Abstract
Heart failure following acute myocardial infarction (AMI) is a major cause of morbidity and mortality. Our previous observation that injection of apoptotic peripheral blood mononuclear cell (PBMC) suspensions was able to restore long-term cardiac function in a rat AMI model prompted us to study the effect of soluble factors derived from apoptotic PBMC on ventricular remodelling after AMI. Cell culture supernatants derived from irradiated apoptotic peripheral blood mononuclear cells (APOSEC) were collected and injected as a single dose intravenously after myocardial infarction in an experimental AMI rat model and in a porcine closed chest reperfused AMI model. Magnetic resonance imaging (MRI) and echocardiography were used to quantitate cardiac function. Analysis of soluble factors present in APOSEC was performed by enzyme-linked immunosorbent assay (ELISA) and activation of signalling cascades in human cardiomyocytes by APOSEC in vitro was studied by immunoblot analysis. Intravenous administration of a single dose of APOSEC resulted in a reduction of scar tissue formation in both AMI models. In the porcine reperfused AMI model, APOSEC led to higher values of ejection fraction (57.0 vs. 40.5%, p < 0.01), a better cardiac output (4.0 vs. 2.4 l/min, p < 0.001) and a reduced extent of infarction size (12.6 vs. 6.9%, p < 0.02) as determined by MRI. Exposure of primary human cardiac myocytes with APOSEC in vitro triggered the activation of pro-survival signalling-cascades (AKT, Erk1/2, CREB, c-Jun), increased anti-apoptotic gene products (Bcl-2, BAG1) and protected them from starvation-induced cell death. Intravenous infusion of culture supernatant of apoptotic PBMC attenuates myocardial remodelling in experimental AMI models. This effect is probably due to the activation of pro-survival signalling cascades in the affected cardiomyocytes.
Electronic supplementary material
The online version of this article (doi:10.1007/s00395-011-0224-6) contains supplementary material, which is available to authorized users.
Keywords: Apoptosis, Myocardial infarction, Cardioprotection, Ischaemia/reperfusion
Introduction
Although prompt reperfusion within a narrow time window has significantly reduced early mortality following acute myocardial infarction (AMI), ischaemic heart failure remains widely prevalent and represents an increasing economic burden [42]. A new field in regenerative cardiovascular medicine emerged when investigators observed that distant stem cells were able to sense sites of damage and promote structural and functional repair after experimental myocardial infarction [6, 9, 10, 17, 29, 31, 38, 44]. This experimental insight into regeneration following AMI triggered the start of randomized, controlled clinical trials demonstrating that cell therapy could improve cardiac function in patients after AMI [14, 27, 36, 43]. In 2005, Thum et al. established “The Dying Stem Cell Hypothesis”, namely, that therapeutic stem cells are already undergoing apoptosis while being infused into the infarcted area, thereby attenuating infarction-induced immunoactivation and remodelling via the induction of immunomodulatory mechanisms [33, 34, 39].
We have previously shown that infusion of cultured irradiated apoptotic peripheral blood mononuclear cell (PBMC) suspensions in a rat acute AMI model caused homing of regenerative FLK+/c-kit+ cells in the early phase of experimental AMI and restored long-term cardiac function [2, 26]. In contrast, infusion of cultured viable PBMC in the same setting had only marginal efficacy in preservation of cardiac function. Moreover, we found that induction of apoptosis in PBMC led to the massive secretion of Interleukin-8 (IL-8) and Matrixmetalloproteinase 9 (MMP9) proteins known to be responsible for neo-angiogenesis and recruitment of pro-angiogenic cells from the bone marrow (BM) to the infarcted myocardium [20, 24, 28].
Our data suggest two possible causes for this in vivo regenerative effect. Either infusion of apoptotic PBMC reduces the immune response after AMI by defined mechanisms [4, 34] or soluble factors secreted by apoptotic PBMC cause induction of neo-angiogenesis and cytoprotection in the acute phase of myocardial infarction. This latter speculation is supported by the recent publications providing evidence that bone marrow cells or endothelial progenitor cells secrete soluble proteins which induce regenerative mechanisms in a paracrine manner [7, 13, 25].
Having shown that infusion of apoptotic PBMC suspensions in an acute rat AMI model prevented ventricular remodelling, we investigated whether simply administering soluble factors derived from irradiated PBMC (APOSEC; this acronym stands for apoptotic secretome) showed similar properties and caused cardioprotection in the same acute rat AMI model. To further strengthen the clinical relevance of APOSEC, we tested this “biological” in cell cultures of cardiac myocytes in vitro and in a porcine closed chest reperfused AMI model in vivo. Here we provide evidence that APOSEC confers cytoprotection directly to cardiac myocytes, and that intravenous application of APOSEC is effective in preventing myocardial damage and tissue remodelling in a dose-dependent manner in the porcine reperfused AMI model.
Methods
Generation of cell culture medium derived from irradiated human apoptotic PBMC (APOSECH) for in vitro assays
Human PBMC were obtained from young healthy volunteers after informed consent (ethics committee vote: EK-Nr 2010/034). Cells were separated by Ficoll-Paque (GE Healthcare Bio-Sciences AB, Sweden) density gradient centrifugation as described previously [2]. Apoptosis of PBMC was induced by Caesium-137 irradiation (Department of Transfusion Medicine, General Hospital Vienna) with 60 Gray (Gy) for in vitro experiments. Induction of apoptosis was measured by Annexin-V/propidium iodine (FITC/PI) co-staining (Becton–Dickinson, Franklin Lakes, NJ, USA) on a flow cytometer. Irradiated and non-irradiated cells were resuspended in serum-free UltraCulture Medium (Lonza, Switzerland) and cultured for 24 h in various cell densities (1 × 106, 2.5 × 106 and 25 × 106 cells/ml, n = 5). After 24 h supernatants were collected and served as experimental entities for ELISA content analysis or were lyophilized as follows: supernatants were dialyzed against ammonium acetate (at a concentration of 50 mM) for 24 h at 4°C. The obtained liquid was sterile filtered (Whatman Filter 0.2 μm FP30/o,2 Ca–S, Dassel, Germany), frozen and lyophilized overnight (Lyophilizator Christ alpha 1-4, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode am Harz, Germany) (Fig. 1).
Fig. 1.
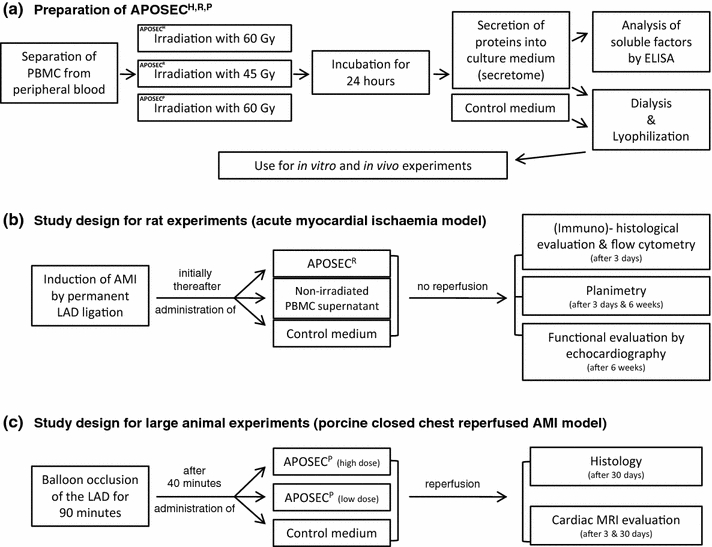
Study design: a The preparation process of APOSEC, starting with cell separation, induction of apoptosis, cell culture, dialysis, lyophilization and its use in in vitro and in vivo experiments is shown. b Study design for in vivo rat experiments. A permanent ligation of the LAD was conducted in male Sprague–Dawley rats, initially thereafter APOSECR, supernatant derived from non-irradiated cells or control medium was injected intravenously into the femoral vein. Evaluations by immunohistology and flow cytometry were performed 3 days after LAD ligation. Planimetric analysis and echocardiography were conducted 6 weeks after MCI. c Experimental setting of closed chest reperfused AMI in a porcine model. Ischaemia was induced by balloon occlusion of the LAD for 90 min. 40 min after balloon inflation, APOSECP was administered intravenously. Cardiac MRI evaluations were performed 3 and 30 days after AMI
To avoid possible cross-species detrimental immune reactions, we opted to utilize APOSEC preparations solely in a syngeneic fashion.
For in vivo rat experiments, syngeneic rat PBMC were separated by density gradient centrifugation from whole blood obtained from heparinized rats. PBMC were irradiated by Caesium-137 (45 Gy) and cultured for 24 h at a cell density of 25 × 106 cells/ml (in UltraCulture Medium, Lonza, Switzerland). APOSEC for rat experiments (APOSECR) was further processed as described for APOSECH. Supernatants of non-irradiated rat PBMC served as controls.
For large animal experiments, blood was obtained from anaesthetized pigs by direct heart puncture. Three pigs were anaesthetized with an IV bolus of 10 mg/kg ketamine and 1.3 mg/kg azaperone and a left thoracic dermal incision was conducted. Direct heart puncture was then performed under sterile conditions using a hollow needle and blood was drawn with 50-ml syringes. Blood obtained during this procedure was transferred into heparinized plastic bags for blood products. PBMC were then obtained according to the protocol described above. CellGro® DC serum-free medium (CellGenix GmbH, Freiburg, Germany), a “Good Manufacturing Practice” certified culture medium, was utilized for porcine PBMC-derived APOSEC production (APOSECP). APOSEC for porcine experiments (APOSECP) was processed as described for APOSECH.
APOSECH content evaluation by membrane arrays and ELISA analysis
APOSECH was screened for cytokines and angiogenic factors using two commercially available array systems (Proteome Profiler Arrays, R&D Systems, Minneapolis, USA). Supernatant levels of cytokines secreted by irradiated and non-irradiated PBMC in various concentrations were measured by utilizing commercially available enzyme-linked immunosorbent assay (ELISA, Duoset, R&D Systems, Minneapolis, USA) kits for the quantification of IL-8, GRO-α, ENA-78, VEGF, IL-16, IL-10, TGF-β, sICAM-1, RANTES, IL-1ra, MIF, PAI-1, IGF-I, HGF, FGF-2, MCP-1, MMP9, SDF-1, G-CSF, GM-CSF and HMGB1 (IBL International GmbH, Hamburg, Germany).
Acute rat ischaemic model and APOSECR treatment
Animal experiments were approved by the committee for animal research, Medical University of Vienna (vote: 66.009/0168-II/10b/2008). Acute myocardial infarction was induced in adult male Sprague–Dawley rats (weight 275–300 g) by ligating the LAD. A left lateral thoracotomy was performed and a ligature using 6-0 prolene was placed around the LAD beneath the left atrium. Immediately after the onset of ischaemia, lyophilized supernatants obtained from 8.5 × 106 either irradiated apoptotic PBMC or non-irradiated viable cells resuspended in 0.3 ml fresh UltraCulture Medium (Lonza, Basel, Switzerland) were injected in the femoral vein. Injection of cell culture medium alone and sham operation served as controls.
Rat immunohistochemistry and determination of myocardial infarction size by planimetry
All animals were sacrificed either 72 h or 6 weeks after experimental infarction. Hearts were explanted and then sliced into three layers at the level of the largest extension of infarcted area (n = 6 for 72 h analyses, n = 9 for 6 weeks analyses). The tissue samples were stained with hematoxylin–eosin (H&E) and Elastica van Gieson (EVG). Short-term immunohistological evaluation (72 h) was performed using antibodies directed to CD68 (MCA341R, AbD Serotec, Kidlington, UK) and c-kit (sc-168, Santa Cruz Biotechnology, CA, USA). Image J planimetry software (Rasband, W.S., Image J, U.S. National Institutes of Health, Bethesda, USA) was utilized to determine the size of myocardial infarct after 6 weeks.
Flow cytometry analysis, homing of CD68+ and c-kit+ cells
Three days after myocardial infarction, rats treated with either APOSECR, viable cell derived supernatant or control medium were sacrificed. Infarcted areas of explanted hearts were cut into small cubes (1 mm) and incubated with collagenase (2.4 U/ml, Sigma, St Louis, Mo) for 12 h at 4°C. After digestion and washing, the cells were incubated with primary antibodies directed to CD68 (MCA341R, AbD Serotec, Kidlington, UK) and c-kit (sc-168, Santa Cruz Biotechnology, CA, USA). After an incubation period with a secondary antibody, cell suspensions were analysed for total CD68+ and c-kit+ cell numbers by flow cytometry (FACS Calibur, Becton–Dickinson, Franklin Lakes, USA).
Rat cardiac function assessment by echocardiography
Six weeks after induction of myocardial infarction, rats were anaesthetized with 100 mg/kg ketamine and 20 mg/kg xylazine. The sonographic examination was conducted on a Vivid 7 system (General Electric Medical Systems, Waukesha, USA). Analyses were performed by an experienced observer blinded to the treatment groups to which the animals were allocated. M-mode tracings were recorded from a parasternal short-axis view and functional systolic and diastolic parameters were obtained. Fractional shortening was calculated as follows: FS (%) = ((LVEDD − LVESD)/LVEDD) × 100%, where LVEDD and LVESD mean left ventricular end-diastolic and end-systolic diameter, respectively.
Porcine closed chest reperfused infarction model and APOSECP
A closed chest reperfused AMI infarction model was applied in a large animal setting [15, 16]. The experiments in the porcine infarction model were carried out at the Institute of Diagnostics and Oncoradiology, University of Kaposvar, Hungary. Animal experiments were approved by the University of Kaposvar (vote: 246/002/SOM2006, MAB-28-2005). After overnight fasting, the pigs (female Large Whites weighing approximately 30 kg) were sedated with 12 mg/kg ketamine hydrochloride, 1.0 mg/kg xylazine and 0.04 mg/kg atropine. After the administration of 200 IU/kg of heparin, a 6F guiding catheter (Medtronic Inc., Minneapolis, USA) was introduced into the left coronary ostium, and selective angiography of the left coronary arteries was performed using Ultravist contrast medium (Bayer Healthcare, Berlin, Germany). A Maverick balloon catheter (diameter: 3.0 mm, length: 15 mm; Boston Scientific, Natick, USA) was inserted into the left anterior descending artery (LAD) after the origin of the second major diagonal branch. The LAD was then occluded by inflating the balloon slowly at 4–6 atm (n = 8 in the control group, n = 7 in the treatment high dose and n = 7 in the treatment low-dose group), controlling the occlusion with angiography. Forty minutes after the start of the LAD occlusion, the lyophilized supernatant obtained from 250 × 106 (low-dose group), 1 × 109 (high-dose group) irradiated apoptotic porcine PBMC or lyophilized serum-free cell culture medium (CellGro® DC, CellGenix GmbH, Freiburg, Germany) was resuspended in 250 ml of 0.9% physiologic sodium chloride solution and administered intravenously over the next 25 min. After 90 min of occlusion, the balloon was deflated and reperfusion was established. Control coronary angiography was performed to prove the patency of the infarct-related artery and to exclude arterial injury. Furthermore, all animals received 75 mg clopidogrel and 100 mg acetylsalicylic acid. After 24 h or after 30 days, euthanasia was performed by the administration of saturated potassium chloride. For the analysis conducted after 24 h, in situ double-staining with 1% Evans blue dye and a 4% solution of 2,3,5-triphenyltetrazolium chloride (TTC) was performed to delineate areas at risk for ischaemia and infarcted (necrotic) areas, respectively. In short, after explantation the LAD was occluded again at same position where the balloon was situated before and both coronary arteries were perfused with Evans blue solution to delineate the area at risk and non-risk region. The hearts were cut into 7 mm thick slices starting from the apex towards the level of the occlusion (6–7 layers per heart). The slices were incubated in 500 ml of TTC solution at 37°C in a shaking water bath for 20 min. Subsequently, all slices underwent an overnight bleach cycle at room temperature using 4.5% formaldehyde. All slices were photographed using a digital camera (Panasonic HDC-HS700, Osaka, Japan) mounted on a fixed stand. Planimetry was performed using Image J software (Rasband, W.S., Image J, US National Institutes of Health, Bethesda, USA). Serum levels of Troponin I were determined by ELISA (Uscn Life Science Inc., Wuhan, China). After 30 days, specimens of infarcted myocardium were obtained after euthanasia, fixed in formaldehyde and embedded in paraffin for histological staining (H&E, Movat’s pentachrome).
Bari scores for all animals were calculated based on LAD and LCX pre-occlusion angiograms according to the method previously described [1, 32].
Quantification of cardiac parameters after reperfused AMI in pigs by magnetic resonance imaging (MRI)
Three and thirty days after the reperfused AMI procedure, cardiac MRI was performed using a 1.5-T clinical scanner (Avanto, Siemens, Erlangen, Germany) together with a phased array coil and a vector ECG system. Cine MR images were acquired using a retrospectively ECG-gated, steady-state free precession cine MRI technique in short-axis and long-axis views of the heart using 1.2 ms echo time (TE), 40 ms repetition time (TR), 50° flip angle, 300 mm field-of-view, 8 mm slice thickness, and 256 × 256 image matrix. Sixteen short-axis images were acquired using ECG-gated, saturation recovery true fast imaging with steady-state precession (FISP) sequences. Delayed enhancement images were obtained after injection of 0.05 mmol/kg of contrast medium using an inversion recovery prepared, gradient-echo sequence. Short-axis and long-axis images were obtained 10–15 min after gadolinium injection. The images were analyzed using Mass 6.1.6 software (Medis, Leiden, The Netherlands). After segmentation of the left ventricular (LV) endocardial and epicardial borders, end-diastolic and end-systolic volumes and global LV ejection fraction were automatically calculated. The left ventricular and infarcted myocardial mass was determined from the cine and delayed enhancement MR images, respectively. The infarct size was determined relative to LV mass. Data analyses and interpretations were performed by an experienced observer blinded to all study results.
Human cardiomyocyte culture and immunoblot analysis
Primary human ventricular cardiac myocytes were obtained from CellSystems (CellSystems Biotechnologie, St. Katharinen, Germany) and cultured in cardiac myocyte medium (CellSystems) at 37°C. To investigate the cytoprotective activity of APOSEC, 3 × 105 human cardiac myocytes were seeded in 6-well plates and cultivated in either basal medium without serum and growth factors or in basal medium supplemented with APOSEC (PBMC cell density, 0.25 × 106, 2.5 × 106 and 25 × 106) for 24 h. For Western Blot analysis, 3 × 105 human cardiac myocytes were incubated with APOSEC (PBMC cell density for APOSEC production, 2.5 × 106) or with lyophilized UltraCulture Medium for 5, 10, 30 and 60 min and for 24 h. Immunodetection was performed with anti-phospho-c-Jun (1 μg/ml, New England Biolabs, Beverly, MA, USA), anti-phospho-CREB (1 μg/ml, New England Biolabs, Beverly, MA, USA), anti-phospho-AKT (1 μg/ml, New England Biolabs, Beverly, MA, USA), anti-phospho-Erk1/2 (1 μg/ml, New England Biolabs, Beverly, MA, USA), anti-phospho-Hsp27 (Ser15) (1 μg/ml, New England Biolabs, Beverly, MA, USA), anti-phospho-Hsp27 (Ser85), anti-BAG1 (C-16) (1 μg/ml, Santa Cruz Biotechnology, Heidelberg, Germany), anti-Bcl-2 (2 μg/ml, Acris, Herford, Germany), followed by horseradish peroxidase-conjugated goat anti-rabbit or goat anti-mouse IgG antisera (both 1:10,000; Amersham BioSciences). In parallel, identical blots were performed with the equivalent non-phosporylated factors as controls. In addition, an apoptosis membrane-array (R&D Systems) was performed with lysates from cardiac myocytes treated with either medium or APOSEC (PBMC cell density 2.5 × 106) for 24 h according to the manufacturer’s instructions.
To identify some of the mayor soluble factors that are responsible for exerting the cytoprotective effect of APOSEC or a subset thereof, we sought to inhibit the activity of selected prominent cytokines by adding neutralizing antibodies. VEGF, IL-8, ENA-78 and MMP9 were selected based on the higher expression levels over controls and their known cytoprotective properties. The activity of these selected factors was blocked using the recommended concentration of the neutralizing antibodies (2 μg/ml anti-VEGF: AF-293-NA, 2 μg/ml anti-IL-8: MAB208, R&D Systems; 2 μg/ml anti-ENA-78: AB-254-PB and 2 μg/ml anti-MMP9: MAB911; all antibodies obtained from R&D Systems, USA). Blocking capacity of neutralizing antibodies was verified (see Supplementary Fig. 5). Mouse and goat isotype antibodies were used as controls (Mouse IgG (Clone 11711), MAB002, Goat IgG, AB-108-C, R&D Systems). For these neutralization assays, 3 × 105 human cardiac myocytes were incubated with APOSEC and neutralizing antibodies, a combination thereof or with lyophilized medium for 60 min (for phospho-CREB and phospho-Hsp-27) or for 24 h (for Bcl-2 and BAG1). Western blots were performed as described above.
Statistical methods
Statistical analysis was performed using Graph Pad Prism software (La Jolla, USA). All data are given as mean ± standard error of the mean (SEM). The Wilcoxon–Mann–Whitney test or Student’s t test were utilized to calculate significances between the groups. The Bonferroni–Holm correction was used to adjust significance levels for ELISA results. In boxplot figures, whiskers indicate minimums and maximums, the upper edge of the box indicates the 75th percentile and the lower one indicates the 25th percentile. p values < 0.05 were considered statistically significant.
Results
An overview of the study design is shown in Fig. 1 and in a supplementary video file (video_aposec.mpg).
Analysis of soluble factors produced by irradiated human PBMC (APOSECH)
To induce apoptosis, PBMC (purity > 98%) were γ-irradiated (60 Gray) and cultured for 24 h. Supernatants of irradiated and non-irradiated cells were collected and secreted proteins associated with tissue repair and neo-angiogenesis were determined by membrane arrays and ELISA. As shown in Table 1, after irradiation of PBMC, higher amounts of IL-8, GRO-alpha, ENA-78, RANTES, sICAM-1, MIF, VEGF, IL-1ra and IL-16 were detected in a cell density-dependent manner as compared to the supernatant of non-irradiated cells. In contrast, little if any secretion was detected for MCP-1, IL-10, IGF-1, HGF, FGF-2, TGF-β, SDF-1, G-CSF and GM-CSF (Table 1), indicating that some of the factors previously associated with cardioprotection might not play a relevant role in this experimental setting [35]. An overview of secreted factors is shown in Supplementary Fig. 1.
Table 1.
Analysis of soluble factors secreted by non-irradiated cells and irradiated apoptotic PBMC (APOSEC)
| Soluble factors (ng/ml) | Viable PBMC | Apoptotic PBMC | Sig. | ||||
|---|---|---|---|---|---|---|---|
| 1 × 106 | 2.5 × 106 | 25 × 106 | 1 × 106 | 2.5 × 106 | 25 × 106 | ||
| IL-8 | 1.74 ± 0.40 | 1.93 ± 0.09 | 10.49 ± 3.53 | 1.22 ± 0.29 | 2.30 ± 0.13 | 18.01 ± 2.87 | ns ns¥ |
| GRO-alpha | 0.17 ± 0.09 | 0.36 ± 0.09 | 2.06 ± 1.58 | 0.07 ± 0.02 | 0.48 ± 0.09 | 3.95 ± 0.93 | ns ns ns |
| ENA-78 | 3.41 ± 1.34 | 29.93 ± 3.41 | 34.89 ± 16.33 | 3.93 ± 1.43 | 37.86 ± 12.73 | 108.86 ± 27.88 | ns ns¥ |
| MCP-1 | 1.66 ± 0.65 | 0.47 ± 0.21 | 0.27 ± 0.00 | 0.76 ± 0.19 | 0.74 ± 0.17 | 0.27 ± 0.00 | ns ns ns |
| RANTES | 8.32 ± 0.18 | 18.62 ± 3.21 | 37.63 ± 2.72 | 4.01 ± 0.05 | 22.25 ± 3.64 | 51.58 ± 4.44 | ns ns ns |
| HMGB1 | 0.63 ± 0.39 | 3.44 ± 2.11 | 33.57 ± 6.45 | 2.74 ± 0.27 | 6.46 ± 1.12 | 20.51 ± 3.62 | ns ns† |
| MMP9 | 4.14 ± 0.91 | 14.59 ± 2.75 | 29.46 ± 8.29 | 0.99 ± 0.16 | 3.61 ± 0.59 | 19.35 ± 5.34 | ns†,‡ |
| sICAM-1 | 0.14 ± 0.04 | 1.43 ± 0.25 | 7.43 ± 0.85 | 0.42 ± 0.25 | 2.09 ± 0.42 | 9.40 ± 1.29 | ns ns¥ |
| VEGF165 | 0.13 ± 0.01 | 0.42 ± 0.04 | 0.82 ± 0.34 | 0.15 ± 0.02 | 0.64 ± 0.04 | 4.39 ± 1.22 | ns ns¥ |
| MIF | 4.84 ± 0.09 | 17.79 ± 0.95 | 13.24 ± 0.85 | 5.85 ± 0.22 | 20.15 ± 1.14 | 58.99 ± 1.17 | ns ns¥ |
| PAI-1 | 1.25 ± 0.35 | 1.93 ± 0.29 | 49.60 ± 9.04 | 0.00 ± 0.00 | 5.06 ± 3.25 | 45.86 ± 1.43 | ns ns ns |
| IL-16 | 0.0 ± 0.0 | 0.11 ± 0.02 | 0.84 ± 0.31 | 0.00 ± 0.00 | 1.25 ± 0.07 | 5.25 ± 0.52 | ns‡, ¥ |
| IL-1ra | 0.35 ± 0.09 | 0.52 ± 0.17 | 2.16 ± 0.96 | 0.13 ± 0.04 | 0.41 ± 0.17 | 6.43 ± 1.33 | ns ns¥ |
| IL-10 | 0.01 ± 0.00 | 0.00 ± 0.0 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.02 ± 0.01 | 0.06 ± 0.01 | ns ns ns |
| IGF-I | 0.00 ± 0.00 | 0.01 ± 0.0 | 0.03 ± 0.02 | 0.00 ± 0.00 | 0.01 ± 0.01 | 0.03 ± 0.03 | ns ns ns |
| HGF | 0.33 ± 0.08 | 0.16 ± 0.01 | 0.69 ± 0.19 | 0.11 ± 0.03 | 0.07 ± 0.02 | 0.79 ± 0.19 | ns ns ns |
| FGF-2 | 0.56 ± 0.02 | 0.53 ± 0.00 | 0.59 ± 0.01 | 0.48 ± 0.01 | 0.53 ± 0.02 | 0.55 ± 0.02 | ns ns ns |
| TGF-β | 0.08 ± 0.01 | 0.10 ± 0.01 | 0.21 ± 0.07 | 0.06 ± 0.01 | 0.09 ± 0.02 | 0.39 ± 0.09 | ns ns ns |
| SDF-1 | 0.17 ± 0.0 | 0.19 ± 0.0 | 0.22 ± 0.03 | 0.16 ± 0.01 | 0.15 ± 0.07 | 0.12 ± 0.04 | ns ns ns |
| G-CSF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | ns ns ns |
| GM-CSF | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.07 ± 0.02 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.08 ± 0.02 | ns ns ns |
Cells were incubated in three different cell concentrations for 24 h. Supernatants were analyzed for cytokines, chemokines and growth factors (n = 5)
ns p > 0.05 viable PBMC versus apoptotic PBMC (of corresponding cell density)
† p < 0.05 1 × 106 viable PBMC versus 1 × 106 apoptotic PBMC
‡ p < 0.05 2.5 × 106 viable PBMC versus 2.5 × 106 apoptotic PBMC
¥ p < 0.05 25 × 106 viable PBMC versus 25 × 106 apoptotic PBMC
Diverted early inflammatory immune response and long-term preservation of ventricular function in AMI rats treated with APOSECR
Since the degree of the inflammatory response after AMI is an important factor which correlates to infarct size and outcome, we investigated whether APOSECR IV (derived from rat cells) modifies the extent of necrosis and the quality of infiltrating cells in the ischaemic myocardium. H&E-staining revealed that rat hearts treated with APOSECR showed a significant reduction of infarction area within 72 h after ligation of the LAD as compared to animals receiving medium or the supernatant from viable cells (Fig. 2a–d).
Fig. 2.
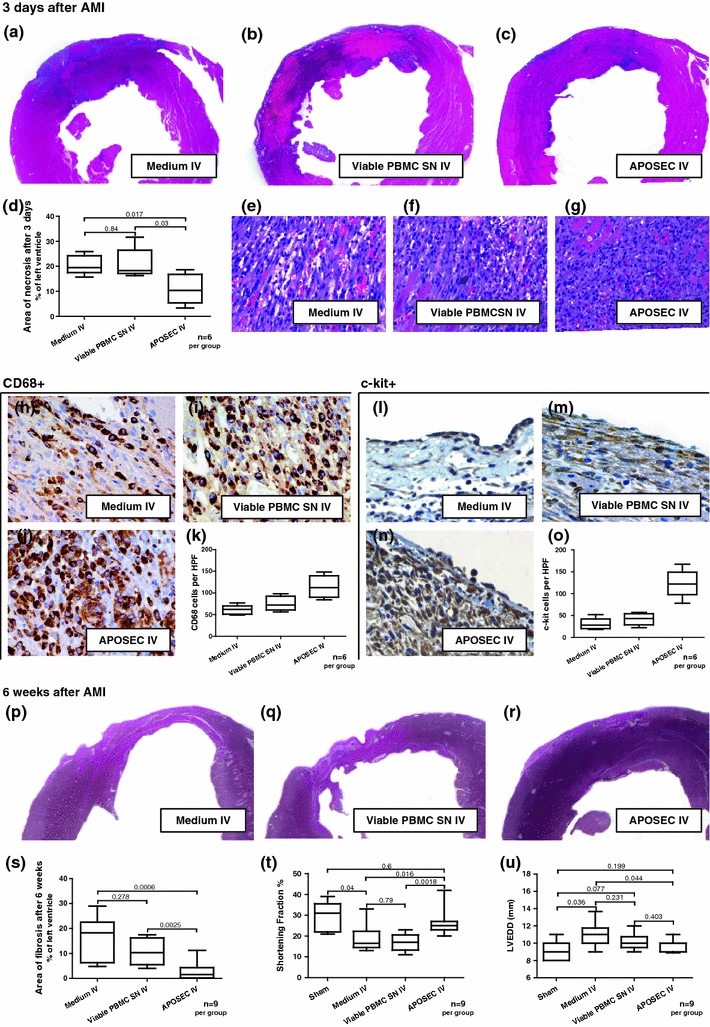
Results of in vivo rat experiments. a–d Hearts of APOSECR-injected animals 3 days after LAD ligation evidenced less myocardial necrosis compared to controls. e–g H&E-stained rat myocardium 3 days after LAD ligation. The cellular infiltrate appears to be more consolidated in APOSECR-injected animals. h–j Myocardial tissue stained for CD68. k Quantification of positively stained cells per high power field (HPF). l–n Specimen obtained 3 days after AMI stained for c-kit, o shows results of cell quantification per HPF. p–r shows size of myocardial infarction 6 weeks after LAD ligation. s Planimetric analyses indicate a significant reduction of scar area compared to controls. t, u show results obtained by echocardiography 6 weeks after AMI. Functional parameters (SF, LVEDD) were improved in comparison to medium or viable cell supernatant injected animals
Compared to APOSEC-treated rats (Fig. 2g), control AMI animals evidenced a more mixed cellular infiltrate in the wound areas similar to granulation tissue within 72 h after AMI (Fig. 2e, f). Immunohistochemical analysis revealed that the cellular infiltrate in the APOSECR AMI rats was composed of abundant CD68+ monocytes/macrophages (Fig. 2j), significantly more than in the control groups (Fig. 2h, i).
In addition, most of these medium-sized monocytoid cells in the APOSECR cellular infiltrate were highly positive for c-kit (CD117) and vascular endothelial growth factor receptor 2 (FLK-1) (data not shown) (Fig. 2n). Expression of both markers was more accentuated in APOSECR-injected rats as compared to control groups (see representative images, Fig. 2l–o, n = 6 per group). To further strengthen these histological data, we obtained hearts 72 h after treatment with either APOSECR or control medium. After homogenisation of myocardium, infiltrating cells were separated and the quantity of c-kit+ and CD68+ cells was determined by flow cytometry analysis. Both the cell populations were enriched in APOSECR-injected animals, as evidenced by a mean increase of 37% of CD68+ cells and 107% of c-kit+ cells compared to control animals injected with medium (n = 4 per group). Figure 2p–r shows that APOSECR-injected rats demonstrate a significantly greater extent of viable myocardium within the anterior free wall of the left ventricle. In contrast, collagen deposition and scar formation extended through almost the entire left ventricular wall in rats receiving control medium.
Six weeks after LAD ligation, the mean left ventricular ejection fractions (LVEF), shortening fractions (SF), left ventricular end-diastolic diameter (LVEDD) and left ventricular end-systolic diameter (LVESD) were determined to be 43.04% ± 4.17 (LVEF), 19.00% ± 2.29 (SF), 10.96 mm ± 0.51 (LVEDD) and 9.00 mm ± 0.63 (LVESD) in animals having received culture medium alone and 39.38% ± 2.89 (LVEF), 16.88% ± 1.45 (SF), 10.17 mm ± 0.33 (LVEDD) and 8.63 mm ± 0.32 (LVESD) in rats injected with the supernatant of non-irradiated cells (p values between 0.23 and 0.79). Remarkably, APOSEC IV-injected AMI rats evidenced significantly improved functional parameters: 56.22% ± 3.05 (LVEF; p = 0.018 vs. medium and p = 0.0006 vs. viable cell supernatant), 26.33% ± 2.11 (SF; p = 0.016 vs. medium and p = 0.0018 vs. viable cell supernatant), 9.77 mm ± 0.23 (LVEDD; p = 0.044 vs. medium) and 7.33 mm ± 0.33 (LVESD; p = 0.025 vs. medium and p = 0.013 vs. viable cell supernatant). Mean levels of cardiac function of healthy rats without myocardial infarction were as follows: 60.40% ± 4.95 (LVEF), 29.20% ± 3.26 (SF), 9.00 mm ± 0.55 (LVEDD) and 6.40 mm ± 0.51 (LVESD). Figure 2t, u shows echocardiographic data for each group.
APOSECP IV protects the myocardium from ischaemic injury in a pig model of closed chest reperfused AMI
BARI scores did not differ significantly between the groups (see Supplementary Fig. 4), indicating a comparable distribution of the area a risk after balloon occlusion.
Hearts explanted from animals infused with APOSECP evidenced less myocardial necrosis as shown by tetrazolium chloride staining after 24 h compared to controls (Fig. 3a–c). Additionally, troponin I release was less than in animals treated with resuspended lyophilized medium as controls (Fig. 3d).
Fig. 3.
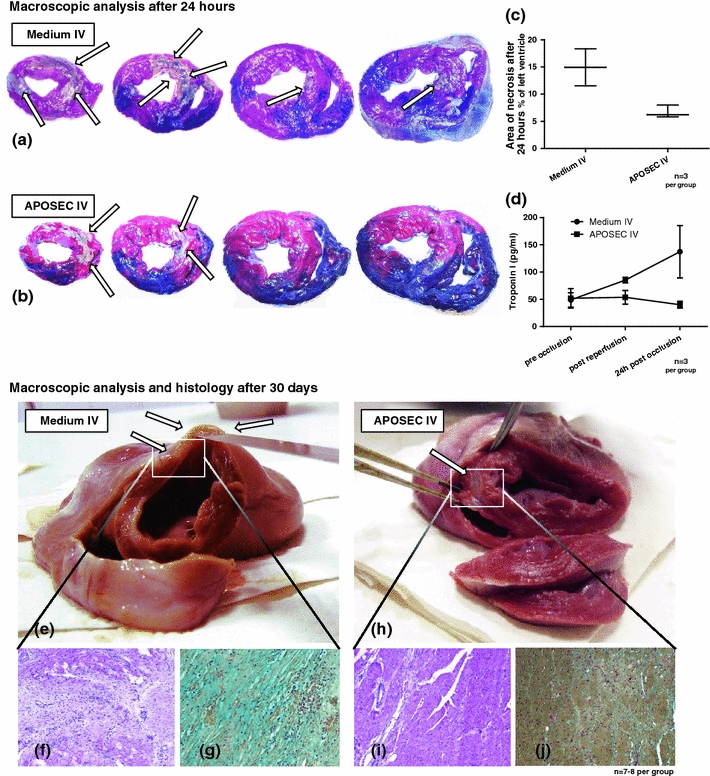
Results of the porcine closed chest reperfused AMI model. a, b Show representative images of porcine hearts explanted 24 h after myocardial infarction stained with tetrazolium chloride and Evans blue solution. The area at risk is stained red and necrotic areas remained unstained (white/grey, arrows). c shows the extent of necrosis for controls and APOSECP-treated animals. d shows ELISA data indicating less troponin I release in treated animals. e, h Show representative images of hearts explanted 30 days after AMI. Hearts of APOSECP-injected pigs evidenced only marginal formation of scar tissue in the myocardium compared to control animals where large infarcts were common. H&E-stained (f, i) and Movat’s pentachrome-stained (g, j) specimens of the infarcted myocardium shown in the lower part of Fig. 3 indicate fewer signs of collagen deposition and more viable cardiomyocytes within the scar tissue (i, j) compared to control animals (f, g)
Intravenous high-dose APOSECP caused a significant short and long-term reduction of myocardial damage as determined by cardiac MRI (Table 2). These results were corroborated by a significant hemodynamic improvement as compared to controls. In the high-dose group, APOSEC infusion reduced the extent of myocardial infarction by approximately 50%, from 18.17 to 8.66% after 3 days (p = 0.0019) and from 12.60 to 6.92% after 30 days (p = 0.015), respectively. Conversely, LVEF, cardiac output and cardiac index were improved significantly compared to controls or low-dose treatment. Figure 3 (see also Supplementary Fig. 2 and supplementary video file) illustrates representative transverse sections of a high-dose APOSECP-injected animal (Fig. 3h) and a control (Fig. 3e). H&E-stained (Fig. 3f, i) and Movat’s pentachrome-stained (Fig. 3g, j) myocardial specimens obtained 30 days after AMI showed less collagen deposition and more viable myocardium in treated pigs as compared to controls.
Table 2.
Cardiac MRI evaluation 3 and 30 days after AMI
| Parameters | Medium control (n = 8) | 250 × 106 apoptotic PBMC (low-dose APOSEC, n = 7) | 1 × 109 apoptotic PBMC (high-dose APOSEC, n = 7) | |
|---|---|---|---|---|
| After 3 days | Weight (kg) | 31.86 ± 9.1 | 30.86 ± 1.6 ns | 33.33 ± 1.3 ns |
| Age (days) | 90 ± 0 | 90 ± 0 ns | 90 ± 0 ns | |
| LVEDV (ml) | 67.59 ± 2.7 | 64.19 ± 5.4 ns | 63.73 ± 1.6 ns | |
| LVESV(ml) | 38.42 ± 2.5 | 35.96 ± 3.0 ns | 33.93 ± 2.1 ns | |
| LVSV (ml) | 29.17 ± 1.3 | 28.23 ± 3.2 ns | 29.77 ± 1.8 ns | |
| LVEF (%) | 43.38 ± 1.9 | 43.63 ± 2.8 ns | 46.65 ± 2.9 ns | |
| HR/min | 111 ± 6 | 109 ± 5 ns | 111 ± 13 ns | |
| CO (l/min) | 3.24 ± 0.1 | 3.03 ± 0.3 ns | 3.28 ± 0.3 ns | |
| CI (l/min/m2) | 3.64 ± 0.1 | 3.59 ± 0.4 ns | 3.82 ± 0.4 ns | |
| Infarct % | 18.17 ± 1.7 | 14.01 ± 1.9 ns | 8.66 ± 1.5** | |
| After 30 days | Weight (kg) | 39.43 ± 0.5 | 37.00 ± 1.9 ns | 48.83 ± 0.7*** |
| Age (days) | 120 ± 0 | 120 ± 0 ns | 120 ± 0 ns | |
| LVEDV (ml) | 54.74 ± 4.1 | 53.43 ± 3.2 ns | 65.99 ± 3.5 ns | |
| LVESV(ml) | 32.93 ± 4.0 | 31.89 ± 2.9 ns | 28.71 ± 3.5 ns | |
| LVSV (ml) | 21.84 ± 1.8 | 21.54 ± 1.9 ns | 37.29 ± 1.7*** | |
| LVEF (%) | 40.54 ± 3.6 | 40.64 ± 3.2 ns | 57.05 ± 3.3** | |
| HR/min | 114 ± 7 | 108 ± 7 ns | 107 ± 5 ns | |
| CO (l/min) | 2.44 ± 0.1 | 2.28 ± 0.1 ns | 3.98 ± 0.2*** | |
| CI (l/min/m2) | 2.46 ± 0.1 | 2.40 ± 0.1 ns | 3.51 ± 0.2*** | |
| Infarct % | 12.60 ± 1.3 | 11.50 ± 1.5 ns | 6.92 ± 1.4* |
Three and 30 days after ischaemia/reperfusion injury, MRI was conducted and parameters of cardiac function were obtained from pigs treated with low and high-dose APOSEC and from control animals
LVEDD left ventricular end-diastolic diameter, LVESD left ventricular end-systolic diameter, LVSV left ventricular stroke volume, LVEF left ventricular ejection fraction, HR heart rate, CI cardiac index, CO cardiac output, ns no significance versus control
* p < 0.05 versus control
** p < 0.01 versus control
*** p < 0.001 versus control
APOSECH induces cytoprotective and anti-apoptotic mechanisms in cardiac myocytes in vitro
Strategies to abrogate the death cascade at the level of intracellular signalling kinases and inhibitors of apoptosis were previously defined in the ischaemia–reperfusion (I/R) and ischaemic postconditioning literature [18, 19, 37, 40], suggesting that targeting anti-apoptotic mechanisms of cellular protection at the time of ischaemia may offer a potential approach for reducing reperfusion-induced myocyte cell death. To investigate whether APOSECH is able to activate pro-survival pathways in cultured cardiac myocytes, we performed Western blot analysis. APOSECH induced phosphorylation of AKT, p42/p44 extra-cellular signal-regulated kinases (Erk1/2), p38 MAPK, Hsp27, c-Jun and CREB in human primary cultured cardiomyocytes within 60 min (Fig. 4a) and in dose-dependent fashion (Fig. 4b). Moreover, the expression of several anti-apoptotic proteins such as Bcl-2 and BAG1 was up-regulated in APOSECH-treated cardiac myocytes after 24 h (Fig. 4c and Supplementary Fig. 3).
Fig. 4.
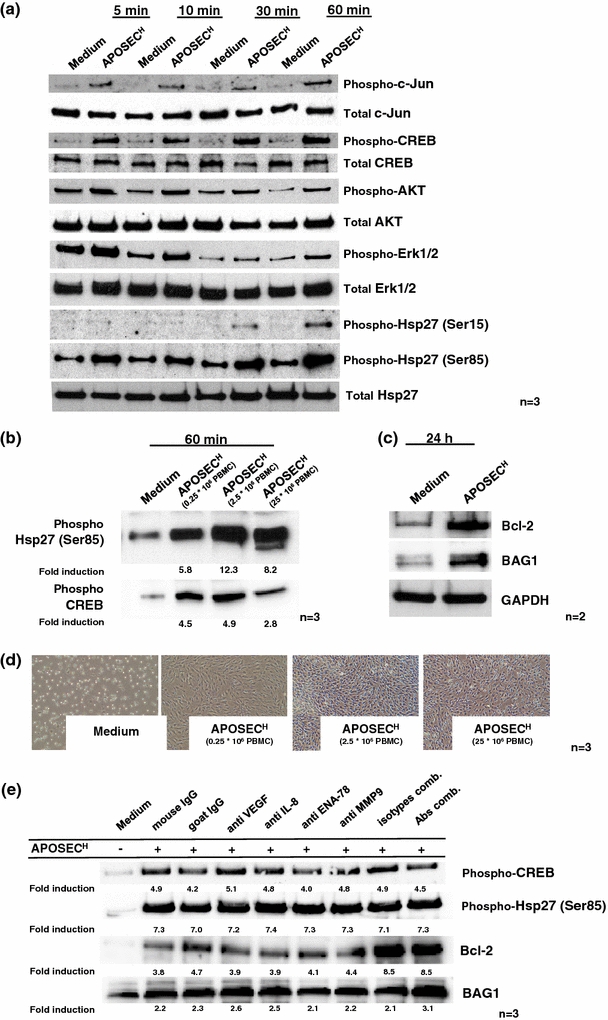
APOSECH induces cytoprotective protein expression and activation. a Cell extracts were prepared after stimulation with APOSECH or control medium after the indicated time intervals. Western blot analysis showed activation of c-Jun, CREB, AKT, ERK1/2 and Hsp27. b shows the dose-dependent induction of Hsp27 and CREB in cultured cardiomyocytes. c 24 h after treatment expression of the anti-apoptotic proteins Bcl-2 and BAG1 was analyzed by Western blotting. Proteins were normalized to the house-keeping gene GAPDH. One representative experiment of two is shown. d shows a dose-dependent cytoprotective effect of APOSEC in a starvation induced cell death model in cardiac myocytes. e shows that neutralization of selected factors (VEGF, IL-8, ENA-78 and MMP9) does not attenuate the induction of CREB and Bcl-2 in cardiomyocytes by APOSEC co-incubation
Furthermore, APOSECH circumvented cardiomyocyte cell death in a starvation assay even at low doses such as APOSEC obtained from 0.25 × 106 irradiated apoptotic PBMC (Fig. 4d). Neutralization of selected factors, e.g. VEGF, IL-8, ENA-78, MMP9 alone or in combination did not result in an obvious decrease with regard to CREB and Hsp27 phosphorylation nor Bcl-2 or BAG1 up-regulation (Fig. 4e).
Discussion
Current medical and invasive therapies after AMI do not address the central problem associated with the massive loss of cardiomyocytes, vascular cells, and interstitial cells, and in consequence these patients continue to experience frequent hospitalizations and premature death. Additionally, although reperfusion after coronary occlusion is undoubtedly prerequisite for tissue salvage, additional myocardial injury can occur. The sudden reinitiation of blood flow leads to an inflammatory response, which results in further endothelial and myocardial injury. No drug has successfully passed clinical trials for cardiac injury despite the 30 years that have passed since the phenomenon of “myocardial injury through reflow” was first acknowledged [11]. This myocardial reperfusion injury is triggered by several distinct soluble and/or cellular components of the inflammatory network [12, 23]. In our previous work we abrogated myocardial remodelling by infusing cultured irradiated apoptotic PBMC [2, 26] and we could demonstrate that an alternative approach, namely, intravenous infusion of soluble factors derived from irradiated PBMC (APOSEC) reduced progressive collagen deposition and scar formation and improved ventricular function in a rodent model of acute myocardial ischaemia and in a large animal closed chest reperfused infarction model.
Recently, there has been a change of thinking in the field of regenerative medicine since publications showed that soluble factors from bone marrow cells, termed BMC-SN, initiated proliferation and migration of coronary artery endothelial cells, endothelial tube formation and cell sprouting in a mouse aortic ring assay [25]. Other investigators demonstrated that endothelial progenitor cell-conditioned medium (EPC-CM) enhanced the formation of capillary outgrowth in a rat aortic ring model and enhanced survival of serum-starved human umbilical cord endothelial cells (HUVEC) [7]. In vivo relevance was demonstrated in this study showing that EPC-CM caused increased blood flow, muscle mitochondrial activity and functional improvement in an ischaemic hindlimb model. More recently Kalka et al. have shown that EPC-CM causes resistance of HUVEC to oxidative stress by inducing anti-oxidative enzymes, and resistance to apoptosis induction in vitro by increasing the Bcl-2/BAX ratio. From these results, the group deduced that EPC-CM contains massive amounts of cytoprotective proteins causing anti-apoptotic and growth factor-mediated effects. Most importantly and similar to our results, neutralization of selective or combined cytokines such as VEGF, HGF, IL-8 and MMP9 did not significantly reverse the cytoprotective effect of EPC-CM. The group concluded that EPC secrete factors which cause broad synergistic effects and exert strong cytoprotective properties on differentiated endothelium through modulation of intracellular antioxidant and defensive mechanisms and pro-survival genes [45].
The mechanism described in the above paragraph for BMC-SN and EPC-CM is similar to that which we describe in this paper concerning secreted factors from PBMC, which documents the first evidence that culture medium from apoptotic PBMC (APOSEC) induces cytoprotective mechanisms in cultured cardiomyocytes in vitro. However, unlike the BMC-SN process, which requires bone marrow stem cell acquisition and preparation, PBMC can be obtained from venous whole blood, which can be much more easily obtained via simple venal puncture.
Based on the fact that infarct dimensions were reduced compared to controls in both in vivo models even within the first 3 days after AMI, we believe that the main mechanism of action induced by APOSEC treatment is conferring cardioprotective effects to cardiomyocytes at risk. In support of this hypothesis, we tested whether APOSEC could induce the activation of intracellular signalling pathways known to be associated with cardiac conditioning or cytoprotection [19, 21].
The co-incubation of human cardiac myocytes with APOSEC led to a rapid phosphorylation of several important survival factors such as AKT, Erk1/2, p38 MAPK (all part of the ischaemia reperfusion injury salvage kinase pathway, RISK), c-JUN, cAMP-response element binding protein (CREB) and Hsp27 [5, 8, 19, 40]. Moreover, Bcl-2 and BAG1, known anti-apoptotic effectors, were massively enhanced within 24 h.
To identify key factors mediating the cytoprotective effect of APOSEC, we tried to inhibit the activity of some factors found in APOSEC at higher concentrations (IL-8, VEGF, ENA-78, MMP9) by utilizing neutralizing antibodies. As shown in Fig. 4e, the neutralization of these selected factors alone or in combination thereof was not sufficient in attenuating the phosphorylation of CREB/Hsp27 and Bcl-2/BAG1 expression in cultured human cardiomyocytes exposed to APOSEC. These results suggest that other or even yet unidentified factors or combinations thereof or other pleiotropic effects might be responsible for the beneficial effects seen by APOSEC treatment.
In addition, as seen in Table 1, we have identified a great variety of proteins in APOSEC defined to be relevant for homing of progenitor cells to sites of ischaemia. We believe that enhanced presence of c-kit+ and CD68+ cells in the infarcted area is a direct consequence of early single-dose intravenous administration of APOSEC after the onset of myocardial ischaemia. Based on these findings, we suggest that APOSEC IV is causative for myocardial resistance to hypoxia-induced cell damage in AMI and circumvented inflammation.
According to the guidelines issued by the European Society of Cardiology and the American Heart Association (AHA), prompt restoration of coronary perfusion within the shortest time is currently considered to be the standard of cardiac care for patients suffering from ST-elevation myocardial infarction (STEMI) [3, 41]. We therefore have chosen a similar approach by utilizing a closed chest reperfused AMI large animal model mimicking the human AMI scenario with “primary PCI for coronary occlusion”. In addition to this standardized treatment of AMI in humans, we applied high and low-dose APOSEC 40 min after starting of the LAD balloon occlusion. These time intervals were selected to accord with the clinical scenario of earliest possible intravenous therapy with approximately 30-40 min delay from symptom to first needle time. Our obtained short and long-term MRI results evidenced that one high-dose APOSEC infusion led to a highly significant improvement of cardiac function and attenuation of myocardial infarction, indicating a dose-dependent effect.
Thus, APOSEC would appear to represent a “biological” which prevents experimental myocardial infarction by causing peri-infarct conditioning and stimulation of regenerative effects in the hypoxic myocardium.
Conclusion and outlook
Beneficiary blood products such as intravenous immunoglobulin (IVIG), coagulation factors or plasma protein concentrates have confirmed their clinical value in recent decades. Unlike these derivatives, APOSEC is a product made of soluble factors secreted by irradiated PBMC. This lyophilized “biological” combines the following clinically favourable features: (a) easily obtainable raw material (PBMC) for APOSEC production from the blood of healthy donors obviating the need to process cell of diseased patients avoiding disadvantageous effects such as stem cell dysfunction or a reduced secretory capacity when using cells of diseased patients [30]; (b) minimal or no antigenicity owing to protein-only content; and (c) “off the shelf” utilization in the clinical setting of AMI diagnosis requiring only single-dose administration. We conclude from our in vitro and in vivo data that APOSEC IV exhibits the features of a highly effective peri-infarct conditioning drug compound. Since the effectiveness of APOSEC has been demonstrated in our pre-clinical studies, the use of APOSEC with human experimental subjects is warranted. With regard to the underlying mechanisms, further studies are necessary to fully understand the mode of action of APOSEC, and we strongly believe that our present study can serve as a good basis for such a study in the future.
Limitations
In all three experimental settings (rat AMI model, porcine reperfused AMI model, in vitro experiments using human cells), APOSEC was tested in a syngeneic fashion only to obviate inter-species influences. However, the composition of the supernatants and its effects on human cardiac myocytes in vitro were exclusively analyzed for APOSEC derived from human cells, any differences in the secretory capacity of rat or porcine PBMC cannot entirely be ruled out.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
We are thankful to Christoph Inci, Barbara Neudert, Andrea Baumgartner, Matthias Hasun, Gregor Werba, Moritz Rauch and Karina Plesch for technical assistance. Funding for editing was provided by the Christian Doppler Research Association. This paper is dedicated to M.P., I.P., M.B. and K.F.
Contributions
M.L. and M.M. designed and performed animal as well as in vitro experiments, and collected and interpreted the data. M.L. and M.M. share the first authorship. K.H. and M.Z. performed laboratory experiments. B.P. provided advice on animal protocols and histology. M.G and W.S. performed large animal experiments. E.B. performed MRI analyses. E.T. and M.D. provided input on developmental aspects. The text was edited by V.B.H.J.A. conceived, designed and oversaw all of the studies, collected results, interpreted the data and wrote the manuscript.
Conflict of interest
The study was funded by the Christian Doppler Research Association, APOSCIENCE AG and the Medical University Vienna. The authors declare competing financial interests. The medical university has claimed financial interest (patent number EP2201954, WO2010070105-A1, filed 18 Dec 2008). H.J.A. is a shareholder of APOSCIENCE AG, which owns the rights to commercialize APOSEC for therapeutic use.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Footnotes
M. Lichtenauer and M. Mildner contributed equally to this work.
References
- 1.Alderman EL, Stadius ML. The angiographic definitions of the bypass angioplasty revascularization investigation. Coron Artery Dis. 1992;3:1189–1207. [Google Scholar]
- 2.Ankersmit HJ, Hoetzenecker K, Dietl W, Soleiman A, Horvat R, Wolfsberger M, Gerner C, Hacker S, Mildner M, Moser B, Lichtenauer M, Podesser BK. Irradiated cultured apoptotic peripheral blood mononuclear cells regenerate infarcted myocardium. Eur J Clin Invest. 2009;39:445–456. doi: 10.1111/j.1365-2362.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- 3.Canadian Cardiovascular Society; American Academy of Family Physicians; American College of Cardiology; American Heart Association, Antman EM, Hand M, Armstrong PW, Bates ER, Green LA, Halasyamani LK, Hochman JS, Krumholz HM, Lamas GA, Mullany CJ, Pearle DL, Sloan MA, Smith SC Jr, Anbe DT, Kushner FG, Ornato JP, Pearle DL, Sloan MA, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Lytle BW, Nishimura R, Page RL, Riegel B, Tarkington LG, Yancy CW (2007) 2007 focused update of the ACC/AHA 2004 guidelines for the management of patients with ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 51:210–247. doi:10.1016/j.jacc.2007.10.001 [DOI] [PubMed]
- 4.Bittencourt MC, Perruche S, Contassot E, Fresnay S, Baron MH, Angonin R, Aubin F, Hervé P, Tiberghien P, Saas P. Intravenous injection of apoptotic leukocytes enhances bone marrow engraftment across major histocompatibility barriers. Blood. 2001;98:224–230. doi: 10.1182/blood.V98.1.224. [DOI] [PubMed] [Google Scholar]
- 5.Breivik L, Helgeland E, Aarnes EK, Mrdalj J, Jonassen AK. Remote postconditioning by humoral factors in effluent from ischemic preconditioned rat hearts is mediated via PI3 K/Akt-dependent cell-survival signaling at reperfusion. Basic Res Cardiol. 2011;106:135–145. doi: 10.1007/s00395-010-0133-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunner S, Engelmann MG, Franz WM. Stem cell mobilisation for myocardial repair. Expert Opin Biol Ther. 2008;8:1675–1690. doi: 10.1517/14712598.8.11.1675. [DOI] [PubMed] [Google Scholar]
- 7.Di Santo S, Yang Z, Wyler von Ballmoos M, Voelzmann J, Diehm N, Baumgartner I, Kalka C. Novel cell-free strategy for therapeutic angiogenesis: in vitro generated conditioned medium can replace progenitor cell transplantation. PLoS One. 2009;4:e5643. doi: 10.1371/journal.pone.0005643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Efthymiou CA, Mocanu MM, de Belleroche J, Wells DJ, Latchmann DS, Yellon DM. Heat shock protein 27 protects the heart against myocardial infarction. Basic Res Cardiol. 2004;99:392–394. doi: 10.1007/s00395-004-0483-6. [DOI] [PubMed] [Google Scholar]
- 9.Fazel SS, Chen L, Angoulvant D, Li SH, Weisel RD, Keating A, Li RK. Activation of c-kit is necessary for mobilization of reparative bone marrow progenitor cells in response to cardiac injury. FASEB J. 2008;22:930–940. doi: 10.1096/fj.07-8636com. [DOI] [PubMed] [Google Scholar]
- 10.Fazel S, Cimini M, Chen L, Li S, Angoulvant D, Fedak P, Verma S, Weisel RD, Keating A, Li RK. Cardioprotective c-kit+ cells are from the bone marrow and regulate the myocardial balance of angiogenic cytokines. J Clin Invest. 2006;116:1865–1877. doi: 10.1172/JCI27019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrans VJ. Morphological methods for evaluation of myocardial protection. Ann Thorac Surg. 1975;20:11–20. doi: 10.1016/S0003-4975(10)63845-3. [DOI] [PubMed] [Google Scholar]
- 12.Frangogiannis NG. The immune system and cardiac repair. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gnecchi M, Zhang Z, Ni A, Dzau VJ. Paracrine mechanisms in adult stem cell signaling and therapy. Circ Res. 2008;103:1204–1219. doi: 10.1161/CIRCRESAHA.108.176826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gyöngyösi M, Lang I, Dettke M, Beran G, Graf S, Sochor H, Nyolczas N, Charwat S, Hemetsberger R, Christ G, Edes I, Balogh L, Krause KT, Jaquet K, Kuck KH, Benedek I, Hintea T, Kiss R, Préda I, Kotevski V, Pejkov H, Zamini S, Khorsand A, Sodeck G, Kaider A, Maurer G, Glogar D. Combined delivery approach of bone marrow mononuclear stem cells early and late after myocardial infarction: the MYSTAR prospective, randomized study. Nat Clin Pract Cardiovasc Med. 2009;6:70–81. doi: 10.1038/ncpcardio1388. [DOI] [PubMed] [Google Scholar]
- 15.Gyöngyösi M, Posa A, Pavo N, Hemetsberger R, Kvakan H, Steiner-Böker S, Petrási Z, Manczur F, Pavo IJ, Edes IF, Wojta J, Glogar D, Huber K. Differential effect of ischaemic preconditioning on mobilisation and recruitment of haematopoietic and mesenchymal stem cells in porcine myocardial ischaemia-reperfusion. Thromb Haemost. 2010;104:376–384. doi: 10.1160/TH09-08-0558. [DOI] [PubMed] [Google Scholar]
- 16.Gyöngyösi M, Hemetsberger R, Posa A, Charwat S, Pavo N, Petnehazy O, Petrasi Z, Pavo IJ, Hemetsberger H, Benedek I, Benedek T, Benedek I, Jr, Kovacs I, Kaun C, Maurer G. Hypoxia-inducible factor 1-alpha release after intracoronary versus intramyocardial stem cell therapy in myocardial infarction. J Cardiovasc Transl Res. 2010;3:114–121. doi: 10.1007/s12265-009-9154-1. [DOI] [PubMed] [Google Scholar]
- 17.Halkos ME, Zhao ZQ, Kerendi F, Wang NP, Jiang R, Schmarkey LS, Martin BJ, Quyyumi AA, Few WL, Kin H, Guyton RA, Vinten-Johansen J. Intravenous infusion of mesenchymal stem cells enhances regional perfusion and improves ventricular function in a porcine model of myocardial infarction. Basic Res Cardiol. 2008;103:525–536. doi: 10.1007/s00395-008-0741-0. [DOI] [PubMed] [Google Scholar]
- 18.Hausenloy DJ, Yellon DM. Reperfusion injury salvage kinase signalling: taking a RISK for cardioprotection. Heart Fail Rev. 2007;12:217–234. doi: 10.1007/s10741-007-9026-1. [DOI] [PubMed] [Google Scholar]
- 19.Hausenloy DJ, Yellon DM. Survival kinases in ischemic preconditioning and postconditioning. Cardiovasc Res. 2006;70:240–253. doi: 10.1016/j.cardiores.2006.01.017. [DOI] [PubMed] [Google Scholar]
- 20.Heissig B, Hattori K, Dias S, Friedrich M, Ferris B, Hackett NR, Crystal RG, Besmer P, Lyden D, Moore MA, Werb Z, Rafii S. Recruitment of stem and progenitor cells from the bone marrow niche requires MMP-9 mediated release of kit-ligand. Cell. 2002;109:625–637. doi: 10.1016/S0092-8674(02)00754-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heusch G, Boengler K, Schulz R. Cardioprotection: nitric oxide, protein kinases, and mitochondria. Circulation. 2008;118:1915–1919. doi: 10.1161/CIRCULATIONAHA.108.805242. [DOI] [PubMed] [Google Scholar]
- 22.Hoffmann PR, Kench JA, Vondracek A, Kruk E, Daleke DL, Jordan M, Marrack P, Henson PM, Fadok VA. Interaction between phosphatidylserine and the phosphatidylserine receptor inhibits immune responses in vivo. J Immunol. 2005;174:1393–1404. doi: 10.4049/jimmunol.174.3.1393. [DOI] [PubMed] [Google Scholar]
- 23.Kleinbongard P, Heusch G, Schulz R. TNFalpha in atherosclerosis, myocardial ischemia/reperfusion and heart failure. Pharmacol Ther. 2010;127:295–314. doi: 10.1016/j.pharmthera.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 24.Kocher AA, Schuster MD, Bonaros N, Lietz K, Xiang G, Martens TP, Kurlansky PA, Sondermeijer H, Witkowski P, Boyle A, Homma S, Wang SF, Itescu S. Myocardial homing and neovascularization by human bone marrow angioblasts is regulated by IL-8/Gro CXC chemokines. J Mol Cell Cardiol. 2006;40:455–464. doi: 10.1016/j.yjmcc.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 25.Korf-Klingebiel M, Kempf T, Sauer T, Brinkmann E, Fischer P, Meyer GP, Ganser A, Drexler H, Wollert KC. Bone marrow cells are a rich source of growth factors and cytokines: implications for cell therapy trials after myocardial infarction. Eur Heart J. 2008;29:2851–2858. doi: 10.1093/eurheartj/ehn456. [DOI] [PubMed] [Google Scholar]
- 26.Lichtenauer M, Mildner M, Baumgartner A, Hasun M, Werba G, Beer L, Altmann P, Roth G, Gyöngyösi M, Podesser BK, Ankersmit HJ. Intravenous and intramyocardial injection of apoptotic white blood cell suspensions prevents ventricular remodelling by increasing elastin expression in cardiac scar tissue after myocardial infarction. Basic Res Cardiol. 2011;106:645–655. doi: 10.1007/s00395-011-0173-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lunde K, Solheim S, Aakhus S, Arnesen H, Abdelnoor M, Egeland T, Endresen K, Ilebekk A, Mangschau A, Fjeld JG, Smith HJ, Taraldsrud E, Grøgaard HK, Bjørnerheim R, Brekke M, Müller C, Hopp E, Ragnarsson A, Brinchmann JE, Forfang K. Intracoronary injection of mononuclear bone marrow cells in acute myocardial infarction. N Engl J Med. 2006;355:1199–1209. doi: 10.1056/NEJMoa055706. [DOI] [PubMed] [Google Scholar]
- 28.Madonna R, Rokosh G, De Caterina R, Bolli R. Hepatocyte growth factor/Met gene transfer in cardiac stem cells—potential for cardiac repair. Basic Res Cardiol. 2010;105:443–452. doi: 10.1007/s00395-010-0102-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oerlemans MI, Goumans MJ, van Middelaar B, Clevers H, Doevendans PA, Sluijter JP. Active Wnt signaling in response to cardiac injury. Basic Res Cardiol. 2010;105:631–641. doi: 10.1007/s00395-010-0100-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orlandi A, Chavakis E, Seeger F, Tjwa M, Zeiher AM, Dimmeler S. Long-term diabetes impairs repopulation of hematopoietic progenitor cells and dysregulates the cytokine expression in the bone marrow microenvironment in mice. Basic Res Cardiol. 2010;105:703–712. doi: 10.1007/s00395-010-0109-0. [DOI] [PubMed] [Google Scholar]
- 31.Orlic D, Kajstura J, Chimenti S, Limana F, Jakoniuk I, Quaini F, Nadal-Ginard B, Bodine DM, Leri A, Anversa P. Mobilized bone marrow cells repair the infarcted heart, improving function and survival. Proc Natl Acad Sci USA. 2001;98:10344–10349. doi: 10.1073/pnas.181177898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ortiz-Pérez JT, Meyers SN, Lee DC, Kansal P, Klocke FJ, Holly TA, Davidson CJ, Bonow RO, Wu E. Angiographic estimates of myocardium at risk during acute myocardial infarction: validation study using cardiac magnetic resonance imaging. Eur Heart J. 2007;28:1750–1758. doi: 10.1093/eurheartj/ehm212. [DOI] [PubMed] [Google Scholar]
- 33.Perruche S, Kleinclauss F, Bittencourt Mde C, Paris D, Tiberghien P, Saas P. Intravenous infusion of apoptotic cells simultaneously with allogenic hematopoietic grafts alters anti-donor humoral immune responses. Am J Transplant. 2004;4:1361–1365. doi: 10.1111/j.1600-6143.2004.00509.x. [DOI] [PubMed] [Google Scholar]
- 34.Saas P, Bonnefoy F, Kury-Paulin S, Kleinclauss F, Perruche S. Mediators involved in the immunomodulatory effects of apoptotic cells. Transplantation. 2007;84(1 Suppl):31–34. doi: 10.1097/01.tp.0000269113.59857.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sanganalmath SK, Abdel-Latif A, Bolli R, Xuan YT, Dawn B. Hematopoietic cytokines for cardiac repair: mobilization of bone marrow cells and beyond. Basic Res Cardiol. 2011;106:709–733. doi: 10.1007/s00395-011-0183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schächinger V, Erbs S, Elsässer A, Haberbosch W, Hambrecht R, Hölschermann H, Yu J, Corti R, Mathey DG, Hamm CW, Süselbeck T, Assmus B, Tonn T, Dimmeler S, Zeiher AM, REPAIR-AMI Investigators Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 37.Skyschally A, van Caster P, Boengler K, Gres P, Musiolik J, Schilawa D, Schulz R, Heusch G. Ischemic postconditioning in pigs: no causal role for RISK activation. Circ Res. 2009;104:15–18. doi: 10.1161/CIRCRESAHA.108.186429. [DOI] [PubMed] [Google Scholar]
- 38.Smart N, Riley PR. The stem cell movement. Circ Res. 2008;102:1155–1168. doi: 10.1161/CIRCRESAHA.108.175158. [DOI] [PubMed] [Google Scholar]
- 39.Thum T, Bauersachs J, Poole-Wilson PA, Volk HD, Anker SD. The dying stem cell hypothesis: immune modulation as a novel mechanism for progenitor cell therapy in cardiac muscle. J Am Coll Cardiol. 2005;46:1799–1802. doi: 10.1016/j.jacc.2005.07.053. [DOI] [PubMed] [Google Scholar]
- 40.Tsang A, Hausenloy DJ, Mocanu MM, Yellon DM. Postconditioning: a form of “modified reperfusion” protects the myocardium by activating the phosphatidylinositol 3-kinase-Akt pathway. Circ Res. 2004;95:230–232. doi: 10.1161/01.RES.0000138303.76488.fe. [DOI] [PubMed] [Google Scholar]
- 41.Van de Werf F, Bax J, Betriu A, Blomstrom-Lundqvist C, Crea F, Falk V, Filippatos G, Fox K, Huber K, Kastrati A, Rosengren A, Steg PG, Tubaro M, Verheugt F, Weidinger F, Weis M, ESC Committee for Practice Guidelines (CPG) Management of acute myocardial infarction in patients presenting with persistent ST-segment elevation: the Task Force on the Management of ST-Segment Elevation Acute Myocardial Infarction of the European Society of Cardiology. Eur Heart J. 2008;29:2909–2945. doi: 10.1093/eurheartj/ehn416. [DOI] [PubMed] [Google Scholar]
- 42.Velagaleti RS, Pencina MJ, Murabito JM, Wang TJ, Parikh NI, D’Agostino RB, Levy D, Kannel WB, Vasan RS. Long-term trends in the incidence of heart failure after myocardial infarction. Circulation. 2008;118:2057–2062. doi: 10.1161/CIRCULATIONAHA.108.784215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wollert KC, Meyer GP, Lotz J, Ringes-Lichtenberg S, Lippolt P, Breidenbach C, Fichtner S, Korte T, Hornig B, Messinger D, Arseniev L, Hertenstein B, Ganser A, Drexler H. Intracoronary autologous bone-marrow cell transfer after myocardial infarction: the BOOST randomised controlled clinical trial. Lancet. 2004;364:141–148. doi: 10.1016/S0140-6736(04)16626-9. [DOI] [PubMed] [Google Scholar]
- 44.Wu J, Li J, Zhang N, Zhang C. Stem cell-based therapies in ischemic heart diseases: a focus on aspects of microcirculation and inflammation. Basic Res Cardiol. 2011;106:317–324. doi: 10.1007/s00395-011-0168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang Z, von Ballmoos MW, Faessler D, Voelzmann J, Ortmann J, Diehm N, Kalka-Moll W, Baumgartner I, Di Santo S, Kalka C. Paracrine factors secreted by endothelial progenitor cells prevent oxidative stress-induced apoptosis of mature endothelial cells. Atherosclerosis. 2010;211:103–109. doi: 10.1016/j.atherosclerosis.2010.02.022. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


