Abstract
Inflammation contributes to metabolic and cardiovascular disease. Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disorder that predominantly affects young women. Cardiovascular disease is a major cause of mortality in patients with SLE. We recently reported that a model of SLE (female NZBWF1 mice) develops characteristics of the metabolic syndrome. In the present study, we tested the hypothesis that high dietary fat during SLE accelerates the development of cardiovascular risk factors such as central obesity and vascular dysfunction. Twenty four week old female SLE mice (NZBWF1) were fed either control diet (SLE, 10% kcal) or high fat diet (SLE+HF, 45% kcal) for a total of 14 weeks. Body weight was similar between SLE (42±1g, n=5) and SLE+HF (45±2g, n=6) and weight gain was not different in the SLE+HF mice (+18.0±3.0%) compared with controls (+15.8±3.6%) and food intake was not different (SLE, 2.2±0.3 vs. SLE+HF, 2.1±0.2 g/24 hours). Fifty seven percent of the SLE+HF mice exhibited signs of albuminuria (>100 mg/dL) compared with only 20% of the control SLE mice at the end of the experiment. Endothelial dependent relaxation in isolated carotid arteries was impaired in the SLE+HF group compared to SLE. Ovarian fat was increased in SLE+HF mice (6.6±0.5g) when compared to control SLE (5.4±0.1g, p<0.05) and liver weight was decreased in SLE+HF (1.6±0.1g) mice compared to control mice (1.9±0.1g, p<0.03). These data suggest that dietary fat accelerates renal injury and peripheral vascular dysfunction and promotes visceral obesity in a disease model with chronic inflammation.
Keywords: Lupus, Endothelial, Adipose
Introduction
Increasing evidence suggests that inflammation plays an important role in the progression of cardiovascular and metabolic disorders. Systemic lupus erythematosus (SLE) is a chronic autoimmune inflammatory disorder that predominantly occurs in young women. Affected individuals exhibit a bimodal pattern of mortality with deaths late in the course of SLE resulting from cardiovascular disease 1. This is somewhat unusual given that the prevalence of cardiovascular disease in young women is typically very low. In addition, women with SLE are at an increased risk for developing metabolic disturbances such as insulin resistance and changes in body composition 2,3 that may contribute to the greater cardiovascular risk.
It is widely accepted that caloric restriction can delay mortality in organisms ranging from single cell to complex mammalian systems. Not surprisingly, diet can also profoundly affect the progression of metabolic, renal and cardiovascular disease. SLE is not different in this regard as it is long established that caloric restriction delays the onset of renal disease and mortality in the NZBWF1 mouse model of the disease 4,5. Although caloric restriction in mouse models of SLE appears to have the greatest impact on mortality, there is also evidence that composition of the diet can affect disease progression. For example, studies report that increasing dietary fat and cholesterol accelerates renal disease in the NZBWF1 model 6,7. Given the increased cardiovascular risk observed in patients with SLE and the established effect of high fat diet on disease progression in mouse models of SLE, we tested the hypothesis that feeding female NZBWF1 SLE mice a high fat diet would accelerate weight gain and exacerbate the progression of peripheral vascular dysfunction, both risk factors for cardiovascular and metabolic disease.
Methods
Animals
Female NZBWF1 mice (Jackson Laboratories, Bar Harbor, ME), an established model of SLE, were placed on either high fat diet (SLE+HF, 45% kcal fat, n=7) or a control diet (SLE, 10% kcal fat, n=5) for a period of 14 weeks (Research Diets, New Brunswick NJ) with access to food and water ad libitum. The control diet consisted of 20% kcal from protein (casein, L-Cystine), 70% kcal from carbohydrate (cornstarch, maltodexrtin 10, sucrose), and 10% from fat (soybean oil, lard). The high fat diet consisted of 20% kcal from protein, 35% carbohydrate, and 45% from fat (from increased lard content). The control and high fat diets were isocaloric. All of the studies were performed with the approval of the University of Mississippi Medical Center Institutional Animal Care and Use Committee and in accordance with National Institutes of Health guidelines.
Body Weight, Food Intake and Albuminuria
Body weight was measured weekly and mice were placed in metabolic cages once per week for overnight collection of urine. Food intake was measured by weighing the food on three consecutive days in mice individually housed in shoebox cages. The bedding was sifted to include any particulate that may have fallen into the cage. We have previously published using this method 8. Urine was assessed using Albustix (Bayer Inc) and animals were considered to have albuminuria at ≥100 mg/dL (++).
Vascular Function
At the conclusion of the experiment, mice were euthanized and the carotid arteries were quickly removed in order to assess vascular relaxation in organ chamber baths as previously described 9. Concentration responses (10−8 to 10−4 M) to acetylcholine and sodium nitroprusside were performed in vessels pre-contracted with the thromboxane mimetic U46619 (0.4 μg/ml) in order to test endothelial dependent and endothelial independent relaxation, respectively. This concentration of U46619 produced similar levels of contraction (in grams of tension) between SLE (SLE 0.24±0.02 g) and SLE+HF (0.28±0.02 g, p=0.18). The spleen, heart, liver, and ovarian fat pads were dissected and weighed at the time of death.
Statistical Analysis
A student’s T test was used to assess statistical differences between SLE and SLE+HF fed mice. The means were considered statistically different at p<0.05. A two-factor ANOVA was used in order to determine whether the diet or concentration significantly altered vascular responses or body weight.
Results
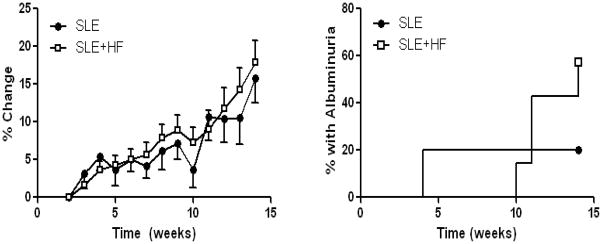
Body weight was measured weekly during the experiment. The mean body weight of each group did not differ at the start or completion of the experiment (Table), and high fat diet did not alter weight gain over this period (Figure 1). Importantly, the equivalent body mass between experimental groups was not likely due to a lower caloric intake in high fat fed mice given that food consumption (Table) and the total kcal for each diet was the same. One mouse from the SLE+HF group was sacrificed at week 12 due to precipitous weight loss that accompanies advanced disease in this model.
Table.
Physical Characteristics
| SLE | SLE+HF | |
|---|---|---|
| Start Weight (g) | 42±1 | 45±2 |
| End Weight (g) | 48±1 | 53±1 |
| Food Intake (g/day) | 2.1±0.3 | 2.1±0.2 |
| Ovarian Fat Weight (g) | 5.4 ±0.1 | 6.6±0.5* |
| Liver Weight (g) | 1.9±0.1 | 1.6±0.1* |
| Spleen Weight (mg) | 191±60 | 180±30 |
| Heart Weight (mg) | 151±10 | 148±10 |
p<0.05 vs SLE
Figure 1.
Left. The increase in body weight over time was not altered in SLE mice fed a high fat diet (SLE+HF) when compared to SLE mice fed a control diet (SLE). Right. The prevalence of albuminuria was increased in SLE mice fed high fat (SLE+HF) compared to SLE mice on a control diet (SLE). Over the course of the experiment 4 out of 7 animals from the SLE+HF group developed albuminuria compared with only 1 out of 5 SLE animals on the control diet.
Mice were housed overnight in metabolic cages each week in order to assess the development of albuminuria. Consistent with the work of others 6,7,10,11, a greater percentage of SLE mice fed a high fat diet exhibited albuminuria as assessed by a dipstick assay (Figure 1). Four out of 7 mice in the SLE+HF group developed albuminuria compared with only 1 out of 5 in the SLE control diet group.
Although body weight and caloric intake were not different between experimental groups, the SLE+HF mice had significantly more visceral fat as assessed by ovarian fat pad weight (Table). Liver weight was significantly reduced in the SLE+HF group compared to controls while spleen and heart weights were not affected by high fat feeding (Table).
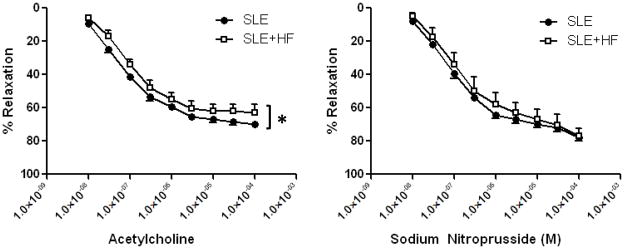
Vascular endothelial function was examined using isolated carotid arteries in organ chamber baths. Figure 2 shows the concentration response to the endothelial dependent agonist acetylcholine and endothelial independent agonist, sodium nitroprusside. Feeding SLE mice with a high fat diet led to a modest, but statistically significant, shift in the response to acetylcholine indicative of impaired endothelial function. The – Log EC50 tended to be lower in SLE+HF (7.22±0.08 M) when compared to the SLE group (7.05±0.09 M, p=0.09), consistent with a reduced responsiveness to ACh in carotid arteries from the SLE+HF group. The high fat diet did not alter the concentration response to sodium nitroprusside, suggesting that the vascular effects were restricted to the endothelium.
Figure 2.
Endothelial dependent relaxation was impaired in the carotid arteries from SLE+HF mice compared to SLE mice (* p<0.002 vs SLE). Carotid artery responses to sodium nitroprusside were not different between SLE+HF and SLE mice.
Discussion
In the present study we tested whether feeding a high fat diet to mice with SLE accelerates or promotes risk factors related to metabolic and cardiovascular disease, the major cause of mortality in women with SLE. The major findings of the study are that an established mouse model of SLE has increased visceral adiposity without increasing body weight after consuming a high fat diet and that vascular endothelial dysfunction is increased. Other noteworthy findings include a reduction in liver mass and an increased proportion of mice with albuminuria after the high fat feeding.
Animal Model of SLE
The female NZBWF1 mouse is a widely used and accepted experimental model of SLE 12,13. The female mice are disproportionately affected compared with males, they produce the anti double stranded DNA antibodies characteristic of SLE in humans, and they develop immune complex glomerulonephritis. We recently demonstrated that this model exhibits characteristics of the metabolic syndrome including visceral obesity and insulin resistance 8 as well as hypertension and impaired endothelial function 9. Therefore, this is an excellent disease model with which to further explore the link between chronic inflammatory disease, metabolic disease and cardiovascular risk factors in response to dietary changes.
Adiposity and Organ Weights
The predicted effect of high fat diet in animal models is to increase body weight and obesity. In the present study, SLE mice fed a high fat diet did not exhibit accelerated weight gain; however, the amount of ovarian fat as an indicator of visceral obesity was increased. The fact that the experimental groups exhibit similar changes in body weight coupled with increased ovarian fat in the SLE+HF mice suggests that a change in body composition occurred in the SLE+HF fed mice. The site of the change is not clear, although it is reasonable to speculate about a possible decrease in lean mass in SLE+HF mice.
Although it has been suggested that high fat diet in the NZBWF1 model causes an initial surge in weight gain 7, this was not evident in our study. In addition, the current work is in agreement with studies showing that the overall pattern of weight gain is not altered during high fat feeding in SLE mice 6,10. To our knowledge, increased central adiposity in response to a high fat diet has not been previously reported in this model. We and others reported the presence of fatty vacuolization of the liver from NZBWF1 mice 12,8. The reason for reduced liver weight in SLE mice fed with high fat is not clear, although it may be related to an increased liver apoptosis. Previous studies in the NZBWF1 model of SLE show that diets enriched with cholesterol induce apoptosis and reduce liver weight 14,15. The diet utilized in this study has 169 mg cholesterol compared with only 19 mg in the control diet. Interestingly, normal liver function is important for peripheral immune tolerance 16. Therefore, one could speculate that impaired liver function resulting from a high fat diet could promote or accelerate the development of SLE.
Renal Disease
The development of albuminuria in the NZBWF1 model of SLE and in women with SLE is widely established. Over the time course of this study, a greater percentage of SLE mice receiving high fat diet developed albuminuria when compared to SLE mice fed the control diet. This is not a new finding. For example, NZBWF1 mice fed a comparable diet (51.7% fat) from four months of age had accelerated development of albuminuria and nephritis 7. Even when the diet consisted of only 20% fat (from lard) the development of nephritis was accelerated in this model 6. This represents an important confirmation that our data and model is consistent with previous work in the field.
Vascular Function
Several studies show that patients with SLE have impaired flow dependent dilation in large vessels (brachial artery, forearm blood flow) 17,18,19. Endothelial dysfunction is a precursor to the development of vascular disease such as atherosclerosis which is prevalent in patients with SLE. While the NZBWF1 mice do not develop atherosclerotic lesions, we have previously shown that they have impaired endothelial dependent relaxation 9. The absence of atherosclerosis in this model is likely the result of species specific differences since mice in general are resistant to the development of atherosclerosis. Nevertheless, the NZBWF1 model has an atherosclerotic plasma lipid profile 20 and we previously reported that the NZBWF1 model has impaired carotid artery responses to acetylcholine, the classic endothelial dependent agonist. The latter was recently confirmed by Kaplan’s group 21. The carotid artery response to acetylcholine in SLE mice fed a control diet was the same as our previous study reaching a maximal relaxation of 61% 9. That the vascular response to sodium nitroprusside is not deteriorated after high fat feeding, further supports a prominent role for the endothelium. The possibility of a further increase in pressure in SLE+HF cannot be ruled out as a contributing factor to the exaggerated endothelial dysfunction. However, the data showing that heart weight is not different between the groups is consistent with no change in arterial pressure. Regardless of the mechanism, these data show that a high fat diet further impairs vessel function in a model of SLE and can contribute to increased risk for peripheral vascular disease.
Conclusions
SLE is a chronic inflammatory disorder that predominantly affects young women and carries an increased risk for cardiovascular, renal, and metabolic disease. Previous studies have reported that a diet high in fat can accelerate the progression of SLE; however, whether increased dietary fat accelerates the development of cardiovascular risk factors such as central obesity and vascular dysfunction is not clear. This study demonstrates an important role for dietary fat in the progression of vascular endothelial dysfunction and obesity and indicates that dietary fat is as an important risk factor for promoting cardiovascular disease during SLE.
Acknowledgments
None
Grant Support
This work was supported by the National Heart Lung and Blood Institute awards (HL085907, HL085907S3, and HL092284) and the American Heart Association (0630089N) to M.J.R. as well as P01HL5197.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Urowitz MB, Bookman AA, Koehler BE, Gordon DA, Smythe HA, Ogryzlo MA. The bimodal mortality pattern of systemic lupus erythematosus. Am J Med. 1976;60:221–225. doi: 10.1016/0002-9343(76)90431-9. [DOI] [PubMed] [Google Scholar]
- 2.Kipen Y, Strauss BJ, Morand EF. Body composition in systemic lupus erythematosus. Br J Rheumatol. 1998;37:514–519. doi: 10.1093/rheumatology/37.5.514. [DOI] [PubMed] [Google Scholar]
- 3.Tso TK, Huang HY, Chang CK, Liao YJ, Huang WN. Clinical evaluation of insulin resistance and beta-cell function by the homeostasis model assessment in patients with systemic lupus erythematosus. Clin Rheumatol. 2004;23:416–420. doi: 10.1007/s10067-004-0908-5. [DOI] [PubMed] [Google Scholar]
- 4.Fernandes G, Yunis EJ, Good RA. Influence of diet on survival of mice. Proc Natl Acad Sci U S A. 1976;73:1279–1283. doi: 10.1073/pnas.73.4.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friend PS, Fernandes G, Good RA, Michael AF, Yunis EJ. Dietary restrictions early and late: effects on the nephropathy of the NZB X NZW mouse. Lab Invest. 1978;38:629–632. [PubMed] [Google Scholar]
- 6.Alexander NJ, Smythe NL, Jokinen MP. The type of dietary fat affects the severity of autoimmune disease in NZB/NZW mice. Am J Pathol. 1987;127:106–121. [PMC free article] [PubMed] [Google Scholar]
- 7.Kelley VE, Izui S. Enriched lipid diet accelerates lupus nephritis in NZB x W mice. Synergistic action of immune complexes and lipid in glomerular injury. Am J Pathol. 1983;111:288–297. [PMC free article] [PubMed] [Google Scholar]
- 8.Ryan MJ, McLemore GR, Jr, Hendrix ST. Insulin Resistance and Obesity in a Mouse Model of Systemic Lupus Erythematosus. Hypertension. 2006;48:988–993. doi: 10.1161/01.HYP.0000243612.02929.df. [DOI] [PubMed] [Google Scholar]
- 9.Ryan MJ, McLemore GR., Jr Hypertension and Impaired Vascular Function in a Female Mouse Model of Systemic Lupus Erythematosus. Am J Physiol Regul Integr Comp Physiol. 2007;292:R736–R742. doi: 10.1152/ajpregu.00168.2006. [DOI] [PubMed] [Google Scholar]
- 10.Lin BF, Huang CH, Chiang BL, Jeng SJ. Dietary fat influences Ia antigen expression, cytokines and prostaglandin E2 production of immune cells in autoimmune-prone NZB x NZW F1 mice. Br J Nutr. 1996;75:711–722. doi: 10.1079/bjn19960175. [DOI] [PubMed] [Google Scholar]
- 11.Muthukumar A, Zaman K, Lawrence R, Barnes JL, Fernandes G. Food restriction and fish oil suppress atherogenic risk factors in lupus-prone (NZB x NZW) F1 mice. J Clin Immunol. 2003;23:23–33. doi: 10.1023/a:1021996130672. [DOI] [PubMed] [Google Scholar]
- 12.Burnet FM, Holmes MC. The natural history of the NZB/NZW F1 hybrid mouse: a laboratory model of systemic lupus erythematosus. Australas Ann Med. 1965;14:185–191. doi: 10.1111/imj.1965.14.3.185. [DOI] [PubMed] [Google Scholar]
- 13.HELYER BJ, HOWIE JB. Renal disease associated with positive lupus erythematosus tests in a cross-bred strain of mice. Nature. 1963;197:197. doi: 10.1038/197197a0. [DOI] [PubMed] [Google Scholar]
- 14.Hsu TC, Chen YC, Tsai CC, Wu JH, Li SL, Tzang BS. Protective effects of taurine against hepatic abnormality in NZB/W F1 mice fed a hypercholesterolemic diet. Food Chemistry. 2010;119:62–68. [Google Scholar]
- 15.Hsu TC, Chiang SY, Wu JH, Tsai CC, Huang CY, Chen YC, Tzang BS. Treatment with taurine attenuates hepatic apoptosis in NZB/W F1 mice fed with a high-cholesterol diet. J Agric Food Chem. 2008;56:9685–9691. doi: 10.1021/jf8020255. [DOI] [PubMed] [Google Scholar]
- 16.Knolle PA, Gerken G. Local control of the immune response in the liver. Immunol Rev. 2000;174:21–34. doi: 10.1034/j.1600-0528.2002.017408.x. [DOI] [PubMed] [Google Scholar]
- 17.Johnson SR, Harvey PJ, Floras JS, Iwanochko M, Ibanez D, Gladman DD, Urowitz M. Impaired brachial artery endothelium dependent flow mediated dilation in systemic lupus erythematosus: preliminary observations. Lupus. 2004;13:590–593. doi: 10.1191/0961203304lu1072oa. [DOI] [PubMed] [Google Scholar]
- 18.Lima DS, Sato EI, Lima VC, Miranda F, Jr, Hatta FH. Brachial endothelial function is impaired in patients with systemic lupus erythematosus. J Rheumatol. 2002;29:292–297. [PubMed] [Google Scholar]
- 19.Tam LS, Fan B, Li EK, Thomas GN, Yim SF, Haines CJ, Tomlinson B. Patients with systemic lupus erythematosus show increased platelet activation and endothelial dysfunction induced by acute hyperhomocysteinemia. J Rheumatol. 2003;30:1479–1484. [PubMed] [Google Scholar]
- 20.Jacobson MS, Trachtman H, Feldman J, Samuel P, Ilowite NT. Dyslipoproteinemia in murine systemic lupus erythematosus. Atherosclerosis. 1989;79:205–211. doi: 10.1016/0021-9150(89)90125-1. [DOI] [PubMed] [Google Scholar]
- 21.Thacker SG, Duquaine D, Park J, Kaplan MJ. Lupus-prone New Zealand Black/New Zealand White F1 mice display endothelial dysfunction and abnormal phenotype and function of endothelial progenitor cells. Lupus. 2010;19:288–299. doi: 10.1177/0961203309353773. [DOI] [PMC free article] [PubMed] [Google Scholar]