Abstract
Background/Aims
Dysfunction of the gastrointestinal tract occurs in about 76% of patients who are diabetic for more than 10 years. Although diabetes-related dysfunctions of the stomach such as gastroparesis have been extensively studied over the recent years, studies about the mechanism underlying colonic symptoms in long-term diabetes models are rare. Therefore, the goal of our study was to clarify the nature of colonic dysfunction in a long-term diabetic rat model.
Methods
The characteristics of colonic smooth muscle were investigated in Otsuka Long-Evans Tokushima Fatty (OLETF) rats, an animal model of type 2 diabetes. These results were compared to those obtained from Long-Evans Tokushima Otsuka (LETO) control rats.
Results
Spontaneous contractility of the proximal colon was significantly decreased in the diabetic rats compared to the controls, while the spontaneous contractility of the distal colon was not. The number of interstitial cells of Cajal networks in the proximal colon was greatly decreased in diabetic rats compared to the controls. Contractility of the proximal colon in response to carbachol, an acetylcholine receptor agonist, was significantly weaker in the diabetic rats. In addition, the degree of relaxation in response to nitric oxide in the proximal colon of diabetic rats also appeared to be attenuated.
Conclusions
The results from our study suggest that the decrease of interstitial cells of Cajal network, cholinergic receptors, and neuronal nitric oxide synthase in the proximal colon plays important roles in diabetes-related dysfunction of colon.
Keywords: Colon, Diabetes mellitus, Gastrointestinal motility, Interstitial cells of Cajal
Introduction
Diabetes and its long-term complications, including cardiovascular, renal, neurologic and ophthalmic diseases, represent a major cause of morbidity and mortality throughout the world.1 It has been reported that 76% of outpatients with diabetes have one or more gastrointestinal (GI) symptoms such as nausea, vomiting, early satiety, abdominal pain, constipation, diarrhea and fecal incontinence.2-8 The prevalence of diabetes-related GI symptoms may negatively impact the patient's quality of life.9
There have been many reports about upper GI symptoms, especially gastroparesis, in diabetic patients.6,8,10-13 However, few studies about the mechanism underlying colonic symptoms in long-term diabetes models have been conducted and produced conflicting results.14,15 In many reports, these GI symptoms are thought to be results of abnormal GI motility that, in turn, may be a manifestation of diabetic autonomic neuropathy involving the GI tract.3,5,6,8 Increasing evidence, however, indicates that GI symptoms in diabetic patients are multifactorial.16,17 Hyperglycemia can impair gastric and small intestinal motility18,19 and autonomic neuropathy cannot be considered as the only reason for GI motility disorders.20
Interstitial cells of Cajal (ICC) have been identified as a pacemaker and mediators of neurotransmission in the GI tract, and so play a central role in GI motility.21 The involvement of ICC in diabetic gastroenteropathies was recognized relatively recently.13,21 Since then, several studies have reported the depletion of ICC networks in both diabetic patients and various diabetic animal models.11,14
In the present study, we analyzed Otsuka Long-Evans Tokushima Fatty (OLETF) rats older than 1 year as a long-term type 2 diabetes mellitus (DM) model. The clinical and pathological features of diabetes in OLETF rats closely resemble those of humans with type 2 DM.22 We evaluated colonic motility, the cholinergic/nitrergic response, and ICC networks in OLETF rats to clarify the pathogenesis of the lower GI symptoms associated with type 2 DM.
Materials and Methods
Experimental Animals
Six male OLETF and 6 male Long-Evans Tokushima Otsuka (LETO) rats older than 60 weeks were used. All animals were allowed free access to water and food, and were maintained in a controlled environment with alternating 12 hour light/dark cycles. All animal procedures were approved by the Institutional Guideline Committee for Animal Experiments, Keimyung university school of medicine.
Tissue Preparations
Tissue specimens were collected from the proximal (about 1 cm from the cecum) and distal (about 1 cm from the rectosigmoid junction) colon. The mucosal layer was peeled off and transferred to a dissecting dish containing oxygenated (95% O2 and 5% CO2) Krebs solution with the following composition: 118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 25 mM NaHCO3, 2.5 mM CaCl, and 11 mM glucose. Colonic circular muscle strips (2 × 10 mm) were prepared.
Functional Studies
One side of the colon strip was pinned to the floor of the recording chamber and the opposite side was connected to an isometric force transducer (Grass Technology, West Warwick, RI, USA). The strips were allowed to equilibrate for 60 minutes under an initial tension of 0.5 g. During this period, the bath solution was changed at every 10 minutes with aerated Krebs solution (95% O2 and 5% CO2). The bath temperature was maintained at 37 ± 0.5℃. Carbachol (1 nM-1 µM; Sigma-Aldrich, St. Louis, MO, USA), a cholinergic agonist, was used to evaluate the cholinergic system in the proximal colon. L-NG-nitroarginine methyl ester (L-NAME, 100 µM; Sigma-Aldrich, St. Louis, MO, USA) was used to evaluate the effect of nitric oxide on colonic motility. Electrical field stimulation (EFS; 1-10 Hz, 120 V, 0.5 ms for 30 seconds) was applied between 2 platinum plates using a Grass S48 stimulator (Grass Technology) to induce a neuronal response. Tetrodotoxin (TTX, 1 µM; Tocris Bioscience, Avonmouth, Bristol, UK) was used to confirm that the EFS-induced responses were neuronal. At the beginning of each EFS experiment, the strips were pretreated with atropine (1 µM; Sigma-Aldrich).
Fluorescent Immunohistochemistry
Colonic tissues were fixed in 4% paraformaldehyde and washed for 5 minutes with phosphate-buffered saline (PBS, pH 7.4) with a 3% dehydroxide solution. Sections were preincubated with blocking solution (Invitrogen, Carlsbad, CA, USA) for 30 minutes before being incubated with the following antibodies at room temperature: anti-ICC (c-Kit; Santa Cruz Biotechnology, CA, USA), anti-M2 (Abcam, Cambridge, UK), anti-M3 (Santa Cruz Biotechnology), and anti-nNOS (Abcam). After the sections were incubated for 1.5 hours with the primary antibodies, they were washed with PBS before being incubated with: an Alexa Fluor 488 goat anti-rabbit (Invitrogen) or Alexa Fluor 546 goat anti-mouse (Invitrogen) secondary antibodies for 1.5 hours at room temperature. After being washed with PBS, the specimens were counter-stained with 4',6-diamidino-2-phenylindole (DAPI) and mounted. The immuno-stained tissues were observed by confocal laser scanning microscope (LSM 5 EXCITER; Carl Zeiss, Jena, Germany), using a C-Apochromat objective lens (×40). The confocal images shown are digital composites of Z-series scans of 30 optical sections (1.0 µm thick) through a depth of full or partial thickness of the musculature. Image analysis was completed with LSM 5 EXCITER software (Carl Zeiss).
Statistical Methods
The results are expressed as the mean ± SEM. The SPSS statistical package (version 14.0; SPSS Inc, Chicago, IL, USA) was used for statistical analyses. The area under the curve (AUC) was calculated using LabChart 7 software (AD Instrument, Colorado Springs, CO, USA). Differences between the 2 groups were analyzed using Student's t test. A P-value less than 0.05 was considered to be statistically significant.
Results
Spontaneous Contractile Activity
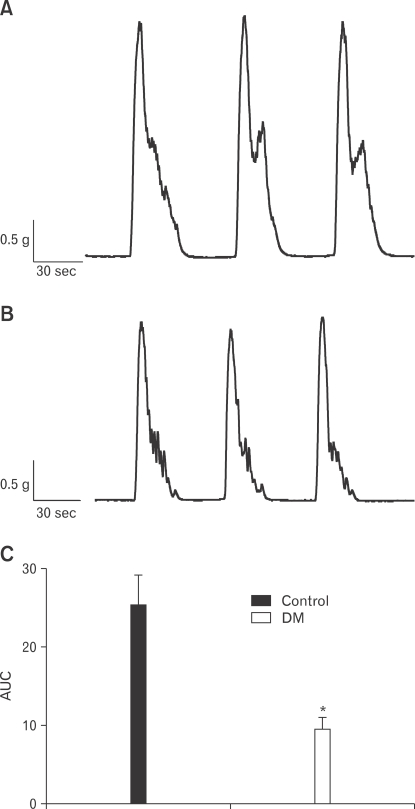
Spontaneous contractility of the proximal and distal colon was examined. Spontaneous contractility of the proximal colon significantly decreased in diabetic rats compared to the control rats, while that of the distal colon was not (Fig. 1A and 1B). The AUC of spontaneous contractility significantly decreased for the diabetic rats compared to the controls (Fig. 1C). Therefore, we performed subsequent experiments to study the proximal colon.
Figure 1.
Spontaneous contractility of the proximal colon in control (A) and diabetes mellitus (B) rats. The degree of contractility was expressed as the area under the curve (AUC) (C). Values are expressed as the means ± SEM (n = 6). *P < 0.05 compared to the control. DM, diabetes mellitus.
Identification of the Interstitial Cells of Cajal Network
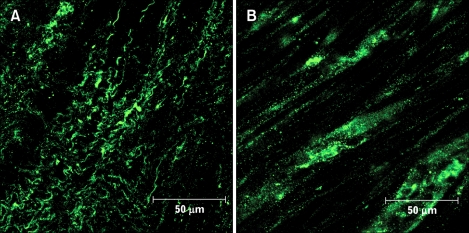
Identification of ICC was made with antibodies against the receptor tyrosine kinase, Kit.21 We observed Kit-positive cells in the proximal colon. The ICC network of the proximal colon was greatly decreased in the diabetic rats compared to the control rats (Fig. 2).
Figure 2.
Network of interstitial cells of Cajal in control (A) and diabetes mellitus (B) rats. Immunofluorescence corresponding to for c-Kit (green) was prepared in the proximal colon.
Response to Cholinergic Agonist
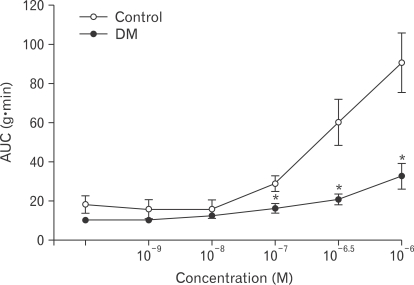
Muscle strips from control rats responded to carbachol with a progressive increase of phasic contractile activity in a dose-dependent manner. The AUC was significantly decreased for diabetic rats compared to the control rats (Fig. 3).
Figure 3.
Effects of carbachol on the contractility of the proximal colon in control and diabetes mellitus rats. Values are expressed as the means ± SEM (n = 6). *P < 0.05 compared to the control value at the same concentration of carbachol. AUC, area under the curve.
Expression of Muscarinic Receptors
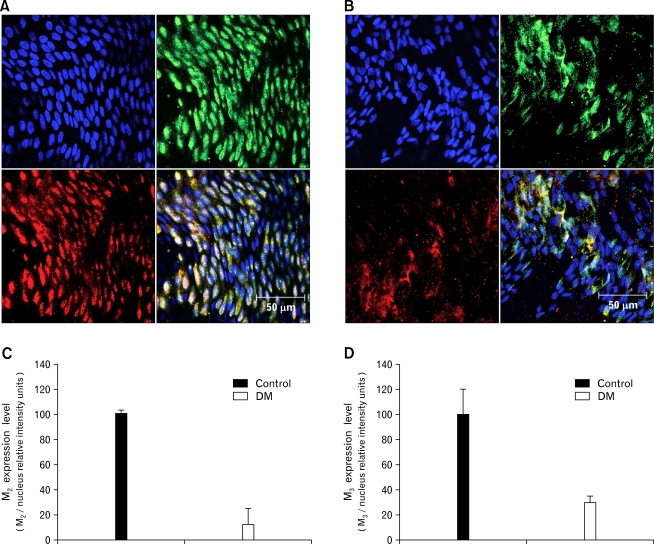
M2 and M3 muscarinic receptors preferentially expressed in GI smooth muscles23,24 were observed in the proximal colon with immunofluorescence. Expressions of receptors relative to the number of nuclei decreased in the diabetic rats compared to the control rats (Fig. 4A and 4B). Co-localizations presented as expression level decreased in the diabetic rats compared to the control rats (Fig. 4C and 4D).
Figure 4.
Expression of M2 and M3 muscarinic receptors in control (A) and diabetes mellitus (DM) (B) rats. Blue: nuclei, Green: M2 receptors, Red: M3 receptors (representative data from n = 6). Co-localization of M2 and nuclei in the control and DM rats (C). Co-localization of M3 and nuclei in control and DM rats (D). Values are expressed as the means ± SEM (n = 6).
Response to Electrical Field Stimulation
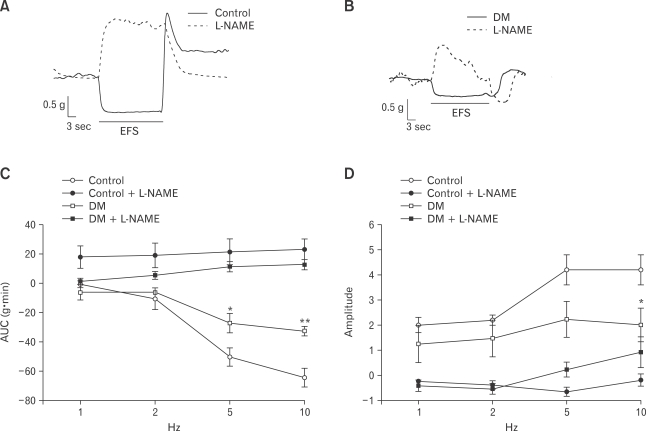
EFS (1, 2, 5 and 10 Hz; 120 V; 0.5 ms) was applied for 30 seconds. In the proximal colon, on-relaxation was noted during EFS. As the EFS frequency increased, the AUC of on-relaxation decreased in the control rats (Fig. 5A and 5C), whereas this response was attenuated in the diabetic rats (Fig. 5B and 5C). To further investigate whether the appearance of on-relaxation during EFS resulted from nitrergic neurotransmission to the smooth muscles, L-NAME was administered for 10 minutes before recording the EFS-induced responses. L-NAME, a non-selective nitric oxide synthase (NOS) inhibitor, prevented the appearance of on-relaxation during EFS in the both the control and diabetic rats (Fig. 5A-5C). EFS caused on-relaxation followed by off-contraction; the amplitude of off-contraction increased in the control rats (Fig. 5A and 5D) whereas this response was attenuated in the diabetic rats (Fig. 5B and 5D). L-NAME also prevented the appearance of off-contraction after EFS in the both the control and diabetic rats (Fig. 5A, 5B and 5D).
Figure 5.
Response to electric field stimulation (EFS) in the proximal colon. (A) EFS (10 Hz) induced normal patterns of on-relaxation and off-contraction response in control rats. These responses disappeared after treatment with L-NG-nitroarginine methyl ester (L-NAME). (B) In diabetes mellitus (DM) rat, the degrees of on-relaxation and off-contraction were less than those observed in the control. Both responses also disappeared after treatment with L-NAME. (C) Response of on-relaxations of the proximal colon in the control was more dependent on the EFS frequency than that of DM rats. (D) Response of off-contractions of the proximal colon in the control and DM rats. Values are expressed as the means ± SEM (n = 6). *P < 0.05 compared to the control value at the stimulation of same frequency. **P < 0.001 compared to the control value at the stimulation of same frequency. AUC, area under the curve.
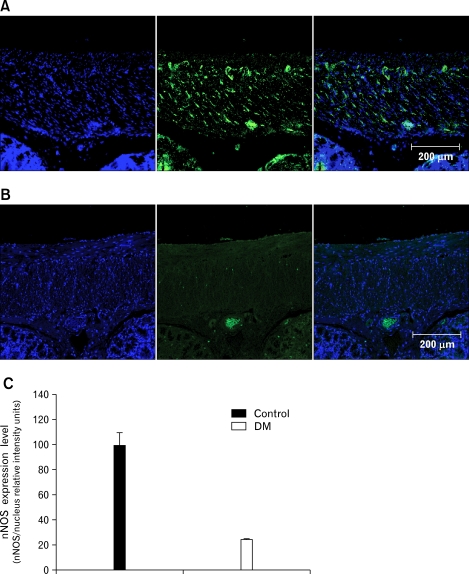
Expression of Neuronal Nitric Oxide Synthase
Non-adrenergic, non-cholinergic relaxation is primarily mediated by nitric oxide in the colon,25 and endogenous neuronal nitric oxide synthase (nNOS) plays an important role in regulating intestinal motility.26 Expression of nNOS relative to the number of nuclei decreased in the diabetic rats compared to the controls (Fig. 6A and 6B). Co-localizations presented as expression level were decreased in the diabetic rats compared to the control rats (Fig. 6C).
Figure 6.
Expression of neuronal nitric oxide synthase (nNOS) in the control (A) and diabetes mellitus (DM) (B) rats. Blue: nuclei, Green: nNOS (representative data from n = 6). Co-localization of nNOS and nuclei in the control and DM rats (C). Values are expressed as the means ± SEM (n = 6).
Discussion
This observational animal study showed that decreases of the ICC network, cholinergic receptors and nNOS expression in the proximal colon play important roles in diabetes-related colonic dysfunction.
A previous study reported higher rates of GI symptoms among 149 patients with type 2 DM who were referred to a university clinic compared to community controls. In this study, the duration of diabetes was the only factor independently associated with GI symptoms.7 Therefore, our experiments were performed on male OLETF and LETO rats older than at least 60 weeks as an animal model of long-term diabetes. The clinico-pathological characteristics of OLETF rats resemble those of humans with type 2 DM including late onset of hyperglycemia, a chronic disease course and resultant end stage.22
In the present study, we initially examined colonic motility of both the proximal and distal colon. However, contractility of the distal colon was not different between the 2 groups. Therefore, we performed subsequent experiments to evaluate the proximal colon. The degree of contractility was estimated with several indicators such as the amplitude, frequency and AUC. Although the frequency of spontaneous contraction seemed to be higher in the diabetic rats and the amplitude of the contraction waves seemed to be larger in control group, these differences were not statistically significant. Therefore, we used the AUC as an indicator of contractility; by doing this, a significant difference between the 2 groups was observed.
In the proximal colon of the diabetic rats, the ICC network was greatly decreased compared to that of the controls. Additionally, expression of cholinergic receptors and nNOS was decreased in the diabetes rats. However, we could not estimate the change in the number of ICC. To estimate the number of ICC or to observe the whole network of ICCs, it is necessary to dissect the specimen horizontally through the myenteric plexus. However, we could not perform this procedure because the specimen was not thick enough. Instead, we observed the morphology and degree of c-Kit expression with confocal microscopy.
ICC are mesenchymal cells that are found throughout the muscular coat of the GI tract. These cells perform functions critical for normal GI motility including the generation and propagation of electrical slow waves and mediation of bidirectional communication between the autonomic nervous system and smooth muscle cells.27 It is also well established that ICC serve as pacemaker cells responsible for promoting spontaneous contractions of the smooth muscle coats.28,29 ICC deficiencies and ultrastructural changes in GI muscle have been reported in diabetic animal models.13,30 A similar deficiency of ICC has also been described in a case report of an insulin-dependent DM patient31 and a study of 7 patients with DM.32 It remains unclear whether this ICC deficiency in colonic smooth muscle is related to the lower rate of GI symptoms associated with type 2 DM. Since ICC are GI pacemaker cells, it can be postulated that impairment of the ICC network can variably affect to the contractile pattern of smooth muscles (eg, dysrrhythmia, absence or decrease). Although we could observe only decreased contractility, further investigation may induce other abnormalities.
ICC within the GI tract express a number of neurotransmitter, their receptors and modulators including acetylcholine receptors (M2 and M3), tachykinin receptors (NK1 and NK3), vasoactive intestinal peptide-1 and nNOS.33-38 In GI smooth muscles, M2 and M3 muscarinic receptors are preferentially expressed with a preponderance of the former subtype.23,24 The contractile response to externally administered agonists or cholinergic nerve stimulation has been shown to be promoted by either M2 or M3 receptors.39,40 Little is known about the alteration of these muscarinic receptors in the long-term diabetic rat model. Endogenous nNOS plays an important role in non-adrenergic, non-cholinergic intestinal relaxation.25,26 Neuronal NOS deficiencies in the GI muscle layer and/or delayed gastric emptying have been observed in both diabetic animal models41-44 and patients with DM.31
Our study had several limitations. First, the number of animals we studied was small because it was difficult to maintain old diabetic rats as well preserved condition. Second, experiments were not performed to examine the distal colon only because no significant difference in simple contractility was observed between the 2 groups. Third, as mentioned above, we could not estimate changes in number of ICC. Despite these limitations, the results from this study still indicated the involvement of several mechanisms in diabetic colonic dysmotility in our long-term diabetic rat model which resemble humans with type 2 DM.
In conclusion, we showed that spontaneous contractility, cholinergic responses and nitrergic responses in the proximal colon were attenuated in a long-term diabetic rat model. We speculated that the loss of the ICC network might have decreased spontaneous contractility. We also hypothesized that decreased numbers of M2/M3 receptors and nNOS expression might have blunted the cholinergic and nitrergic responses, respectively. These results suggest that decreases of the ICC network, the number of cholinergic receptors and nNOS expression in the proximal colon affect DM-related symptoms of the lower GI in long-term diabetic rats. Further investigation is required to identify the precise mechanism underlying lower GI symptoms in patients with diabetes.
Footnotes
Financial support: This study was supported by SK chemical grant from the Korean Society of Neurogastroenterology and Motility, Korea (2010) and a grant (KRF-2006-00008) from the Korea Research Foundation Grant funded by the Korean Government (MOEHRD).
Conflicts of interest: None.
References
- 1.Mokdad AH, Ford ES, Bowman BA, et al. Diabetes trends in the U.S.: 1990-1998. Diabetes Care. 2000;23:1278–1283. doi: 10.2337/diacare.23.9.1278. [DOI] [PubMed] [Google Scholar]
- 2.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–1996. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 3.Enck P, Rathmann W, Spiekermann M, et al. Prevalence of gastrointestinal symptoms in diabetic patients and non-diabetic subjects. Z Gastroenterol. 1994;32:637–641. [PubMed] [Google Scholar]
- 4.Farup CE, Leidy NK, Murray M, Williams GR, Helbers L, Quigley EM. Effect of domperidone on the health-related quality of life of patients with symptoms of diabetic gastroparesis. Diabetes Care. 1998;21:1699–1706. doi: 10.2337/diacare.21.10.1699. [DOI] [PubMed] [Google Scholar]
- 5.Feldman M, Schiller LR. Disorders of gastrointestinal motility associated with diabetes mellitus. Ann Intern Med. 1983;98:378–384. doi: 10.7326/0003-4819-98-3-378. [DOI] [PubMed] [Google Scholar]
- 6.Horowitz M, Fraser R. Disordered gastric motor function in diabetes mellitus. Diabetologia. 1994;37:543–551. doi: 10.1007/BF00403371. [DOI] [PubMed] [Google Scholar]
- 7.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670–674. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 8.Koch KL. Diabetic gastropathy: gastric neuromuscular dysfunction in diabetes mellitus: a review of symptoms, pathophysiology, and treatment. Dig Dis Sci. 1999;44:1061–1075. doi: 10.1023/a:1026647417465. [DOI] [PubMed] [Google Scholar]
- 9.Talley NJ, Young L, Bytzer P, et al. Impact of chronic gastrointestinal symptoms in diabetes mellitus on health-related quality of life. Am J Gastroenterol. 2001;96:71–76. doi: 10.1111/j.1572-0241.2001.03350.x. [DOI] [PubMed] [Google Scholar]
- 10.Horváth VJ, Vittal H, Lörincz A, et al. Reduced stem cell factor links smooth myopathy and loss of interstitial cells of Cajal in murine diabetic gastroparesis. Gastroenterology. 2006;130:759–770. doi: 10.1053/j.gastro.2005.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Iwasaki H, Kajimura M, Osawa S, et al. A deficiency of gastric interstitial cells of Cajal accompanied by decreased expression of neuronal nitric oxide synthase and substance P in patients with type 2 diabetes mellitus. J Gastroenterol. 2006;41:1076–1087. doi: 10.1007/s00535-006-1909-8. [DOI] [PubMed] [Google Scholar]
- 12.James AN, Ryan JP, Crowell MD, Parkman HP. Regional gastric contractility alterations in a diabetic gastroparesis mouse model: effects of cholinergic and serotoninergic stimulation. Am J Physiol Gastrointest Liver Physiol. 2004;287 doi: 10.1152/ajpgi.00431.2003. [DOI] [PubMed] [Google Scholar]
- 13.Ordög T, Takayama I, Cheung WK, Ward SM, Sanders KM. Remodeling of networks of interstitial cells of Cajal in a murine model of diabetic gastroparesis. Diabetes. 2000;49:1731–1739. doi: 10.2337/diabetes.49.10.1731. [DOI] [PubMed] [Google Scholar]
- 14.Forrest A, Huizinga JD, Wang XY, Liu LW, Parsons M. Increase in stretch-induced rhythmic motor activity in the diabetic rat colon is associated with loss of ICC of the submuscular plexus. Am J Physiol Gastrointest Liver Physiol. 2008;294:G315–G326. doi: 10.1152/ajpgi.00196.2007. [DOI] [PubMed] [Google Scholar]
- 15.Imaeda K, Takano H, Koshita M, Yamamoto Y, Joh T, Suzuki H. Electrical properties of colonic smooth muscle in spontaneously non-insulin-dependent diabetic rats. J Smooth Muscle Res. 1998;34:1–11. doi: 10.1540/jsmr.34.1. [DOI] [PubMed] [Google Scholar]
- 16.Clouse RE, Lustman PJ. Gastrointestinal symptoms in diabetic patients: lack of association with neuropathy. Am J Gastroenterol. 1989;84:868–872. [PubMed] [Google Scholar]
- 17.Quan C, Talley NJ, Jones MP, Spies J, Horowitz M. Gain and loss of gastrointestinal symptoms in diabetes mellitus: associations with psychiatric disease, glycemic control, and autonomic neuropathy over 2 years of follow-up. Am J Gastroenterol. 2008;103:2023–2030. doi: 10.1111/j.1572-0241.2008.01943.x. [DOI] [PubMed] [Google Scholar]
- 18.Abrahamsson H. Gastrointestinal motility disorders in patients with diabetes mellitus. J Intern Med. 1995;237:403–409. doi: 10.1111/j.1365-2796.1995.tb01194.x. [DOI] [PubMed] [Google Scholar]
- 19.De Boer SY, Masclee AA, Lamers CB. Effect of hyperglycemia on gastrointestinal and gallbladder motility. Scand J Gastroenterol. 1992;27(suppl 194):13–18. doi: 10.3109/00365529209096020. [DOI] [PubMed] [Google Scholar]
- 20.Pozzi M, Rivolta M, Gelosa M, et al. Upper gastrointestinal involvement in diabetes mellitus: study of esophagogastric function. Acta Diabetol Lat. 1988;25:333–341. doi: 10.1007/BF02581132. [DOI] [PubMed] [Google Scholar]
- 21.Sanders KM, Koh SD, Ward SM. Interstitial cells of Cajal as pacemakers in the gastrointestinal tract. Annu Rev Physiol. 2006;68:307–343. doi: 10.1146/annurev.physiol.68.040504.094718. [DOI] [PubMed] [Google Scholar]
- 22.Kawano K, Hirashima T, Mori S, Saitoh Y, Kurosumi M, Natori T. Spontaneous long-term hyperglycemic rat with diabetic complications. Otsuka Long-Evans Tokushima Fatty (OLETF) strain. Diabetes. 1992;41:1422–1428. doi: 10.2337/diab.41.11.1422. [DOI] [PubMed] [Google Scholar]
- 23.Eglen RM, Peelle B, Pulido-Rios MT, Leung E. Functional interactions between muscarinic M2 receptors and 5-hydroxytryptamine (5-HT)4 receptors and beta 3-adrenoceptors in isolated oesophageal muscularis mucosae of the rat. Br J Pharmacol. 1996;119:595–601. doi: 10.1111/j.1476-5381.1996.tb15714.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ehlert FJ, Ostrom RS, Sawyer GW. Subtypes of the muscarinic receptor in smooth muscle. Life Sci. 1997;61:1729–1740. doi: 10.1016/s0024-3205(97)00433-5. [DOI] [PubMed] [Google Scholar]
- 25.Takahashi T, Owyang C. Regional differences in the nitrergic innervation between the proximal and the distal colon in rats. Gastroenterology. 1998;115:1504–1512. doi: 10.1016/s0016-5085(98)70029-0. [DOI] [PubMed] [Google Scholar]
- 26.Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277(2 Pt 1):G275–G279. doi: 10.1152/ajpgi.1999.277.2.G275. [DOI] [PubMed] [Google Scholar]
- 27.Ordög T. Interstitial cells of Cajal in diabetic gastroenteropathy. Neurogastroenterol Motil. 2008;20:8–18. doi: 10.1111/j.1365-2982.2007.01056.x. [DOI] [PubMed] [Google Scholar]
- 28.Huizinga JD. Gastrointestinal peristalsis: joint action of enteric nerves, smooth muscle, and interstitial cells of Cajal. Microsc Res Tech. 1999;47:239–247. doi: 10.1002/(SICI)1097-0029(19991115)47:4<239::AID-JEMT3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 29.Thuneberg L. Interstitial cells of Cajal: intestinal pacemaker cells? Adv Anat Embryol Cell Biol. 1982;71:1–130. [PubMed] [Google Scholar]
- 30.Long QL, Fang DC, Shi HT, Luo YH. Gastro-electric dysrhythm and lack of gastric interstitial cells of Cajal. World J Gastroenterol. 2004;10:1227–1230. doi: 10.3748/wjg.v10.i8.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He CL, Soffer EE, Ferris CD, Walsh RM, Szurszewski JH, Farrugia G. Loss of interstitial cells of Cajal and inhibitory innervation in insulin-dependent diabetes. Gastroenterology. 2001;121:427–434. doi: 10.1053/gast.2001.26264. [DOI] [PubMed] [Google Scholar]
- 32.Nakahara M, Isozaki K, Hirota S, et al. Deficiency of KIT-positive cells in the colon of patients with diabetes mellitus. J Gastroenterol Hepatol. 2002;17:666–670. doi: 10.1046/j.1440-1746.2002.02756.x. [DOI] [PubMed] [Google Scholar]
- 33.Epperson A, Hatton WJ, Callaghan B, et al. Molecular markers expressed in cultured and freshly isolated interstitial cells of Cajal. Am J Physiol Cell Physiol. 2000;279:C529–C539. doi: 10.1152/ajpcell.2000.279.2.C529. [DOI] [PubMed] [Google Scholar]
- 34.Horiguchi K, Sanders KM, Ward SM. Enteric motor neurons form synaptic-like junctions with interstitial cells of Cajal in the canine gastric antrum. Cell Tissue Res. 2003;311:299–313. doi: 10.1007/s00441-002-0657-1. [DOI] [PubMed] [Google Scholar]
- 35.Iino S, Ward SM, Sanders KM. Interstitial cells of Cajal are functionally innervated by excitatory motor neurones in the murine intestine. J Physiol. 2004;556(Pt 2):521–530. doi: 10.1113/jphysiol.2003.058792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith VC, Sagot MA, Wong H, Buchan AM. Cellular expression of the neurokinin 1 receptor in the human antrum. J Auton Nerv Syst. 2000;79:165–172. doi: 10.1016/s0165-1838(99)00092-2. [DOI] [PubMed] [Google Scholar]
- 37.Ward SM, Beckett EA, Wang X, et al. Interstitial cells of Cajal mediate cholinergic neurotransmission from enteric motor neurons. J Neurosci. 2000;20:1393–1403. doi: 10.1523/JNEUROSCI.20-04-01393.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward SM, Morris G, Reese L, Wang XY, Sanders KM. Interstitial cells of Cajal mediate enteric inhibitory neurotransmission in the lower esophageal and pyloric sphincters. Gastroenterology. 1998;115:314–329. doi: 10.1016/s0016-5085(98)70198-2. [DOI] [PubMed] [Google Scholar]
- 39.Unno T, Matsuyama H, Izumi Y, Yamada M, Wess J, Komori S. Roles of M2 and M3 muscarinic receptors in cholinergic nerve-induced contractions in mouse ileum studied with receptor knockout mice. Br J Pharmacol. 2006;149:1022–1030. doi: 10.1038/sj.bjp.0706955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unno T, Matsuyama H, Sakamoto T, et al. M(2) and M(3) muscarinic receptor-mediated contractions in longitudinal smooth muscle of the ileum studied with receptor knockout mice. Br J Pharmacol. 2005;146:98–108. doi: 10.1038/sj.bjp.0706300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cellek S, Foxwell NA, Moncada S. Two phases of nitrergic neuropathy in streptozotocin-induced diabetic rats. Diabetes. 2003;52:2353–2362. doi: 10.2337/diabetes.52.9.2353. [DOI] [PubMed] [Google Scholar]
- 42.Takahashi T, Nakamura K, Itoh H, Sima AA, Owyang C. Impaired expression of nitric oxide synthase in the gastric myenteric plexus of spontaneously diabetic rats. Gastroenterology. 1997;113:1535–1544. doi: 10.1053/gast.1997.v113.pm9352855. [DOI] [PubMed] [Google Scholar]
- 43.Watkins CC, Sawa A, Jaffrey S, et al. Insulin restores neuronal nitric oxide synthase expression and function that is lost in diabetic gastropathy. J Clin Invest. 2000;106:373–384. doi: 10.1172/JCI8273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yu WJ, Juang SW, Chin WT, Chi TC, Chang CJ, Cheng JT. Insulin restores neuronal nitric oxide synthase expression in streptozotocin-induced diabetic rats. Life Sci. 2000;68:625–634. doi: 10.1016/s0024-3205(00)00967-x. [DOI] [PubMed] [Google Scholar]