Abstract
Robotic surgical system provides many unique advantages which might compensate the limitations of usual laparoscopic surgery. By using robotic surgical system, we performed robot-assisted laparoscopic pancreaticoduodenectomy (PD). A Sixty-two year old female patient with an ampullary mass underwent robot assisted PD due to imcomplete treatment of endoscopic ampullectomy. The removal of specimen and reconstruction were performed through small upper midline skin incision. Robot working time was about 8 hours, and blood loss was about 800 ml without blood transfusion. She returned to an oral diet on postoperative day 3. Grade B pancreatic leak was noted during the postoperative period, but was successfully managed by conservative management alone. We successfully performed da Vinci-assisted laparoscopic PD, and robot surgical system provided three-dimensional stable visualization and wrist-like motion of instrument facilitated complex operative procedures. More experiences are necessary to address real role of robot in far advanced laparoscopic pancreatic surgery.
Keywords: Robotics, Pancreaticoduodenectomy
INTRODUCTION
In the past, pancreaticoduodenectomy (PD) was not preferred due to high mortality and morbidity rate after surgery. For example, Crile [1] even suggested advantages of bypass surgery over radical PD in treatment of pancreatic carcinoma in 1970. However, with the advances of experiences in pancreatic surgery, operative techniques, and perioperative managements, the morbidity and mortality rate after PD has been dramatically reduced in high volume centers [2]. Therefore, none deny the fact that PD can be treatment of choice for periampullary lesions requiring resection.
In addition, laparoscopic surgery is recently replacing many fields of conventional open surgery. Though Gagner and Pomp [3] have attempted first laparoscopic PD and early reports suggested the feasibility of laparoscopic PD, many surgeons still did not prefer applying laparoscopic approach as far as PD was concerned [4]. However, with more accumulating laparoscopic experiences and updated instruments, far advanced laparoscopic attempts have been safely performed and laparoscopic PD also apparently has begun to be reconsidered as safe and appropriate surgical modality for selected patients.
Along with recent advances of computer technology, master-slave type of surgical robot system has been introduced in laparoscopic era. It can provide 3-dimensional operative field and free movement of effector instrument with seven-degree of freedom ("endo-wrist"), which compensates the limitations of conventional laparoscopy and possibly results in precise and safe laparoscopic performance. Here, we present a case of robot (da Vinci)-assisted laparoscopic pancreaticoduodenectomy and a brief discussion on the feasibility and safety of robot-assisted surgery in the era of PD.
CASE REPORT
Patient
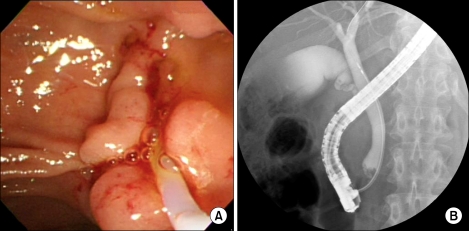
A 62- year-old female patient was admitted to the department of surgery in Yonsei University Health System, in Seoul, Korea because of the residual bile duct tumor after endoscopic ampullectomy for ampullary adenoma with high grade dysplasia. She had been in good health without any previous medical history. The ampullary mass was incidentally found during her routine medical check-up. Endoscopic ampullectomy was attempted in a local hospital, but the tumor was not completely removed. Pathologic examination reported it as tubulo-villous adenoma with high grade dysplasia. Endoscopic retrograde cholangiopancreatography was done after admission. It showed polypoid mass protruded out of the orifice of common bile duct (CBD) (Fig. 1A). On cholangiogram, filling defect was noted at distal CBD area (Fig. 1B). Abdominal computed tomography scan revealed suspicious mass of about 0.9 cm in size in the ampulla of vater portion without evidence of significant lymph node enlargement. Routine chemistry and tumor marker levels (CA 19-9 [carbohydrate antigen 19-9], CEA [carcinoembryonic antigen]) were all within normal limits. She seemed to required PD and minimal invasive technique was regarded as appropriate. Finally, we decided to perform da Vinci robot-assisted laparoscopic PD for her.
Fig. 1.
(A) Endoscopic retrograde cholangiopancreatography showed polypoid mass protruding out of the orifice of common bile duct (CBD). (B) On cholangiogram, filling defect was noted at distal CBD area.
Surgery
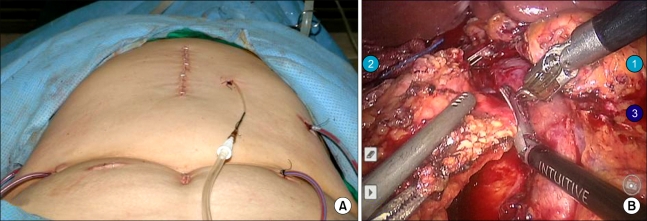
After the da Vinci system and the patient was prepared, a 12-mm subumbilical camera port was placed through a vertical mini-laparotomy and pneumoperitoneum was achieved by CO2 insufflation. Under the laparoscopy- providing vision, three additional 8-mm robotic instrument ports and two conventional laparoscopic ports were placed: port 1 on the right mid portion, port 2 on the mid portion of left side abdomen, port 3 on the left upper portion under the left subcostal area, and additional 5-mm and 12-mm accessory laparoscopic ports (ports 4 and 5) were placed for suction, surgical clipping and Endo-GIA by the table-side assisting surgeon (Fig. 2A). All three working robotic arms were used. The patient was placed in supine position with the head and the right side slightly elevated. The da Vinci surgical cart was rolled into the table over the patient. The robotic camera arm and other three instrument arms were then connected to their respective ports. At this point, the surgeon sat at the surgical console located about 3 m from the operating table. The assistant surgeon was positioned on the patient's right side for temporary use of laparoscopic instruments through ports 4 and 5. The robotic procedure was nearly the same as conventional PD. Preservation of pylorus and division of right gastric artery are our policy for PD. We began by dividing of the gastrocolic ligament. Duodenum was mobilized to the extent where the inferior vena cava and the left renal vein could be seen. Right gastroepiploric vessels were carefully dissected and individually ligated. After trimming the duodenal first portion, Endo-GIA was introduced through port 5 and divided duodenum at the point of about 2 cm distal to pylorus. Division of the right gastric artery facilitates the traction effect of the stomach to the left upper quadrant to ensure surgical field. Lower border of the pancreatic neck portion was carefully dissected to separate the portal vein and the pancreatic neck area. Good surgical field and endo -wrist could lead to safe and accurate dissection in this process. After making complete window between the pancreas and the portal vein, the pancreas was divided by Harmonic scalpel. Upper part of the pancreatic head was then sharply dissected to identify the common hepatic artery and the gastroduodenal artery. The gastroduodenal artery was safely ligated by intracorporeal tie, suture tie, and 5-mm endoclip. About 20 cm sized proximal jejunum from Treiz ligament was divided with another Endo-GIA, and mesenteric vessels were ligated by Harmonic scalpel. Then the proximal jejunum was placed to the right through retromesenteric root. With the duodenum retracted toward the patient's right side, uncinate portion of the pancreas beneath the portal vein was carefully divided by Harmonic scalpel. The robot system was thought to be benefitial in this part. Three-dimensional surgical field, stable portal vein retraction by another robotic arm without tremor helped the surgeon fully focus on sharp dissection of the uncinate process from retroperitoneal reflection (Fig. 2B). Several branches to the portal vein from the head of the pancreas were carefully divided and ligated by intracorporeal tie or endo-clip. After dividing the pancreatic uncinate process, the CBD was dissected from the portal vein and hepatic arteries upto the level of the common hepatic duct (CHD) just above the cystic duct. The cystic artery was then ligated and resected, and cholecystectomy was performed. 10-mm endo-clip was applied to the CHD. Lastly, CHD was divided. About 7-8 cm sized upper mid-line skin incision was made to remove the specimen and reconstruction. Roux-en-Y hepaticojejunostomy, pancreaticojejunostomy, and duodenojejunostomy could be perfomed through mini-laparotomy incision. Two closed suction drains were placed around the surgical field. Robot working time was about 8 hours, and blood loss was about 800 mL without blood transfusion.
Fig. 2.
(A) Port sites and postoperative wound, which was much smaller than that of conventional open pancreaticoduodenectomy. (B) Three-dimensional surgical field, stable portal vein retraction by another robotic arm without tremor helped the surgeon fully focus on sharp dissection of the uncinate process from retroperitoneal reflection.
Postoperative course
She started to an oral diet on postoperative day 3. Grade B pancreatic leak was noted during the postoperative period, but was successfully managed by conservative management alone. She was discharged 1 month after surgery. The body weight was not reduced during her hospital stay. The permanent pathologic report showed that the patient had a tubular adenoma with focal high grade epithelial dysplasia involving the duodenum. The cosmetic effect of postoperative wound was better than that of conventional open PD (Fig. 2A).
DISCUSSION
Several reports have recently suggested the safety and feasibility of laparoscopic PD [5]. We also believed this laparoscopic PD could be appropriate and ideal approach for a well selected patient group, such as benign and borderline malignant tumor of the pancreas based on our limited experiences (Total 5 cases of minimal invasive laparoscopic PD have been performed recently in our institution, not published). However, laparoscopic PD surely calls for far advanced laparoscopic techniques and experiences. In addition, several limitations of conventional laparoscopic surgery made worldwide laparoscopic surgeons reluctant to perform this complex procedure. It contains a loss of dexterity, haptic feedback, natural hand-eye coordination (fulcrum effect), and movement based on a 2-D video monitor, which are all somewhat counterintuitive. Physiologic tremors in the surgeon can be transmitted through the length of the rigid instrument. The poor ergonomic position for the surgeon is another issue for laparoscopic surgery. Therefore, most laparoscopic gastrointestinal operations are difficult to learn, master, and perform routinely and surgeons have to face a long period of learning curve [6].
Da Vinci surgical robot system was developed to overcome such issues of laparoscopic surgery. One of the most outstanding points of the da Vinci system is that the tips of the laparoscopic equipment have seven degrees of freedom of motion, which means that the exact same movements of a human hand can be produced by the system. Another is the 3-dimentional visualization of the operative field which is similar to real open surgery.
In our case, we believe these advantages of the robot system could be enhanced in managing major vessels, creating a window between the neck of the pancreas and the portal vein, and the dissection of the uncinate process. Gastroduodenal artery was able to be safely managed by additional intracorporeal tie and suture tie as it had been done in open surgery. Manual reconstruction after PD was perfomed through the small upper midline mini-laparotomy, which was much smaller than our conventional inverted L-skin incision and was used to retrieve the specimen instead of a new Pfannelstiel incision, in the current case to shorten overall operation time, but robotic intracorporeal anastomosis was also thought to be feasible. In fact, we already published a report on the feasibility of hepaticojejunostomy in treating choledochal cyst and pancreaticogastrostomy in central pancreatectomy [7]. Reconstruction phase in pancreaticoduodenectomy may be highlighted in pancreatic anastomosis, because important morbidity is closely related to this procedure. Therefore, by the time reconstruction phase starts, surgeons may get tired and lose the power of concentration after long time of operation, which may result in morbidity after surgery. We are planned to apply this robot system even during reconstruction phase in a near future if we could shorten the robot working time in the resection phase.
Some surgeons may feel they do not need a robot system at all during this whole procedure because a robot system must have several drawbacks such as cost benefit problem, a total loss of tactile sense, lack of prompt response to critical issues such as intraoperative bleeding. However, it is clear that the surgical robot system does have functions to compensate conventional laparoscopic surgery, even thought the high cost is yet the great obstacle. Advances in experience, technique and instruments of robot surgery will help conventional laparoscopic surgeons more greatly because it is believed to provide precise and accurate laparoscopic performances. However, the goal of laparoscopic and robot-assisted surgery is thought to be same (that is, safe and effective minimally invasive surgery). Surgeons and patients might take advantages of these two surgical options according to surgeons' experiences and disease entities. In addition, for more popular clinical application, more concrete clinical evidences are required and cost of robot surgical system need to be lower. We need to accumulate more experiences of a robot surgery to address those issues exactly.
In summary, we performed successful laparoscopic PD by using the da Vinci robot system. Three-dimensional visualization and wrist-like motion of instrument helped this complex procedure be safely performed. Reconstruction was manually performed via the mini-laparotomy site, but robot assisted anastomosis was thought to be also available. More experiences are needed to identify the real benefits of the robot system in this procedure.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Crile G., Jr The advantages of bypass operations over radical pancreatoduodenectomy in the treatment of pancreatic carcinoma. Surg Gynecol Obstet. 1970;130:1049–1053. [PubMed] [Google Scholar]
- 2.Cameron JL, Riall TS, Coleman J, Belcher KA. One thousand consecutive pancreaticoduodenectomies. Ann Surg. 2006;244:10–15. doi: 10.1097/01.sla.0000217673.04165.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc. 1994;8:408–410. doi: 10.1007/BF00642443. [DOI] [PubMed] [Google Scholar]
- 4.Park A, Schwartz R, Tandan V, Anvari M. Laparoscopic pancreatic surgery. Am J Surg. 1999;177:158–163. doi: 10.1016/s0002-9610(98)00325-0. [DOI] [PubMed] [Google Scholar]
- 5.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg. 2010;145:19–23. doi: 10.1001/archsurg.2009.243. [DOI] [PubMed] [Google Scholar]
- 6.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy -assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kang CM, Chi HS, Kim JY, Choi GH, Kim KS, Choi JS, et al. A case of robot-assisted excision of choledochal cyst, hepaticojejunostomy, and extracorporeal Roux-en-y anastomosis using the da Vinci surgical system. Surg Laparosc Endosc Percutan Tech. 2007;17:538–541. doi: 10.1097/SLE.0b013e318150e57a. [DOI] [PubMed] [Google Scholar]