G, Cotter JR, Breathnach CS, Barron DH. 1965. The hemoglobin, oxygen, carbon dioxide, and hydrogen ion concentrations in the umbilical bloods of sheep and goats as sampled via indwelling plastic catheters. Q J Exp Physiol Cogn Med Sci 50:185–195 [DOI] [PubMed] [Google Scholar]
- 21.Robertson P, Faber JJ, Brace RA, Samantha L, Hohimer AR, Davis LE, Anderson DF. 2009. Responses of amniotic fluid volume and its 4 major flows to lung liquid diversion and amniotic infusion in the ovine fetus. Reprod Sci 16:88–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rudolph AM, Heymann MA. 1967. The circulation of the fetus in utero. Methods for studying distribution of blood flow, cardiac output, and organ blood flow. Circ Res 21:163–184 [DOI] [PubMed] [Google Scholar]
- 23.Srinivas SK, Macones GA. 2005. Preterm premature rupture of the fetal membranes: current concepts. Minerva Ginecol 57:389–396 [PubMed] [Google Scholar]
- 24.Tomoda S, Brace RA, Longo LD. 1987. Amniotic fluid volume regulation: basal volumes and responses to fluid infusion or withdrawal in sheep. Am J Physiol 252:R380–R387 [DOI] [PubMed] [Google Scholar]