Abstract
Many animals are organized into social groups and have to synchronize their activities to maintain group cohesion. Although activity budgets, habitat constraints, and group properties may impact on behavioural synchrony, little is known regarding how members of a group reach a consensus on the timing of activities such as foraging bouts. Game theory predicts that pair partners should synchronize their activities when there is an advantage of foraging together. As a result of this synchronization, differences in the energetic reserves of the two players develop spontaneously and the individual with lower reserves emerges as a pacemaker of the synchrony. Here, we studied the behavioral synchrony of pair-living, nocturnal, red-tailed sportive lemurs (Lepilemur ruficaudatus). We observed 8 pairs continuously for ≥1 annual reproductive cycle in Kirindy Forest, Western Madagascar. During focal observations, one observer followed the female of a pair and, simultaneously, another observer followed the male. We recorded the location and behavioral state of the focal individual every 5 min via instantaneous sampling. Although behavioral synchrony of pair partners appeared to be due mainly to endogenous activity patterns, they actively synchronized when they were in visual contact (<10 m). Nevertheless, red-tailed sportive lemurs benefit from synchronizing their activity only for 15% of the time, when they are close together. The lack of an early warning system for predators and weak support for benefits via social information transfer in combination with energetic constraints may explain why red-tailed sportive lemurs do not spend more time together and thus reap the benefits of behavioral synchrony.
Keywords: Behavioral synchrony, Coordination, Red-tailed sportive lemurs, Sociality
Introduction
Many animals are organized into social groups that differ in size from a few individuals to several millions, as well as in composition, permanence, and cohesion. Group living implies several benefits, but also costs for individual group members. Many of the benefits are related to reduced per capita predation risk, e.g., shared vigilance or predator confusion, whereas costs of group living include increased intragroup feeding competition (Alexander 1974; Bertram 1978; Fichtel in press). In general, to reap the benefits of group living, animals need to coordinate their activities to stay cohesive (Conradt and Roper 2003, 2005). To maintain cohesion individuals need to synchronize their activities, such as foraging, resting, and locomotion (Conradt and Roper 2000; King and Cowlishaw 2009; Rands et al. 2008; Ruckstuhl and Neuhaus 2000). Synchronized behavior occurs when individuals perform the same activity simultaneously. Because individuals differ in their interests and needs (Altmann 1980; Cheney 1987; Fichtel et al. 2010; King and Cowlishaw 2009; Rands et al. 2003; Sueur et al. 2010), lower behavioral synchrony is expected at the group level. In general, activity, habitat and group-related processes may contribute to patterns of variability in behavioral synchrony at the group level.
The activity-budget hypothesis suggests that differing physiological demands among individuals as a consequence of differences in reproductive state, age, or sex can influence synchrony patterns within groups. This can then result in segregation, as shown in many ungulates (Barrett et al. 2006; Conradt and Roper 2000; Fischhoff et al. 2007; King and Cowlishaw 2009; Ruckstuhl 1999). Temporal group-related processes such as visual or auditory isolation, as a consequence of interindividual distances, may also result in reduced synchrony due to a reduced opportunity for the use of socially transmitted information such as signals or cues (group structure hypothesis: Cortopassi and Bradbury 2006; Dostálková and Spinka 2007; Fichtel and Manser 2010; King and Cowlishaw 2009). Finally, the habitat-constraints hypothesis suggests that synchrony breaks down in groups that feed on scattered food items in a heterogeneous habitat because not all group members are able to forage together (King and Cowlishaw 2009; Vahl et al. 2007).
Although these hypotheses explain which factors may hamper behavioral synchrony, little is known of how members of a group reach a consensus on the timing of activities such as foraging bouts (Rands et al. 2003). A dynamic game theoretical model of foraging and resting patterns in pairs, the smallest social unit, suggests that each individual chooses between resting and foraging to maximize its own survival, but that individuals synchronize their activities when there is an advantage of foraging together (Rands et al. 2003, 2008). As a result of this synchronization, differences in the energetic reserves of the two players spontaneously develop and the individual with lower reserves emerges as a pacemaker of synchrony, suggesting a simple process of how consensus can be reached within pairs.
Here, we studied behavioral synchrony in pair-living, nocturnal red-tailed sportive lemurs (Lepilemur ruficaudatus). Because most studies of behavioral synchrony have been conducted in group-living species, we asked whether the activity-budget, habitat, or group structure hypotheses can account for variation in behavioral synchrony in a pair-living species. Red-tailed sportive lemurs are an interesting model species because they are organized into dispersed pairs, which means that a male and a female occupy and defend a common home range but pair partners move mostly solitarily throughout their home range, spending only 15% of their time at <10 m of one another (Zinner et al. 2003). Because red-tailed sportive lemurs show vigilance (Fichtel 2007) and benefit from the confusion and dilution effect, we assume that behavioral synchronization might be advantageous. Thus, red-tailed sportive lemurs should synchronize their behavior to reap benefits of group living, i.e., reduced individual predation risk.
According to the activity-budget hypothesis, variation in energetic states might be lowest and less variable at the beginning of their active period, when individuals are hungry. We predict that red-tailed sportive lemurs’ foraging synchrony is highest during their first activity bout (activity-budget hypothesis). During the course of the night, energetic states and hunger levels might be more variable (Limmer and Becker 2007; King and Cowlishaw 2009), resulting in more variable foraging synchrony. Because group-related processes impact on synchrony when individuals are out of visual or auditory reach, we predict that synchrony should decrease when pair partners are out of sight (group-structure hypothesis).
The habitat-constraints hypothesis predicts that scattered food distribution in a heterogeneous habitat leads to a decrease in synchrony. Because red-tailed sportive lemurs are folivorous and food is not scattered (Ganzhorn 2002), pair partners should be able to feed in the same tree, facilitating synchrony. However, Madagascar is characterized by a pronounced seasonal variation with a short rainy season and a long dry season in which food is less abundant (Dewar and Richard 2007). Because feeding competition also plays an important role in folivorous primates (König et al. 1998; Snaith and Chapman 2008), we predict that behavioral synchrony is lower in the dry season owing to lower food availability.
Finally, red-tailed sportive lemurs are sexually monomorphic and differ in neither body size nor body mass (Hilgartner et al. 2008). According to the game-theoretical model, females should emerge as the pacemakers of the synchrony during gestation and lactation due to higher nutritional demands.
Methods
Study Site
We conducted this study in Kirindy Forest, Western Madagascar, where the German Primate Center (DPZ) operates a field research station. The local climate is characterized by pronounced seasonality with a short rainy season from November to March, followed by a longer dry season with little or no rain from April to October (Kappeler and Fichtel 2012; Sorg et al. 2003).
The core area has small trails every 25 m, and is surrounded by additional trails at 50 m along the edges of the core area. Each trail intersection is marked with a plastic tag for orientation, so that the spatial location of subjects could be assessed easily. We mapped the entire grid system and determined the coordinates of each intersection.
Capture and Marking
We caught red-tailed sportive lemurs by hand or by placing a live trap at the tree hole entrance. We briefly anesthetized them with GM2 (Rensing 1999) and marked subjects with a unique subcutaneously injected transponder (Trovan, Usling, Germany). We equipped adult individuals captured within the core area of our study site with 9-g radio collars (Biotrack, Wareham Dorset, UK), which is <3% of the individual’s body mass. We removed radio collars at the end of the project. Capture and marking complied with the current laws of Madagascar.
Data Collection
We collected data from 8 pairs that we observed over 24 mo between 2002 and 2004, for a total of 2080 observation hours. We observed each pair for ≥1 reproductive cycle, including premating (February–April), mating (May–June), gestation (June–October), and birth/weaning period (November–January) (Hilgartner et al. 2008). We followed radio-tagged individuals with radio-tracking equipment from Telonics (Mesa, AZ). We observed the subjects mainly during the first half of the night (18:00–02:00 h) with the aid of a headlamp and the occasional use of a strong flashlight and binoculars. R. Hilgartner and a Malagasy field assistant followed the pair partners simultaneously for 2 h using focal animal sampling (Altmann 1974). At 5-min intervals, we recorded the exact location, as well as the behavioral state (foraging, resting, locomotion) of each focal individual (instantaneous sampling: Altmann 1974). Observer distance from the focal individuals varied between 1 and 15 m. We recorded social interactions between pair partners and among neighbors via all-occurrence sampling.
Data Analysis
We analyzed the spatial data with the Animal Movement extension for ArcView® (Hoge and Eichenlaub 1997). For the analysis of behavioral synchrony we included only observation bouts in which both pair partners could be observed, resulting in a total of 4673 observation bouts. To estimate synchrony between pair partners, we estimated the probability of pair partners exhibiting the same activity across 2 distance categories: near (<10 m apart) and far (>10 m apart, ranging from 11 to 180 m). The intrapair distance of <10 m was selected as the criterion for synchronization, because it most likely permits visual contact between partners (Hilgartner 2006). To assess whether the behavior of the male and female in a pair is synchronized we compared the observed and expected frequencies of synchrony assuming male and female behaviors are independent (Table I). Synchrony is indicated if the observed frequencies in the diagonal of the table are larger than the corresponding expected frequencies. Cohen’s κ (Cohen 1960) provides a measure of the extent to which there is synchrony in the behavior of males and females; a value >0 indicates more synchrony than is expected purely by chance.
Table I.
Observed frequencies for a single pair in the time interval 20:00–21:00 h and the corresponding expected frequencies under the hypothesis of independent behavior
| Observed frequencies | Expected frequencies | |||||
|---|---|---|---|---|---|---|
| Male resting | Male foraging | Male locomoting | Male resting | Male foraging | Male locomoting | |
| Female resting | 41 | 15 | 4 | 28.9 | 24.4 | 6.8 |
| Female foraging | 21 | 36 | 9 | 31.8 | 26.8 | 7.4 |
| Female locomoting | 2 | 3 | 2 | 3.4 | 2.8 | 0.8 |
To test whether behavioral synchrony of red-tailed sportive lemurs is influenced by time, season, and distance between pair partners, we fitted a generalized linear mixed model (logit link function). Synchrony of activity, i.e., male and female of a given pair exhibiting the same activity was used as the binomial response term. Time of night (18:00–19:00, 19:00–20:00, 20:00–21:00, 21:00–22:00, 22:00–23:00, 23:00–24:00, >24:00 h), season (rainy or dry), and distance of the pair partner (near or far) were used as fixed factors. Pair identity was used as a random factor to control for potential pair effects. We used maximum likelihood ratio tests to test the model with fixed factors against the null model including only the intercept and random factors (Faraway 2006). The GLMM were fitted using R (R Development Core Team, Vienna, Austria, 2010) with the lme4-package (Zuur et al. 2009). We entered all predictor variables simultaneously because stepwise procedures lead to inflated type I error rates (Mundry and Nunn 2009).
To determine which sex emerges as a pacemaker of the synchrony we looked at each observation point at which both pair partners performed the same behavior and calculated how often each sex already exhibited this behavior at the observation bout before, considering this as an initiation of behavioral synchrony. We compared the number of initiations by sex in each behavioral season (mating, gestation, and lactation) with an exact binomial test (Agresti 2002). The (2-sided) test was implemented using the function binom.test of R (R Development Core Team, Vienna, Austria, 2010).
Results
Behavioral Synchrony
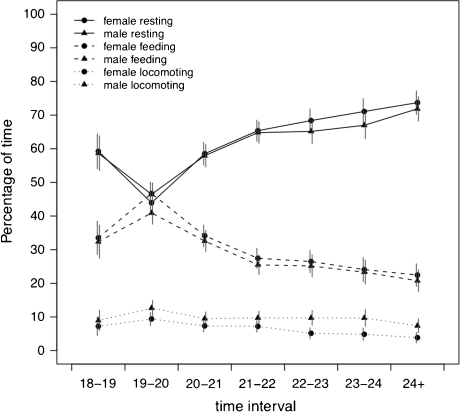
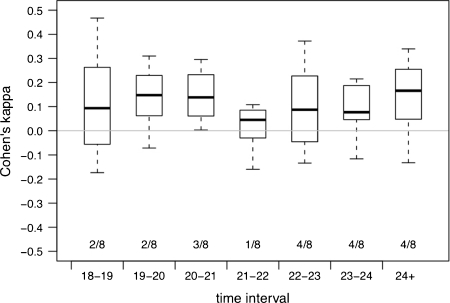
Red-tailed sportive lemurs spent most of their time resting, less time foraging, and the least time locomoting. Females and males showed highly synchronized behavior throughout their activity period, with an increase in foraging at the beginning of their active time between 19:00 and 20:00 h (Fig. 1). The Cohen’s κ index revealed that a given pair synchronized its behavior more often than expected by chance (Fig. 2). The observed frequencies of synchronized behaviors were higher than the expected frequencies in all time intervals (Table II).
Fig. 1.
Mean percentage of time spent resting, foraging, and locomoting for females (N = 8) and males (N = 8). The vertical lines represent approximate 95% confidence intervals.
Fig. 2.
Boxplots of Cohen’s κ; 0 corresponds to independent behavior. Each boxplot summarizes the coefficients for the 8 pairs in a given time interval. The numbers (2/8, 2/8, …,4/8) represent the number of pairs for which the hypothesis of independent behavior is rejected at 10% level of significance using Fisher's test.
Table II.
Number of times that the female and male displayed the same behavior (summed over all 8 pairs), the expected frequencies under the hypothesis of independent behavior, and the differences between the observed and expected frequencies
| Time interval | Observed | Expected | Difference |
|---|---|---|---|
| 18–19 | 188 | 160 | 28 |
| 19–20 | 434 | 338 | 96 |
| 20–21 | 433 | 380 | 53 |
| 21–22 | 452 | 438 | 14 |
| 22–23 | 394 | 352 | 42 |
| 23–24 | 311 | 288 | 23 |
| 24– | 367 | 346 | 21 |
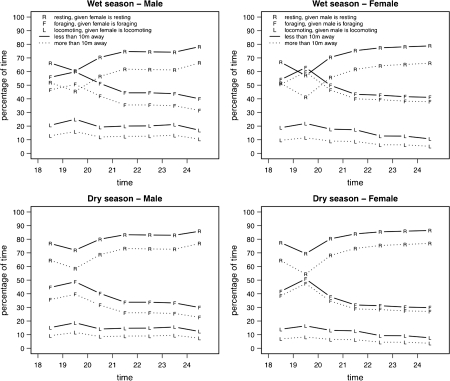
The probability that red-tailed sportive lemurs were foraging was higher when the partner was also foraging close by (<10 m) than when he or she was far away (>10 m; Fig. 3; Table III). In addition, there was a clear pattern during the course of the night, with the highest probability of foraging synchrony around 19:00 h and the lowest probability of foraging synchrony around 24:00 h, independent of the partners’ activities at near or far distance (Fig. 3; Table III; females foraging: GLMM: χ2 = 287.1, df = 10, p < 0.001; males foraging: GLMM: χ2 = 222.26, df = 10, p < 0.001). The probabilities of red-tailed sportive lemurs resting or locomoting also varied during the course of the night and were highest when the partner exhibited the same behavior when he or she was near (Fig. 3; Table III; females resting: GLMM: χ2 = 378.12, df = 10, p < 0.001; males resting: GLMM: χ2 = 316.43, df = 10, p < 0.001; females locomoting: GLMM: χ2 = 36.72, df = 10, p < 0.001; males locomoting: GLMM: χ2 = 35.89, df = 10, p < 0.001). Thus, red-tailed sportive lemurs appear to synchronize their behavior when they are close together, but their activity patterns appear to be controlled by time of the night, i.e., most likely by a strong endogenous activity rhythm.
Fig. 3.
Probabilities of female and male red-tailed sportive lemurs resting, foraging, or locomoting when the partner did the same or not, respectively, within 10 m or when the partner did the same or not when they were far (>10 m) away during the rainy and the dry season.
Table III.
Estimate, SE, and p-value for each model describing the probability that a red-tailed sportive lemur is resting, foraging, or locomoting as a function of time of the night and the partner’s activity and season at the 2 distance classes
| Fixed effects | Estimate (SE) | p-value | Estimate (SE) | p-value |
|---|---|---|---|---|
| Model for foraging | Females | Males | ||
| Intercept | 0.16 (0.21) | 0.44 | 0.23 (0.21) | 0.26 |
| 19:00–20:00 h | 0.37 (0.14) | 0.008 | 0.16 (0.14) | 0.23 |
| 20:00–21:00 h | −0.17 (0.14) | 0.23 | −0.18 (0.14) | 0.19 |
| 21:00–22:00 h | −0.43 (0.14) | 0.002 | −0.46 (0.14) | 0.001 |
| 22:00–23:00 h | −0.46 (0.15) | 0.002 | −0.46 (0.15) | 0.002 |
| 23:00–24:00 h | −0.50 (0.16) | 0.001 | −0.48 (0.16) | 0.002 |
| 24:00 + h | −0.52 (0.15) | <0.001 | −0.64 (0.16) | <0.001 |
| Dry season | −0.50 (0.07) | <0.001 | −0.45 (0.07) | <0.001 |
| Partner foraging far (>10 m) | −0.14 (0.17) | 0.42 | −0.37 (0.17) | 0.03 |
| Partner not foraging near (<10 m) | −1.18 (0.20) | <0.001 | 0.20 (0.20) | <0.001 |
| Partner not foraging far (>10 m) | −0.51 (0.17) | 0.002 | 0.17 (0.17) | <0.001 |
| Model for resting | Females | Males | ||
| Intercept | 0.70 (0.19) | <0.001 | 0.68 (0.17) | <0.001 |
| 19:00–20:00 h | −0.41 (0.13) | 0.002 | −0.25 (0.13) | 0.06 |
| 20:00–21:00 h | 0.17 (0.14) | 0.21 | 0.19 (0.14) | 0.16 |
| 21:00–22:00 h | 0.42 (0.14) | 0.002 | 0.41 (0.14) | 0.002 |
| 22:00–23:00 h | 0.53 (0.14) | <0.001 | 0.39 (0.14) | 0.006 |
| 23:00–24:00 h | 0.57 (0.15) | <0.001 | 0.38 (0.15) | 0.009 |
| 24:00+ h | 0.61 (0.15) | <0.001 | 0.60 (0.15) | <0.001 |
| Dry season | 0.53 (0.07) | <0.001 | 0.52 (0.07) | <0.001 |
| Partner resting far (>10 m) | −0.64 (0.12) | <0.001 | −0.60 (0.12) | <0.001 |
| Partner not resting near (<10 m) | −1.24 (0.18) | <0.001 | −1.29 (0.18) | <0.001 |
| Partner not resting far (>10 m) | −1.08 (0.13) | <0.001 | −1.03 (0.12) | <0.001 |
| Model for locomoting | Females | Males | ||
| Intercept | −1.46 (0.46) | 0.001 | −1.35 (0.42) | 0.001 |
| 19:00–20:00 h | 0.19 (0.24) | 0.43 | 0.25 (0.22) | 0.25 |
| 20:00–21:00 h | −0.07 (0.25) | 0.78 | −0.08 (0.23) | 0.72 |
| 21:00–22:00 h | −0.10 (0.25) | 0.69 | −0.03 (0.22) | 0.88 |
| 22:00–23:00 h | −0.46 (0.28) | 0.09 | 0.03 (0.23) | 0.9 |
| 23:00–24:00 h | −0.47 (0.29) | 0.11 | 0.03 (0.24) | 0.89 |
| 24:00 + h | −0.67 (0.30) | 0.03 | −0.24 (0.23) | 0.33 |
| Dry season | −0.36 (0.13) | 0.005 | −0.37 (0.10) | <0.001 |
| Partner locomoting far (>10 m) | −0.78 (0.42) | 0.06 | 0.56 (0.42) | 0.18 |
| Partner not locomoting near (<10 m) | −0.85 (0.40) | 0.04 | −0.01 (0.40) | 0.01 |
| Partner not locomoting far (>10 m) | −0.85 (0.38) | 0.02 | −0.63 (0.38) | 0.09 |
The reference categories are for time: 18:00−19:00 h, for season: rainy season, partner’s activity: resting, foraging or locomoting near (<10m).
Estimates of the variance for the random effects are: foraging females (0.040), foraging males (0.018), resting females (0.072), resting males (0.007), locomoting females (0.158), and locomoting males (0.029).
Season also influenced synchrony patterns: the probability of foraging was lower in the dry season than it was in the rainy season (Fig. 3; Table III). Again, activity patterns during the course of the night influenced the probability of foraging, independent of partners’ activities across distance categories. Similarly, probabilities that red-tailed sportive lemurs were resting were higher in the dry season than in the rainy season (Fig. 3; Table III).
Pacemaker
Because red-tailed sportive lemurs actively synchronized their behavior when they were within 10 m, we used the exact binomial test to test the hypothesis that a given behavior was as likely to be initiated by the female as it was by the male (Table IV). We could not reject this hypothesis during any of the 3 periods: mating (p = 0.125), gestation (p = 1.000), and lactation (p = 1.000). Thus there is no evidence to support the notion that either sex acted as pacemaker of the synchrony.
Table IV.
Percentage (number) of initiations of foraging, resting, or locomotion when both pair partners were at a range of <10 m
| Season | Females | Males |
|---|---|---|
| Mating | 21% (6) | 3% (1) |
| Gestation | 17% (2) | 25% (3) |
| Lactation | 17% (5) | 17% (5) |
Discussion
The results of this study show that overall behavioral synchrony in red-tailed sportive lemurs is high, suggesting that synchrony might be triggered mainly by endogenous activity rhythms. As predicted by the activity-budget hypothesis, foraging synchrony was highest during the first activity bout around 19:00 h. In addition, as predicted by the group-structure hypothesis, behavioral synchrony was higher when pairs were in visual contact than when they were far away. In contrast to the habitat-constraints hypothesis, seasonal differences in food abundance influenced red-tailed sportive lemurs only insofar as they exhibited lower probabilities of foraging synchrony during the dry season when food is less abundant but higher probabilities of resting synchrony in comparison to the rainy season. Thus, seasonal changes merely influenced the distribution of activity patterns but not behavioral synchrony. Finally, in contrast to the predictions of the game-theoretical model and the activity-budget-hypothesis, females did not emerge as pacemakers of the synchrony during gestation and lactation due to higher nutritional demands. Because we recorded the behavior of red-tailed sportive lemurs only every 5 min, it is likely that this time interval was too long to detect any pacemaker; shorter time intervals between observation bouts might have been more appropriate for this question. The sample sizes available for testing this hypothesis were also rather small and our analyses therefore lacked statistical power.
Although behavioral synchrony in red-tailed sportive lemurs seems to be due mainly to an endogenous activity rhythm, they nevertheless appear to synchronize their behavior actively when they are close. However, pairs spent only 15% of observation time within 10 m (Hilgartner 2006). Hence, the question arises: If behavioral synchrony is beneficial, why do red-tailed sportive lemurs not spend more time close together to reap its benefits? In particular, the shift from a solitary to a pair-living life style entails the highest benefits in terms of individually reduced predation risk through shared vigilance (Caro 2005; Elgar 1989; Lima 1995), predator confusion, and dilution effect (Hamilton 1971; Miller 1922). Advantages of reduced individual predation risk have also been suggested to be one of the driving forces in the evolution of social monogamy (van Schaik and Kappeler 2003).
Because red-tailed sportive lemurs are nocturnal and might be constrained in their ability to detect predators reliably due to reduced visibility at night, one may argue that vigilance may be less beneficial in nocturnal species. However, studies of antipredator strategies in red-tailed sportive lemurs and another nocturnal primate, gray mouse lemurs (Microcebus murinus), demonstrated that they do increase the rate of vigilance after presentation of predator calls or alarm calls (Fichtel 2007; Rahlfs and Fichtel 2010), suggesting that vigilance may also provide benefits in nocturnal primates. Thus, in theory, the benefits of vigilance and the confusion and dilution effects should drive red-tailed sportive lemurs to spend more time together. Because red-tailed sportive lemurs do not rely on early warning of predators and produce alarm calls only when predators directly attack them (Fichtel 2007), lacking benefits of early warning of predators may outweigh the benefits of vigilance and the confusion and dilution effects.
Other benefits of group-living species are joint resource defense and increasing foraging benefits through transmission of social information (Alexander 1974; Bertram 1978; Valone 2007). Although joint territorial defense has not been observed (Hilgartner 2006), red-tailed sportive lemurs may nevertheless benefit from both pair partners defending the territory independently. Another advantage of sociality is the acquisition of socially transmitted information about locations and qualities of food resources by foraging at the same time and by monitoring foraging success of others. Because female red-tailed sportive lemurs park their infants in the vegetation during the first months of life while they themselves are foraging (Kappeler 1998), social learning of food items may not play such a prominent role as in diurnal primates that carry their infants during the first months of life (Jaeggi et al. 2010; van Schaik 2010). Although future studies are required to understand the extent to which social learning is important for nocturnal primates, the ontogenetic perspective suggests that social information of food resources may not play may an important role in these infant-parking nocturnal primates. In principle, competition over food resources may explain avoidance of pair partners. However, red-tailed sportive lemurs show overall low rates of aggression, and aggression occurred only in 2% of the observations during foraging (Hilgartner 2006), indicating that feeding competition does not compensate for spending more time together.
In support of the activity-budget hypothesis, we found that, independent of season, red-tailed sportive lemurs foraging synchrony was highest after they have become active around 19:00 h and decreased throughout the night. In contrast, synchrony in resting increased steadily throughout the night. Because, in general, red-tailed sportive lemurs need a relatively large proportion of resting to sustain the slow processes of leaf fermentation and detoxification of the highly folivorous diet in specialized digestive tracts (Schmid and Ganzhorn 1996), resting synchrony might be a pseudo-synchrony resulting from physiological factors rather than an active synchrony to reach a consensus.
Interestingly, red-tailed sportive lemurs exhibit the lowest metabolic rates among mammals (Schmid and Ganzhorn 1996), suggesting that energy constraints may hamper behavioral synchrony because adjusting the endogenous activity rhythm to that of the partner might be too costly. Because such constraints of variation in individual activity budgets affect behavioral synchrony in several ungulates and primates (King and Cowlishaw 2009; Michelena et al. 2006; Ruckstuhl 1999), it is important to incorporate such costs into future models to bridge the gap between theoretical and empirical work on group coordination and decision making (Kerth 2010; Pyritz et al. 2010).
In summary, we found mixed evidence for the game-theoretical model and the activity-budget, group-structure, and habitat-constraints hypotheses. Behavioral synchrony in pair-living red-tailed sportive lemurs appears to be due mainly to endogenous rhythms, supporting the activity-budget hypothesis. Seasonal differences influenced only the distribution of activities but not synchrony per se, and thus provide no direct support of the habitat-constraints hypothesis. In support of the game-theoretical model and group structure hypothesis, red-tailed sportive lemurs actively synchronized their behavior when they were near one another, although they spent only 15% of time in close contact. The lack of an early warning system against predators, which may outweigh benefits of other antipredator behaviors, and weak support for benefits via social information transfer in combination with energetic constraints, may have led to red-tailed sportive lemurs not spending more time together to reap the benefits of behavioral synchrony.
Finally, synchrony may be triggered either by environmental processes, where similar ecological constraints result in individuals exhibiting similar behaviors independently (Engel and Lamprecht 1997), or by social processes. In chacma baboons (Papio ursinus), social mechanisms promoting stronger group cohesion resulted in higher levels of behavioral synchrony (King and Cowlishaw 2009), suggesting that social processes play an important role in triggering synchronization. Because red-tailed sportive lemurs are organized into dispersed pairs whereas other nocturnal pair-living primates such as avahis (Avahi laninger) or tarsiers (Tarsius spp.) spent more time together (Gursky 2000; Norscia and Borgognini-Tarli 2008), comparative studies of these primates may provide important insights into the evolution of behavioral synchrony and sociality (Fichtel and Hilgartner in press).
Acknowledgments
We thank the Département de Biologie Animale, Université d’Antananarivo, the CAFF of the Direction des Eaux et Forêts, and the CNFEREF Morondava for the authorization of this study. We thank Mario Ramohavelo Heriniaina for assistance in the field and Mamisolo Hilgartner-Rasoilison for support of the study. We also thank Peter Kappeler, Andrew King, Lennart Pyritz, Cédric Sueur, Joanna Setchell, and 2 anonymous referees for constructive comments on the manuscript. R. Hilgartner was supported by the German Research Foundation (DFG: KA 1082/6-1).
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- Agresti A. Categorical data analysis. 2. New York: Wiley; 2002. [Google Scholar]
- Alexander RD. The evolution of social behavior. Annual Review of Ecology and Systematics. 1974;5:325–383. doi: 10.1146/annurev.es.05.110174.001545. [DOI] [Google Scholar]
- Altmann J. Observational study of behavior: sampling methods. Behaviour. 1974;49:227–269. doi: 10.1163/156853974X00534. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon mothers and infants. Cambridge: Harvard University Press; 1980. [Google Scholar]
- Barrett L, Halliday J, Henzi P. The ecology of motherhood: the structuring of lactation costs by chacma baboons. Journal of Animerican Ecology. 2006;75:875–886. doi: 10.1111/j.1365-2656.2006.01105.x. [DOI] [PubMed] [Google Scholar]
- Bertram BCR. Living in groups: Predators and prey. In: Krebs JR, Davies NB, editors. Behavioural ecology. Oxford: Blackwell; 1978. pp. 64–96. [Google Scholar]
- Caro T. Antipredator defenses in birds and mammals. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Cheney DL. Interactions and relationships between groups. In: Smuts BB, Cheney DL, Seyfarth RM, Wrangham RW, Struhsaker TT, editors. Primate societies. Chicago: University of Chicago Press; 1987. pp. 267–281. [Google Scholar]
- Cohen J. A coefficient of agreement for nominal scales. Educational and Psychological Measurement. 1960;20(1):37–46. doi: 10.1177/001316446002000104. [DOI] [Google Scholar]
- Conradt L, Roper T. Activity synchrony and social cohesion: a fission-fusion model. Proceeding of the Royal Society B: Biological Sciences. 2000;267:2213–2218. doi: 10.1098/rspb.2000.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conradt L, Roper TJ. Group decision-making in animals. Nature. 2003;421:155–158. doi: 10.1038/nature01294. [DOI] [PubMed] [Google Scholar]
- Conradt L, Roper TJ. Concensus decision making in animals. TREE. 2005;20:449–456. doi: 10.1016/j.tree.2005.05.008. [DOI] [PubMed] [Google Scholar]
- Cortopassi KA, Bradbury JW. Contact call diversity in wild ornage-fronted parakeet pairs, Aratinga canicularis. Animal Behavior. 2006;71:1141–1154. doi: 10.1016/j.anbehav.2005.09.011. [DOI] [Google Scholar]
- Dewar RE, Richard A. Evolution of the hypervariable environment of Madagascar. PNAS. 2007;104:13723–13727. doi: 10.1073/pnas.0704346104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dostálková I, Spinka M. Synchronization of behaviour in pairs: the role of communication and consequences in timing. Animal Behaviour. 2007;74:1735–1742. doi: 10.1016/j.anbehav.2007.04.014. [DOI] [Google Scholar]
- Elgar MA. Predator vigilance and group size in mammals and birds: a critical review of the empirical evidence. Biological Review. 1989;64:13–33. doi: 10.1111/j.1469-185X.1989.tb00636.x. [DOI] [PubMed] [Google Scholar]
- Engel J, Lamprecht J. Doing what everybody does? A procedure for investigating behavioural synchronisation. Journal of Theoretical Biology. 1997;185:255–262. doi: 10.1006/jtbi.1996.0359. [DOI] [PubMed] [Google Scholar]
- Faraway JJ. Extending the linear model with R. London: Chapman & Hall; 2006. [Google Scholar]
- Fichtel C. Avoiding predators at night: antipredator strategies in red-tailed sportive lemurs (Lepilemur ruficaudatus) American Journal of Primatology. 2007;69:611–624. doi: 10.1002/ajp.20363. [DOI] [PubMed] [Google Scholar]
- Fichtel, C. (in press). Predation on primates. In J. C. Mitani, J. Call, P. Kappeler, R. Palombit, & J. Silk (Eds.), The evolution of primate societies. Chicago: University of Chicago Press.
- Fichtel, C., & Hilgartner, R. (In press). Noises in the dark: Vocal communication in nocturnal pair-living primates. In J. Master, M. Gamba, & F. Génin (Eds.), Leaping ahead: Advances in prosimian biology. Developments in primatology. New York: Springer.
- Fichtel C, Manser MB. Communication in social groups. In: Kappeler PM, editor. Animal behaviour: Evolution and mechanisms. Berlin: Springer; 2010. pp. 29–54. [Google Scholar]
- Fichtel C, Pyritz L, Kappeler PM. Coordination of group movements in non-human primates. In: Boos M, Kolbe M, Ellwart S, Kappeler PM, editors. Coordination in human and non-human primate groups. Heidelberg: Springer; 2010. pp. 37–56. [Google Scholar]
- Fischhoff IR, Sundaresan SR, Cordingley J, Larkin HM, Sellier M-J, Rubenstein DI. Social relationships and reproductive state influence leadership roles in movements of plains zebra, Equus burchellii. Animal Behaviour. 2007;73:825–831. doi: 10.1016/j.anbehav.2006.10.012. [DOI] [Google Scholar]
- Ganzhorn J. Distribution of a folivorous lemur in relation to seasonally varying food resources: integrating quantitative and qualitative aspects of food characteristics. Oecologica. 2002;131:427–435. doi: 10.1007/s00442-002-0891-y. [DOI] [PubMed] [Google Scholar]
- Gursky S. Sociality in the spectral tarsier, Tarsius spectrum. American Journal of Primatology. 2000;51:89–101. doi: 10.1002/(SICI)1098-2345(200005)51:1<89::AID-AJP7>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Hamilton WD. Geometry of the selfish herd. Journal of Theoretical Biology. 1971;31:295–311. doi: 10.1016/0022-5193(71)90189-5. [DOI] [PubMed] [Google Scholar]
- Hilgartner R. Living apart together: Pair-living in red-tailed sportive lemurs (Lepilemur ruficaudatus) Ulm: University of Ulm; 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgartner R, Zinner D, Kappeler PM. Life history traits and parental care in Lepilemur ruficaudatus. American Journal of Primatology. 2008;69:1–15. doi: 10.1002/ajp.20410. [DOI] [PubMed] [Google Scholar]
- Hooge, P., & Eichenlaub, B. (1997). Animal movement extension to ArcView. Ver. 1.1. Anchorage, AL, Alaska Biological Science Center, U.S. Geological Survey.
- Jaeggi A, Dunkel LP, van Noordwijk MA, Wich SA, Sura AAL, van Schaik C. Social learning of diet and foraging skills by wild immature Bornean orangutans: implications for culture. American Journal of Primatology. 2010;72:62–71. doi: 10.1002/ajp.20752. [DOI] [PubMed] [Google Scholar]
- Kappeler P. Nests, tree holes, and the evolution of primate life histories. American Journal of Primatology. 1998;46:7–33. doi: 10.1002/(SICI)1098-2345(1998)46:1<7::AID-AJP3>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Kappeler PM, Fichtel C. A 15-year perspective on the social organization and life history of Sifaka in Kirindy Forest. In: Kappeler PM, Watts DP, editors. Long-term field studies of primates. Berlin: Springer; 2012. [Google Scholar]
- Kerth G. Group decision-making in fission-fusion societies. In: Kappeler PM, editor. Animal behaviour: Evolution and mechanisms. Berlin: Springer; 2010. pp. 241–266. [Google Scholar]
- King AJ, Cowlishaw G. All together now: behavioural synchrony in baboons. Animal Behavior. 2009;78:1381–1387. doi: 10.1016/j.anbehav.2009.09.009. [DOI] [Google Scholar]
- König A, Beise J, Chalise M, Ganzhorn JU. When females should contest for food—testing hypotheses about resource density, distribution, size, and quality with Hunuman langurs (Presbytis entellus) Behavioral Ecology and Sociobiology. 1998;42:225–237. doi: 10.1007/s002650050434. [DOI] [Google Scholar]
- Lima S. Back to the basis of anti-predatory vigilance: the group-size effect. Animal Behaviour. 1995;49:11–20. doi: 10.1016/0003-3472(95)80149-9. [DOI] [Google Scholar]
- Limmer B, Becker PH. The relative role of age and experience in determining variation in body mass during the early breeding career of the common tern (Sterna hirundo) Behavioral Ecology and Sociobiology. 2007;61:1885–1896. doi: 10.1007/s00265-007-0429-8. [DOI] [Google Scholar]
- Michelena P, Noel S, Gautrais J, Gerard J-F, Deneubourg J-L, Bon R. Sexual dimorphism, activity budget and synchrony in groups of sheep. Oecologia. 2006;148:170–180. doi: 10.1007/s00442-005-0347-2. [DOI] [PubMed] [Google Scholar]
- Miller RC. The significance of the gregarious habit. Ecology. 1922;3:122–126. doi: 10.2307/1929145. [DOI] [Google Scholar]
- Mundry R, Nunn CL. Stepwise model fitting and statistical inference: turning noise into signal pollution. American Naturalist. 2009;173:119–123. doi: 10.1086/593303. [DOI] [PubMed] [Google Scholar]
- Norscia I, Borgognini-Tarli SM. Ranging behavior and possible correlates of pair-living in Southeastern Avahis (Madagascar) International Journal of Primatology. 2008;29:153–171. doi: 10.1007/s10764-007-9219-4. [DOI] [Google Scholar]
- Pyritz L, Fichtel C, Kappeler PM. Conceptual and methodological issues in the comparative study of collective group movements. Behavioural Processes. 2010;84:681–684. doi: 10.1016/j.beproc.2010.02.025. [DOI] [PubMed] [Google Scholar]
- Rahlfs M, Fichtel C. Anti-predator behaviour in a nocturnal primate, the grey mouse lemur (Microcebus murinus) Ethology. 2010;116:429–439. doi: 10.1111/j.1439-0310.2010.01756.x. [DOI] [Google Scholar]
- Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. Spontaneous emergence of leaders and followers in foraging pairs. Nature. 2003;423:432–434. doi: 10.1038/nature01630. [DOI] [PubMed] [Google Scholar]
- Rands SA, Cowlishaw G, Pettifor RA, Rowcliffe JM, Johnstone RA. The emergence of leaders and followers in foraging pairs when the qualities of individuals differ. BMC Evolutionary Biology. 2008;8:51. doi: 10.1186/1471-2148-8-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing S. Immibilization and anaesthesia of nonhuman primates. Primate Report. 1999;55:33–38. [Google Scholar]
- Ruckstuhl KE. To synchronise or not synchronise: a dilemma for young bighorn males? Behaviour. 1999;136:805–818. doi: 10.1163/156853999501577. [DOI] [Google Scholar]
- Ruckstuhl KE, Neuhaus P. Sexual segregation in ungulates: a new approach. Behaviour. 2000;137:361–377. doi: 10.1163/156853900502123. [DOI] [Google Scholar]
- Schmid J, Ganzhorn JU. Resting metabolite rates of Lepilemur ruficaudatus. American Journal of Primatology. 1996;38:169–174. doi: 10.1002/(SICI)1098-2345(1996)38:2<169::AID-AJP5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Snaith TV, Chapman C. Towards an ecological solution to the folivore paradox: patch depletion as an indicator of within-group scramble competition in red colobus. Behavioral Ecology and Sociobiology. 2005;59:185–190. doi: 10.1007/s00265-005-0023-x. [DOI] [Google Scholar]
- Sorg J-P, Kappeler PM, Ganzhorn JU. Forestry and research in the Kirindy forest/Centre de formation professionelle forestiere. In: Goodman SM, Benstead JP, editors. The natural history of Madagascar. Chicago: University of Chicago Press; 2003. pp. 1512–1519. [Google Scholar]
- Suer, C., Deneubourg, J.-L., Petit, O., & Couzin, I. D. (2010). Differences in nutrient requirements imply a non-linear e,ergence of leaders in animal groups. PLOS Computational Biology, e1000917. [DOI] [PMC free article] [PubMed]
- Vahl WK, Can der Meer J, Meijer K, Piersma T, Weissing F. Interference competition the spational distribution of food and free-living foragers. Animal Behavior. 2007;74:1493–1503. doi: 10.1016/j.anbehav.2007.03.006. [DOI] [Google Scholar]
- Valone TJ. From eavesdropping on performance to copying the behavior of oters: a review of public information use. Behavioral Ecology and Sociobiology. 2007;62:1–14. doi: 10.1007/s00265-007-0439-6. [DOI] [Google Scholar]
- van Schaik C, Kappeler P. The evolution of pair-living in primates. In: Reichard U, Boesch C, editors. Monogamy: Partnerships in birds, humans and other mammals. Cambridge: Cambridge University Press; 2003. pp. 559–580. [Google Scholar]
- van Schaik C. Social learning and culture in animals. In: Kappeler PM, editor. Animal behaviour: Evolution and mechanisms. Berlin: Springer; 2010. pp. 623–654. [Google Scholar]
- Zinner D, Hilgartner RD, Kappler PM, Pietsch T, Ganzhorn JU. Social organization of Lepilemur ruficaudatus. International Journal of Primatology. 2003;24:869–888. doi: 10.1023/A:1024684907250. [DOI] [Google Scholar]
- Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed effects models and extensions in ecology with R. New York: Springer; 2009. [Google Scholar]