Abstract
Purpose
To conduct a systematic literature review of the epidemiological and economic burden of surgical site infection (SSI) in Korea.
Methods
A search of the EMBASE, Medline and KoreaMed databases for English and Korean language publications was conducted. Searches for epidemiological and economic studies were conducted separately and limited to 1995 to 2010 to ensure the pertinence of the data.
Results
Twenty-six studies were included. The overall incidence of SSI in Korea was 2.0 to 9.7%. The National Nosocomial Infections Surveillance risk index was positively correlated with the risk of developing an SSI. Specific risk factors for SSI, identified through multivariate analyses included; diabetes, antibiotic prophylaxis and wound classification. SSIs were associated with increased hospitalisation cost, with each episode of SSI estimated to cost about an additional ₩2,000,000. A substantial portion of the increased cost was attributed to hospital room costs and the need for additional medication. Studies also found that post-operative stays for patients with SSIs were 5 to 20 days longer, while two studies reported that following cardiac surgery, patients with SSIs spent an additional 5 to 11 days in the intensive care unit, compared to patients without SSIs.
Conclusion
Data from the included studies demonstrate that SSI represents a significant clinical and economic burden in Korea. Consequently, the identification of high-risk patient populations and the development of strategies aimed at reducing SSI may lead to cost-savings for the healthcare system.
Keywords: Surgical site infection, Epidemiology, Cost
INTRODUCTION
A surgical site infection (SSI) is a type of hospital-acquired infection that arises following surgery and is specifically related to the surgical site. Patients who develop an SSI are more likely to have an extended hospital stay, which results in additional healthcare costs. Indirect costs, such as productivity, further add to the burden of SSI.
The aim of this review is to summarise recent evidence pertaining to the clinical and economic burden of SSI in Korea. This systematic review will follow the general format of the publication by Leaper et al. [1] which describes the epidemiological and economic burden of SSI in Europe.
METHODS
Literature search
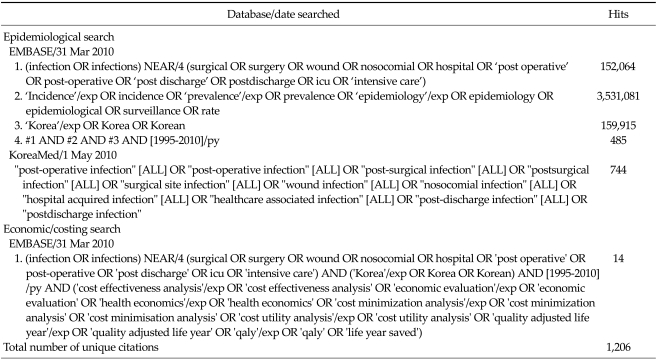
In order to identify relevant epidemiological and economic data for this review, a systematic search of the literature was undertaken. A search of Embase (which includes the EMBASE and Medline databases) and a Korean medical journal database (KoreaMed) was conducted. Separate searches were conducted to identify epidemiological and economic data. The search was limited to the last 15 years (1995 to 2010) to ensure the relevance of these data. The search strategy and results are presented in Table 1.
Table 1.
Search strategy
Identification of studies
There were 1,206 unique citations identified from the literature search. The titles/abstracts of all citations were reviewed to identify publications most relevant to this systematic review. The following exclusion criteria were applied to determine eligibility:
Does not describe the rate, incidence, prevalence, bur den or cost of SSI.
Describes the effect of an intervention to reduce SSI.
Not conducted in Korea.
Not conducted in a hospital setting.
Includes <90 patients/procedures.
Following application of the exclusion criteria to the titles/abstracts, 32 publications were retrieved for full text review. Following detailed assessment of these publications, a further six were excluded leaving 26 studies.
Data extraction and analysis
SSI data from the 26 included studies were compiled into data extraction tables. The overall incidence of SSI was recorded, as well as the incidence of SSI by surgical procedure, wound classification and National Nosocomial Infections Surveillance (NNIS) risk score. Wound classifications were based on definitions by the Centre for Disease Control (CDC). SSIs were classified according to the location in which they occurred: 1) superficial (i.e., skin and subcutaneous tissue); 2) deep (i.e., fascia, muscle); and 3) organ / space. The NNIS categorizes patients according to their likelihood of developing an SSI. The NNIS system risk index comprises three components: 1) the American Society of Anaesthesiologists (ASA) score; 2) wound classification; and 3) the duration of surgery. Based on these factors, patients are assigned an NNIS risk score of 0, 1, 2 or 3. The NNIS risk index is scored as follows: 1) an ASA score of 3 to 5 is allocated 1 point; 2) wound sites classified as contaminated or dirty are allocated 1 point; and 3) surgeries exceeding specified time cut-off points are allocated 1 point. Risk factors for SSIs were also recorded, along with common pathogens associated with SSI. To evaluate the economic impact of SSI, SSI costs and extended hospital stay associated with SSI were summarised.
RESULTS
Characteristics of the included studies
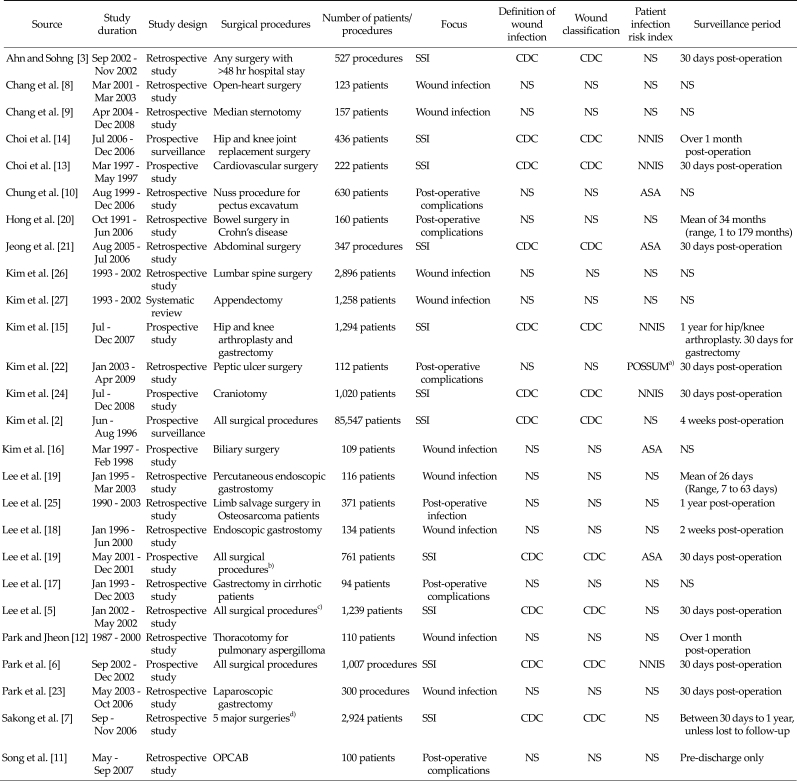
A summary of the characteristics of included studies is presented in Table 2. The majority of the studies were retrospective cohort studies investigating SSI or wound infection following a range of hospital surgical procedures. There was significant variation in the size of the populations investigated, with the number of patients included ranging from 94 to 85,547. There were also differences in the surveillance period, which is likely to influence the opportunity to detect a SSI. SSIs were most commonly defined and classified using the CDC criteria.
Table 2.
Summary of included studies
ASA, American Society of Anaesthesiology; CDC, Centre for Disease Control and Prevention; NNIS, National Nosocomial Infection Surveillance; NS, not stated; OPCAB, off-pump coronary artery bypass; SSI, surgical site infection.
a)POSSUM (physiological and operative severity score for enumeration of mortality and morbidity) score was developed to predict post-operative mortality and morbidity rates. b)Includes surgery of the colon, rectum, small bowel, hepato-biliary-pancreas, stomach and appendix. c)Includes orthopaedic surgery, plastic surgery, general surgery, neurosurgery, chest surgery, obstetrics and gynaecology, otolaryngology and ophthalmology. d)Includes cardiac, colon and gastric surgery, hysterectomy, hip/knee replacement surgery.
Prevalence of SSI in Korea
None of the studies included in this systematic review reported the prevalence of SSI in Korea. However, the multicentre study by Kim et al. [2], involving 15 hospitals, reported that the prevalence of nosocomial infection was 3.7% in 2000, with SSIs accounting for 17.2% of all nosocomial infections.
Incidence of SSI in Korea
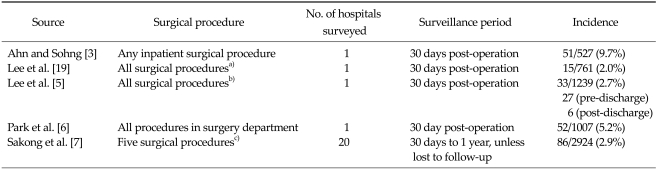
As shown in Table 3, five included studies reported the overall incidence rate of SSIs [3-7]. Each study followed up patients who had undergone a variety of different surgical procedures. Four of the studies examined the incidence of SSI at a single hospital [3-6] during a 30 day post-operative observation period, while one study examined the incidence of SSI across 20 hospitals during a one year post-operative follow-up period (Sakong et al. [7]). The incidence of SSI ranged from 2.0 to 9.7% across the five included studies.
Table 3.
Overall incidence of surgical site infection
a)Includes surgery of the colon, rectum, small bowel, hepato-biliary-pancreas, stomach and appendix. b)Includes orthopedic surgery, plastic surgery, general surgery, neurosurgery, chest surgery, obstetrics and gynecology, otolaryngology and ophthalmology. c)Includes cardiac, colon and gastric surgery, hysterectomy, hip/knee replacement surgery.
Incidence by surgical procedure
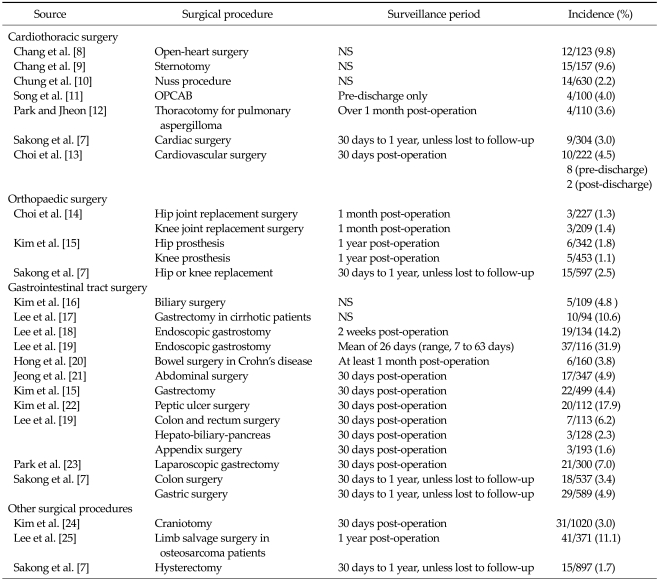
As shown in Table 4, the incidence of SSI varied by surgical procedure. To facilitate comparison, groups were divided into four broad categories, namely, cardiothoracic surgery, orthopaedic surgery, gastrointestinal surgery, and other surgical procedures.
Table 4.
Incidence of surgical site infection by surgical procedure
NS, not stated; OPCAB, off-pump coronary artery bypass.
There were seven studies that reported SSI following cardiothoracic surgery [8-13]. Surgical procedures investigated included open-heart surgery, sternotomy, nuss procedure, off-pump coronary artery bypass, thoracotomy for pulmonary aspergilloma and non-specific cardiac and cardiovascular surgery. The incidence of SSI ranged from 2.2 to 9.8%, with the highest incidence of SSI occurring in patients who had undergone open-heart surgery [8] and the lowest incidence in those undergoing the nuss procedure [10]. Choi et al. [13] reported an incidence of SSI of 4.5% following cardiovascular surgery, of which, 80% (8/10) occurred pre-discharge and 20% (2/10) post-discharge from hospital.
The three included orthopaedic studies examined knee or hip replacement surgery with patients monitored for up to one year post-operation (Table 4). Overall, the incidence of SSI was lower for patients undergoing orthopaedic surgery compared to those undergoing cardiothoracic surgery. The incidence of SSI ranged from 1.1 to 2.5%, with low variation between surgery types and duration of follow-up [7,14,15].
The majority of the included studies in this systematic review examined SSI following gastrointestinal tract surgery [4,7,15-23]. Overall, the incidence of SSIs was generally higher and the range of rates was larger for patients undergoing gastrointestinal tract surgery compared with other surgery types. The lowest rate of SSI was reported by Lee et al. [4] for patients undergoing appendix surgery (1.6% after 30 days follow-up). In contrast, Lee et al. [19] reported that 31.9% of patients undergoing endoscopic gastrostomy experienced a SSI up to 2 months after surgery. Although the range of SSI rates was larger than rates from other surgery types, the majority of studies still reported SSI rates less than 7.
Among the included studies investigating SSIs following other surgical procedures, Kim et al. [24], Lee et al. [25] and Sakong et al. [7] examined craniotomy, limb salvage surgery in osteosarcoma patients and hysterectomy, respectively. The incidence of SSI up to one year following surgery was 3.0%, 11.1% and 1.7%, respectively.
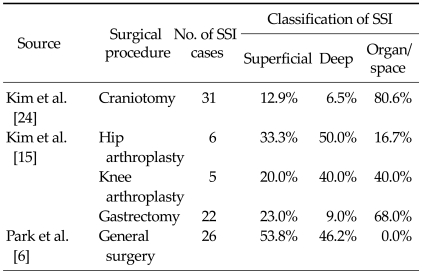
Classification of surgical site infection
As shown in Table 5, three studies reported information on the classification of SSI [6,15,24]. In patients undergoing general surgery, the majority of SSIs occurred in superficial tissue (53.8%) [6]. In contrast, organ/space SSIs were most frequent following craniotomy and gastrectomy [15,24]. The incidence of superficial, deep and organ/space SSI appeared to be similar among patients undergoing hip and knee replacement surgery. However, due to the small number of SSI cases observed for these surgeries, N = 6 and 5, respectively, the results should be interpreted with caution.
Table 5.
Classification of surgical site infections
SSI, surgical site infection.
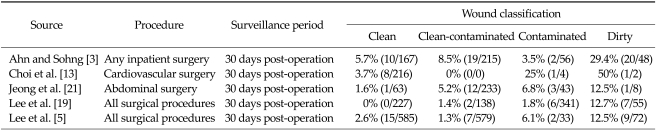
Incidence by wound classification
The incidence of SSI by wound classification is shown in Table 6. A study by Ahn and Sohng [3] examined various inpatient surgeries and observed that 5.7% of patients with a clean wound had an SSI within 30 days post-operation. In comparison, the incidence of SSI was 29.4% among patients with wounds classified as dirty. Similar trends were observed in the other included studies, where the incidence of SSIs increased as wound conditions worsened.
Table 6.
Incidence of surgical site infection by wound classification
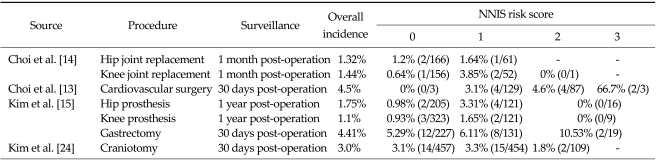
Incidence by NNIS risk score
Four studies included in this review compared the incidence of SSI between NNIS risk categories (Table 7). Overall, the results showed that the higher the NNIS risk score, the greater the risk of SSI. In a study by Choi et al. [14], the incidence of SSI in those with a NNIS risk score of 0 and 1 following hip replacement was 1.2% and 1.6%, respectively. In the same study, the incidence of SSI for NNIS risk score 0 and 1 following knee replacement was 0.6% and 3.9%, respectively. A similar trend was observed in the studies by Kim et al. [15] and Kim et al. [24] that examined SSI following a variety of surgery types.
Table 7.
Incidence of surgical site infections by NNIS risk score
NNIS, National Nosocomial Infections Surveillance; NS, not stated.
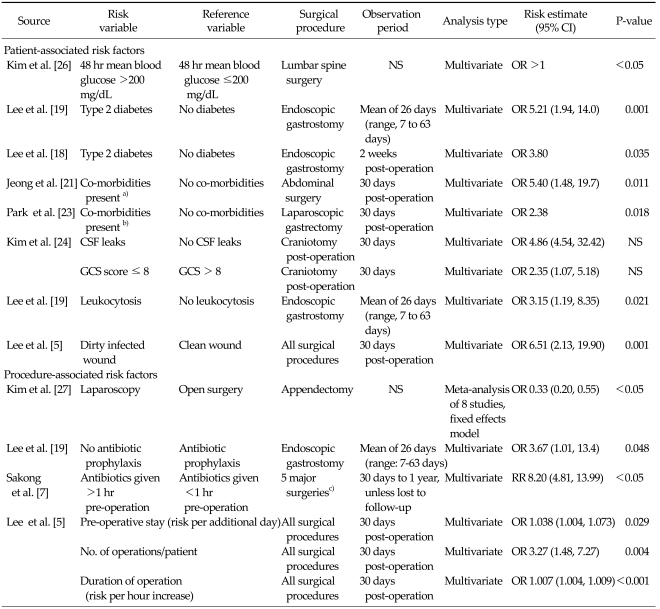
Risk factors associated with SSI in Korea
The risk factors for SSI are shown in Table 8. Nine studies included in this systematic review examined the association of specific risk factors with the incidence of SSI. Only factors which showed significant association with SSI through multivariate analysis were included, as univariate analysis does not take into account the possible confounding effects of other variables.
Table 8.
Risk factors for surgical site infections
CI, confidence interval; CSF, cerebrospinal fluid; GCS, Glasgow Coma Scale; NS, not stated; OR, odds ratio.
a)Includes hypertension, cancer and diabetes. b)Includes diabetes, hypertension, heart/liver/renal disease. c)Includes cardiac, colon and gastric surgery, hysterectomy, hip/knee replacement surgery.
Patient-associated factors
Diabetes was identified as a patient-associated risk factor in three studies. Kim et al. [26] reported that following lumbar spine surgery, patients with a mean blood glucose level greater than 200 mg/dL at 48 hours post-surgery had a significantly higher incidence of SSI (P < 0.05, effect estimate not reported). Lee et al. [19] and Lee et al. [18] examined patients undergoing endoscopic gastrostomy. Type 2 diabetes was significantly associated with an increased risk of SSI in both studies (odds ratio [OR], 5.21; P = 0.001 and OR, 6.51; P = 0.001, respectively). Similarly, Jeong et al. [21] and Park et al. [23] both reported that co-morbidities (e.g., diabetes, hypertension, cancer) significantly increased the risk of SSI in patients undergoing abdominal surgery (OR, 5.4; P = 0.011) and laparoscopic gastrectomy (OR, 2.38; P = 0.018).
Lee et al. [5] showed that surgical patients with dirty infected wounds were at increased risk of developing SSIs, compared to surgical patients with clean wounds (OR, 6.51; P = 0.001). The incidence of SSI was associated with cerebrospinal fluid leaks (OR, 4.86; P < 0.05) and a Glasgow Coma score of >8 (OR, 6.51; P < 0.05) in patients undergoing craniotomy [24], while leukocytosis increased the risk of SSIs among patients undergoing endoscopic gastrostomy (OR, 3.15; P = 0.021), Lee et al. [19].
Procedure-associated factors
Kim et al. [27] conducted a meta-analysis of eight studies and found that the use of laparoscopy instead of open surgery for appendectomies reduced the risk of SSIs (OR, 0.33; 95% confidence interval, 0.20 to 0.55). Lee et al. [19] and Sakong et al. [7] showed that the absence of antibiotic prophylaxis and administration of antibiotics >1 hour before surgery significantly increased the risk of SSI (OR, 3.67; P = 0.048 and OR, 8.2; P < 0.05, respectively). A study by Lee et al. [5] identified several other procedure-associated risk factors that increased the risk of SSI. These included length of pre-operative stay (risk per additional day: OR, 1.038; P = 0.029), number of operations performed on the patient (risk per additional operation: OR, 3.27; P = 0.004) and duration of operation (risk per hour increase: OR, 1.007; P < 0.001).
SSI associated mortality
Only one study identified in this review examined the incidence of SSI-associated mortality. A study by Lee et al. [25] found no significant differences in the 5-year survival between patients with deep wound infections compared to patients with no infection following limb salvage surgery for osteosarcoma (88.9% vs. 82%, P = 0.49).
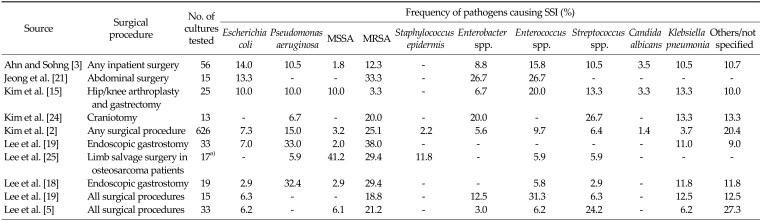
Pathogens associated with SSI in Korea
Ten of the included studies, comprising a range of surgical procedures, reported information on the pathogens present at the SSI (Table 9). The pathogens most commonly identified were Staphylococcus aureus (MRSA and MSSA), Enterobacter spp., Enterococcus spp. and Klebsiella pneumonia. The relative proportions of methicillin-resistant and methicillin-sensitive S. aureus varied between studies, but MRSA tended to be more common.
Table 9.
Common pathogens associated with surgical site infection
SSI, surgical site infection; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-susceptible Staphylococcus aureus; NS, not stated.
a)Cultures from deep infections only.
Economic burden data
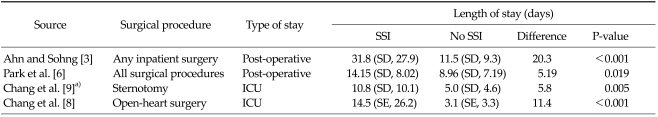
Extended hospital stay
A substantial portion of the economic cost of SSI is attributable to increased length of hospital stay. As shown in Table 10, this review identified four studies that examined the association between hospital stay and SSI. Ahn and Shong [3] reported that following inpatient surgery, patients with SSIs experienced a significantly longer stay in hospital (31.8 days vs. 11.5 days, P < 0.001). Similarly, Park et al. [6] reported significantly longer post-operative stays in patients with SSIs (14.15 days vs. 8.96 days, P = 0.019). Chang et al. [8,9] reported that intensive care unit stay was longer in patients with SSI following sternotomy and open heart surgery, respectively.
Table 10.
Extended hospital stay associated with surgical site infection
ICU, intensive care unit; SD, standard deviation; SE, standard error; SSI, surgical site infection.
a)Comparing patients with mediastinitis to those with no infection.
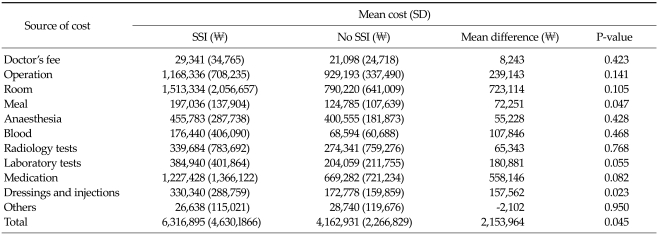
Cost of surgical site infections
Hospitalisation cost associated with SSI for patients undergoing general surgery at Severance Hospital in Seoul was reported in a publication by Park et al. [6] (Table 11). The cost of hospitalisation was, on average, ₩2,153,964 higher in patients with SSI, compared to patients with no SSI (P = 0.045). A substantial portion of the increased cost was due to hospital room costs and the need for additional medication. The authors noted that the costs are likely an underestimation of the actual economic cost of SSI, as only hospital expenditure data were considered. In a study by Ahn and Shong [3], the cost of antibiotics in patients undergoing inpatient surgical procedures was ₩735,155 (SD, 526,336) among those who developed an SSI, compared to ₩174,087 (SD, 171,326) for patients without SSI. The increased cost of antibiotics among patients with SSI was found to be statistically significant (₩561,068 P < 0.001).
Table 11.
Hospitalisation cost associated with surgical site infection in Korea [6]
SD, standard deviation; SSI, surgical site infection.
DISCUSSION
The overall incidence of SSI in Korea ranged between 2.0 to 9.7%. The wide range may be due to differences in the types of surgical procedures examined, or the levels of risk factors in the patients included in the studies. In particular, surgery involving the gastrointestinal system was generally associated with higher rates of SSI. Patient-associated risk factors such as diabetes, wound conditions and patient health were associated with a significantly greater risk of SSI. Similarly, procedure-associated factors such as antibiotic treatment and surgery duration were also found to influence the risk of SSI.
There are a number of limitations with this review. The inclusion of studies was assessed based on the information presented in the title and abstract. As such, studies that reported the rates of SSI as a secondary outcome may not have been identified. However, expanding the search to include all publications assessing post-surgical outcomes in patients would have substantially increased the total number of publications identified in the literature search. This would have likely resulted in the identification of additional publications, generally related to case series of specific surgical techniques. Case series often have small sample sizes and would have only served to provide incidence rates of SSI over a broader range of surgeries than those reported here.
Differences in study designs made it difficult to combine the data and obtain summary estimates of the incidence of SSI. These include differences in follow-up duration, method of data collection, and consideration for the use of antibiotics in the estimates. Most of the studies identified in this systematic review assessed SSI during the hospitalisation (pre-discharge) period, as well as during the post-discharge period, typically for 30 days, according to the CDC definition for post-operative infection. However, some studies only assessed SSI prior to discharge [11], while others conducted follow-up over one year [25]. With a substantial portion of SSI often occurring after discharge from hospital, studies that do not conduct post-discharge surveillance are likely to underestimate the incidence of SSI. As shown in the studies by Lee et al. [5] and Choi et al. [13], approximately 20% of SSIs occurred post-discharge. The manner that data is collected (e.g., method of post-discharge surveillance) influences the accuracy of the estimates, which in turn compromises inter-study comparisons. For example, in the studies by Kim et al. [24] and Kim et al. [15], patients who did not return for outpatient checks after discharge were contacted by telephone by infection control nurses. In comparison, Jeong et al. [21] derived post-discharge information solely from the patients' medical records. The use of antibiotic prophylaxis has been shown to be significantly associated with risk of SSI [7]. Consequently, studies that do not consider or account for the use of antibiotic prophylaxis may result in biased estimates, making their comparison between studies inappropriate.
SSIs represent a substantial economic burden, mainly attributable to the extended length of stay in hospital. In Korea, the incremental cost of an SSI is estimated at ₩2,153,964 (approximately US$2,025) [6]. In comparison, the cost per case of SSI in Japan has been estimated at approximately US$1,600 in patients undergoing colorectal surgery [28], while in Australia, the cost per case of SSI is estimated at US$2,200 [29]. In addition, SSIs result in the loss of productivity in patients and carers. As reported by two studies in this review [5,13], a significant proportion (-20%) of SSIs was identified after discharge from hospital. Consequently, SSIs have the potential to further increase the burden on community healthcare services and the families of patients.
This review has shown that SSIs represent a significant clinical and economic burden in Korea. In particular, certain patient populations appear to be at increased risk of developing SSI, such as patients undergoing gastric surgery or patients with dirty/contaminated wounds. Consequently, strategies and interventions during surgery that reduce the incidence of SSIs would likely translate. For example, recent clinical trials [30-32] have shown that the use of anti-bacterial coated sutures may reduce the incidence of SSIs by up to 40%. The development of such interventions would reduce patient morbidity associated with the development of SSIs, in turn, this could potentially translate into cost-savings for the healthcare system.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Leaper DJ, van Goor H, Reilly J, Petrosillo N, Geiss HK, Torres AJ, et al. Surgical site infection - a European perspective of incidence and economic burden. Int Wound J. 2004;1:247–273. doi: 10.1111/j.1742-4801.2004.00067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim JM, Park ES, Jeong JS, Kim KM, Kim JM, Oh HS, et al. Nosocomial Infection Surveillance Committee of the Korean Society for Nosocomial Infection Control. Multicenter surveillance study for nosocomial infections in major hospitals in Korea. Am J Infect Control. 2000;28:454–458. doi: 10.1067/mic.2000.107592. [DOI] [PubMed] [Google Scholar]
- 3.Ahn YJ, Sohng KY. Factors related to surgical site infections in patients undergoing general surgery. J Korean Acad Fundam Nurs. 2005;12:113–120. [Google Scholar]
- 4.Lee JH, Han HS, Min SK, Lee HK, Lee JH, Kim YW, et al. Surveillance of surgical wound infections among patients from the department of surgery: prospective trial. J Korean Surg Soc. 2004;66:133–137. [Google Scholar]
- 5.Lee S, Kim S, Lee J, Lee K. Risk factors for surgical site infection among patients in a general hospital. Korean J Nosocomial Infect Control. 2007;12:9–20. [Google Scholar]
- 6.Park ES, Kim KS, Lee WJ, Jang SY, Choi JY, Kim JM. The economical impacts of surgical site infections. Korean J Nosocomial Infect Control. 2005;10:57–64. [Google Scholar]
- 7.Sakong P, Lee JS, Lee EJ, Ko KP, Kim CH, Kim Y, et al. Association between the pattern of prophylactic antibiotic use and surgical site infection rate for major surgeries in Korea. J Prev Med Public Health. 2009;42:12–20. doi: 10.3961/jpmph.2009.42.1.12. [DOI] [PubMed] [Google Scholar]
- 8.Chang W, Park HG, Kim H, Youm W. Analysis of risk factors in poststernotomy sternal wound infection and mediastinitis after open-heart surgery. Korean J Thorac Cardiovasc Surg. 2003;36:583–589. [Google Scholar]
- 9.Chang WH, Dong WY, Kim H, Oh HC, Han JW, Kim HJ. An influence of modified robicsek sternal wiring after median sternotomy on the postoperative sternal wound infection. Korean J Thorac Cardiovasc Surg. 2009;42:763–769. [Google Scholar]
- 10.Chung JH, Ahn KR, Kim MN, Kim CS, Kang KS, Yoo SH, et al. Intraoperative and postoperative complications in the patients undergoing the pectus excavatum repair by the Nuss procedure: a retrospective study. Korean J Anesthesiol. 2008;54:646–650. [Google Scholar]
- 11.Song SW, Yi G, Lee S, Youn YN, Sul SY, Yoo KJ. Perioperative indicators of stress response and postoperative inflammatory complications in patients undergoing off-pump coronary artery bypass surgery: a prospective observational study. Circ J. 2008;72:1966–1974. doi: 10.1253/circj.cj-08-0291. [DOI] [PubMed] [Google Scholar]
- 12.Park CK, Jheon S. Results of surgical treatment for pulmonary aspergilloma. Eur J Cardiothorac Surg. 2002;21:918–923. doi: 10.1016/s1010-7940(02)00104-5. [DOI] [PubMed] [Google Scholar]
- 13.Choi YH, Park ES, Chang KH, Yeom JS, Song YG, Chang B, et al. Surgical site infection rates according to patient risk index after cardiovascular surgery. Korean J Nosocomial Infect Control. 1998;3:11–22. [Google Scholar]
- 14.Choi HJ, Park JY, Jung SY, Park YS, Cho YK, Park SY, et al. Multicenter surgical site infection surveillance study about prosthetic joint replacement surgery in 2006. Korean J Nosocomial Infect Control. 2008;13:42–50. [Google Scholar]
- 15.Kim ES, Chang YJ, Park YS, Kang JH, Park SY, Kim JY, et al. Multicenter surgical site infections surveillance system report, 2007: in total hip and total knee arthroplasties and gastrectomies. Korean J Nosocomial Infect Control. 2008;13:32–41. [Google Scholar]
- 16.Kim YW, Han HS, Lee RA, Choi YM, Kim OY. Risk factors of wound infection in biliary surgery: a prospective study. J Korean Surg Soc. 1999;57:94–99. [Google Scholar]
- 17.Lee JH, Kim J, Cheong JH, Hyung WJ, Choi SH, Noh SH. Gastric cancer surgery in cirrhotic patients: result of gastrectomy with D2 lymph node dissection. World J Gastroenterol. 2005;11:4623–4627. doi: 10.3748/wjg.v11.i30.4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JH, Kim JJ, Kim YH, Jang JK, Son HJ, Peck KR, et al. Increased risk of peristomal wound infection after percutaneous endoscopic gastrostomy in patients with diabetes mellitus. Dig Liver Dis. 2002;34:857–861. doi: 10.1016/s1590-8658(02)80256-0. [DOI] [PubMed] [Google Scholar]
- 19.Lee HH, Shim KN, Kwon JM, Jung SA, Yoo K. Complications of percutaneous endoscopic gastrostomy and risk factors of peristomal wound infection. Korean J Med. 2004;67:15–21. [Google Scholar]
- 20.Hong DH, Yu CS, Kim DD, Jung SH, Choi PH, Park IJ, et al. Postoperative complications and recurrence in patients with Crohn's disease. J Korean Soc Coloproctol. 2008;24:13–19. [Google Scholar]
- 21.Jeong YI, Mun SP, Chang JH, Kim KC, Min YD, Kim SH, et al. The risk factors associated with surgical site infection after an abdominal operation. J Korean Surg Soc. 2008;75:177–183. [Google Scholar]
- 22.Kim HB, Ahn HS, Kwon JS, Jung IM, Ahn YJ, Heo SC, et al. Validation of POSSUM-physiological score as predictors of post-operative morbidity and mortality after emergency operation for peptic ulcer complications. J Korean Surg Soc. 2009;77:391–398. [Google Scholar]
- 23.Park JM, Jin SH, Lee SR, Kim H, Jung IH, Cho YK, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc. 2008;22:2133–2139. doi: 10.1007/s00464-008-9962-4. [DOI] [PubMed] [Google Scholar]
- 24.Kim HY, Kim YK, Uh Y, Whang K, Jeong HR, Choi HJ, et al. Risk factors for neurosurgical site infections after craniotomy: a nationwide prospective multicenter study in 2008. Korean J Nosocomial Infect Control. 2009;14:88–97. [Google Scholar]
- 25.Lee JA, Kim MS, Kim DH, Lim JS, Park KD, Cho WH, et al. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol. 2009;16:147–151. doi: 10.1245/s10434-008-0184-8. [DOI] [PubMed] [Google Scholar]
- 26.Kim BO, Kim SW, Lee SM, Shin H. Relationship of glucose control and wound infection in diabetics after lumbar spine surgery. J Korean Neurosurg Soc. 2005;37:44–47. [Google Scholar]
- 27.Kim CB, Kim MS, Hong JH, Lee HY, Yu SH. Is laparoscopic appendectomy useful for the treatment of acute appendicitis in Korea? A meta-analysis. Yonsei Med J. 2004;45:7–16. doi: 10.3349/ymj.2004.45.1.7. [DOI] [PubMed] [Google Scholar]
- 28.Kashimura N. Surgical site infection (SSI) prevention and its medicoeconomic effect. J Jpn Soc Surg Infect. 2005;2:67–72. [Google Scholar]
- 29.Graves N, Halton K, Paterson D, Whitby M. Economic rationale for infection control in Australian hospitals. Healthc Infect. 2009;14:81–88. [Google Scholar]
- 30.Justinger C, Moussavian MR, Schlueter C, Kopp B, Kollmar O, Schilling MK. Antibacterial [corrected] coating of abdominal closure sutures and wound infection. Surgery. 2009;145:330–334. doi: 10.1016/j.surg.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 31.Rozzelle CJ, Leonardo J, Li V. Antimicrobial suture wound closure for cerebrospinal fluid shunt surgery: a prospective, double-blinded, randomized controlled trial. J Neurosurg Pediatr. 2008;2:111–117. doi: 10.3171/PED/2008/2/8/111. [DOI] [PubMed] [Google Scholar]
- 32.Fleck T, Moidl R, Blacky A, Fleck M, Wolner E, Grabenwoger M, et al. Triclosan-coated sutures for the reduction of sternal wound infections: economic considerations. Ann Thorac Surg. 2007;84:232–236. doi: 10.1016/j.athoracsur.2007.03.045. [DOI] [PubMed] [Google Scholar]