The report published in this month’s edition of Circulation by Dr. Chen and colleagues, Cardiomyocyte-Specific Deletion Of The Vitamin D Receptor Gene Results In Cardiac Hypertrophy, is important and timely (1.) This study extends and supports other studies that have demonstrated vitamin D and the vitamin D receptor (VDR) signaling system possess anti-hypertrophic activity in heart. The study also furthers our knowledge of this action by demonstrating an involvement of the pro-hypertrophic calcineurin/NFAT/MCIP 1 pathway. Thereby they identify a potential mechanism to account for the reported beneficial effects of vitamin D on the cardiovascular system. To respond to the question posed in the title of my editorial, ‘Is The Heart A Drugable Target For Vitamin D Receptor Agonists?’ the report published in this month’s Circulation makes an affirmative response more plausible.
The first studies to demonstrate a connection between cardiovascular homeostasis and vitamin D status utilized the established rat model of vitamin D deficiency (2–4). The notion of an involvement of the vitamin D endocrine system with cardiovascular function began more than 25 years ago by our identification of the VDR in rat cardiac myocytes and this led us to the physiological studies to establish relevance (5). These animal studies established a connection between vitamin D deficiency and cardiovascular dysfunctions including cardiac hypertrophy, fibrosis, hypertension as well as elevation of serum calcium, PTH and renin levels. These reports supported a role for vitamin D in maintaining cardiovascular homeostasis through both a direct action of 1,25-dihydroxyvitamin D on cardiomyocyte VDR and indirect actions on circulating hormones and calcium. Our studies in rats maintained on a vitamin D-deficient diet revealed that ventricular muscle mass and contractile function were both markedly enhanced. Increased systolic blood pressure (BP), renin levels, hydroxyproline and serum creatine phosphokinase were reported and coincided with a reduction in serum calcium (2–4). More recently, the VDR was subsequently identified in the human heart cells (6).
Other important preclinical studies demonstrating the involvement of the VDR in the cardiovascular system came with the creation of the global VDR knockout (VDRKO) mouse. As with the vitamin D deficient rat these VDR-KO mice were hypertensive and their heart weight/body weight ratios were significantly increased (7). In addition, renal renin mRNA levels of adult VDRKO mice were higher than that of wild-type mice. The size of left ventricular cardiomyocytes in VDRKO mice was markedly increased compared with WT mice. Also, levels of atrial natriuretic peptide (ANP) mRNA and circulating ANP and the cardiac renin mRNA level were significantly increased in the VDRKO mice. (8) These data strongly supported VDR involvement in regulation of cardiovascular functions, at least in part, through modulation of the local cardiac renin–angiotensin system (RAS) and expression of naturetic peptides. Similar observations were made in mice lacking CYP27B1, the key enzyme involved in the synthesis of 1,25-Dihydroxyvitmin D or calcitriol. (9) As a result of ablation of calcitriol synthesis, vehicle-treated CYP27B1 KO mice developed hypertension, cardiac hypertrophy and impaired cardiac function along with an up-regulation of the RAS in both renal and cardiac tissues. These abnormalities were normalized by calcitriol treatment. Rahman et al. (2007) confirmed the cardiac hypertrophic phenotype of the VDRKO mice and showed that tissue inhibitors of metalloproteinases such as TIMP-1 and TIMP-3 were significantly under-expressed, while metalloproteinases such as MMP-2 and MMP-9 were up regulated in VDR KO mice. (10)
Extracellular matrix remodeling mediated by matrix metalloproteinases is known to contribute to progressive left ventricular remodeling, dilation and heart failure. The data suggest that MMPs and TIMPs expression may be regulated by VDR and that modulation of heart extracellular matrix metabolism may be one of the mechanisms involved in vitamin D’s cardiovascular activities. (Reviewed in: 11) Fibroblasts are principally responsible for deposition of the excessive fibrotic ECM and activated fibroblasts may directly cause hypertrophy of cardiomyocytes via paracrine mechanisms further contributing to impaired cardiac function. (11) The fibrotic ECM causes increased stiffness and induces pathological signaling within cardiomyocytes resulting in progressive cardiac failure. Also, the excessive ECM impairs mechano-electric coupling of cardiomyocytes and increases the risk of arrhythmias (11). The data presented in the report by Chen and colleagues suggest that the mechanisms involved in cardiac ECM changes resulting from vitamin D signaling ablation are not solely a result of direct effects on cardiomyocyte VDR. From this report, as might be expected; fibrosis may involve other cells such as the cardiac fibroblast.
Preclinical intervention studies of cardiovascular dyshomeostatsis in rodent models have also been reported. Paricalcitol, an analog of calcitriol, was tested in the Dahl-salt sensitive (DSS) rat model of hypertension and heart failure (12). The DSS rat is an established animal model in which high-salt diet induces hypertension, cardiac hypertrophy and heart failure. DSS rats became vitamin D-deficient during the development of cardiac dysfunction. Paricalcitol therapy prevented the appearance of both pathological and echocardiographic evidence of cardiac hypertrophy and cardiac dysfunction. In addition, serum brain natriuretic peptide and cardiac ANF mRNA expression levels were normalized after paricalcitol treatment. One relevent observation made in this study was that the effect of paricalcitol in the DSS rat was independent of BP control. Recently, calcitriol treatment was studied in spontaneously hypertensive heart failure (SHHF) rats that possess one or two copies of the corpulent gene (cp), a mutant form of the leptin receptor. Calcitriol treatment of the SHHF rats fed a high-salt diet resulted in a reduction in heart weight, myocardial collagen levels, left ventricular diameter and cardiac output despite higher serum leptin levels (13).
Direct evidence that human vitamin D deficiency could lead to cardiovascular disease initially came from patients with end stage renal disease (ESRD). In ESRD the damaged kidney fails to convert 25-Hydroxyvitamin D to 1,25-Dihydroxyvitamin D resulting in a severe calcitriol deficiency (14–17). In the absence of adequate calcitirol levels, secondary hyperparathyroidism (SHPT) develops resulting in elevated levels of circulating parathyroid hormone (PTH). The sustained stress on myocardial tissue leads to cardiac hypertrophy, myocardial fibrosis, and heart failure. Additionally, studies have also linked elevated levels of PTH with hyperlipidemia and an increased risk of atherosclerosis and vascular disease. Administration of activated forms of vitamin D to patients with ESRD and secondary hyperparathyroidism has resulted in decreased left ventricular hypertrophy along with a decrease in cardiovascular mortality (14–17).
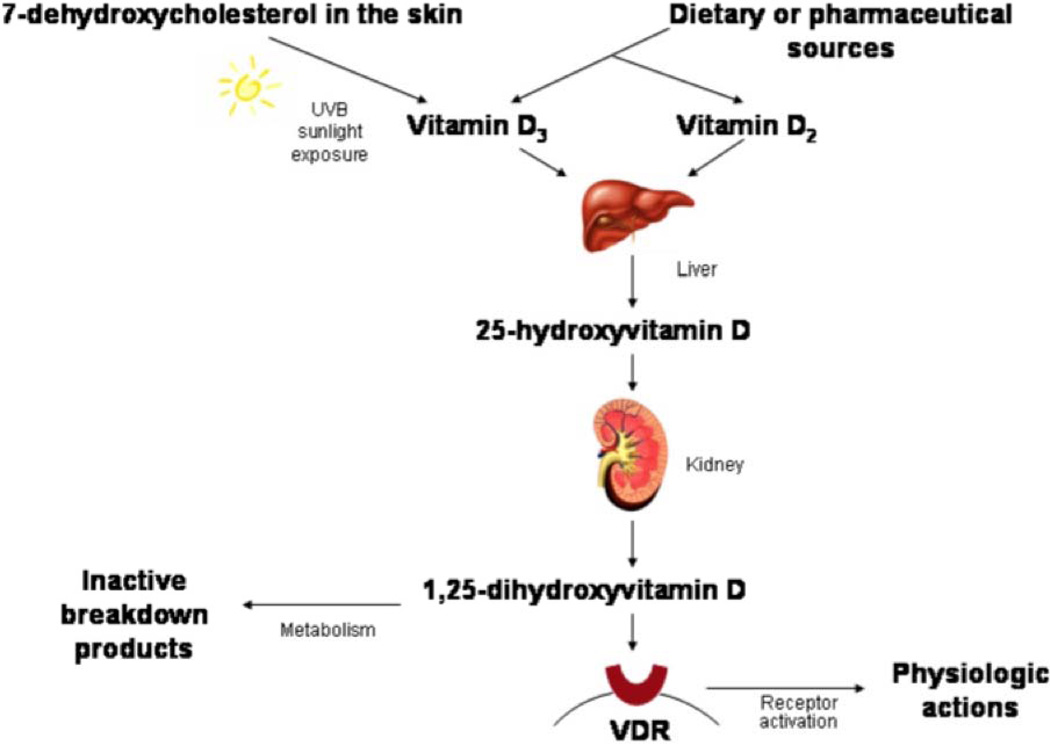
With respect to human health it is important to note that vitamin D itself does one thing and it does this perfectly, it treats and can cure vitamin D deficiency. Figure 1 depicts the activation of vitamin D to form the steroidal hormone 1,25-Dihydroxyvitamin D or calcitriol. This activation of vitamin D to a hormone is similar to the synthesis of other steroid hormones in that P450 enzymes metabolize precursor forms to the active hormone. However, unlike all other steroid hormones that use plentiful cholesterol for substrate, vitamin D (cholecalciferol) deficiency occurs in humans and disease states exist because insufficient calcitriol is then produced. Diseases linked with vitamin D deficiency include cardiovascular disease, stroke, osteoporosis, osteomalacia, several forms of cancer, autoimmune diseases such as multiple sclerosis, rheumatoid arthritis and type I diabetes, type 2 diabetes and depression. The study reported in this volume demonstrates that the cardiomyocyte vitamin D receptor (VDR), a classic member of the steroid/ thyroid/ retinoid hormone receptor family, has a physiological function and that it can control cardiac hypertrophy. The study furthers our understanding of cardiac fibrosis associated with preclinical studies’ models of vitamin D deficiency or VDR global knockout mice by demonstrating that ablation of just the cardiomyocyte is insufficient to yield ECM changes. Thus, cardiac fibroblast as discussed above may be regulated by their VDR that affects fibrosis in the heart.
Figure 1.
Vitamin D metabolism and activation to the steroidal hormone calcitriol [1,25-dihydroxyvitamin D]. VDR = Vitamin D receptor
As shown in Figure 1, 25-hydroxyvitamin D metabolites are further hydroxylated, primarily in the kidneys, to form 1,25-dihydroxyvitamin D2 and 1,25-dihydroxyvitamin D3, which are the active forms of the hormone that bind to specific vitamin D receptors in target tissues. Formation of 1,25-dihydroxyvitamin D is tightly regulated and has a short half-life, making it unsuitable for assessing vitamin D status. In vitamin D deficient states, there is in fact excess production of parathyroid hormone (secondary hyperparathyroidism) stimulating the kidneys to produce even more 1,25-dihydroxyvitamin D, such that levels can appear to be normal or even elevated. The 25-hydroxyvitamin D metabolite, which reflects total body bioavailability of the prohormone, is therefore the commonly accepted measure of vitamin D status. The serum concentration of 25-hydroxyvitamin D is typically used to determine vitamin D status. It reflects vitamin D produced in the skin as well as that acquired from the diet, and has a fairly long circulating half-life of 15 days. The level of serum 1,25-dihydroxyvitamin D is not usually used to determine vitamin D status because it has a short half-life of several hours and is tightly regulated by parathyroid hormone, calcium, and phosphate, such that it does not decrease significantly until vitamin D deficiency is already well advanced. (18) Interest in the preclinical studies of VDR dependent regulation of cardiovascular function and morphology has led to several clinical reports and studies. (Reviewed in: 19–21) The majority of these studies are retrospective and epidemiological however intervention studies are now being reported. It is critical that the above discussion of the biology of vitamin D be kept in mind. Clinical studies in which vitamin D status, as assessed by 25-hydroxyvitamin D levels, are not measured or insignificantly affected are simply not relevant.
Currently my research has focused on developing an analog of calcitriol, a selective VDR agonist [SVDRA] that has selectivity and high efficacy to treat heart failure phenotype and high-renin associated dysfunctions. The hormone calcitriol has toxic hypercalcemic actions and this limits its therapeutic utility. Our preclinical pharmacology and toxicology studies have yielded a SVDRA drug candidate, CARD-024, with efficacy and safety sufficient to obtain FDA Investigational New Drug (IND) status. We will begin first-in-human Phase 1 studies in the coming weeks. A wonderful part of focusing ones research on vitamin D is its great history of doing translation research long before the term was used, it just what we have been doing. The report by Chen and colleagues strongly supports the notion that cardiovascular diseases are targets for VDR agonist therapeutics. I am confident that clear and unambiguous studies will soon determine if this notion is accurate.
Acknowledgments
Disclosures: The author is President of Cardiavent Inc., he has received funding from the National Institutes of Health (HL074894), The American Heart Association, The American Dairy Council, The Michigan Institute of Clinical and Health Research, Abbott Laboratories, Health and Human Services: Qualified Therapeutics Discovery Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Chen S, Law CS, Grigsby CL, Olsen K, Hong TT, Zhang Y, Yeghiazarians Y, Gardner D. Cardiomyocyte-Specific Deletion of the Vitamin D Receptor Gene Results in Cardiac Hypertrophy. Circulation. 2011 doi: 10.1161/CIRCULATIONAHA.111.032680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weishaar RE, Kim SN, Saunders DE, Simpson RU. Involvement of vitamin D3 with cardiovascular function III. Effects on physical and morphological properties. American Journal of Physiology. 1990;258:E134–E142. doi: 10.1152/ajpendo.1990.258.1.E134. [DOI] [PubMed] [Google Scholar]
- 3.Weishaar RE, Simpson RU. Vitamin D3 and cardiovascular function in rats. The Journal of Clinical Investigation. 1987;79:1706–1711. doi: 10.1172/JCI113010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weishaar RE, Simpson RU. Involvement of vitamin D3 with cardiovascular function II. Direct and indirect effects. American Journal of Physiology Endocrinology and Metabolism. 1987;253:E675–E683. doi: 10.1152/ajpendo.1987.253.6.E675. [DOI] [PubMed] [Google Scholar]
- 5.Simpson RU, Thomas GA, Arnold AJ. Identification of 1,25 dihydroxyvitamin D3 receptors and activities in muscle. J. Biol. Chem. 1985;260:8882–8891. [PubMed] [Google Scholar]
- 6.O'Connell TD, Simpson RU. Receptors for 1,25 Dihydroxyvitamin D3 in human heart. Cell Biology Int. 1996;20:621–624. doi: 10.1006/cbir.1996.0081. [DOI] [PubMed] [Google Scholar]
- 7.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110:229–238. doi: 10.1172/JCI15219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC. Cardiac hypertrophy in vitamin D receptor knockout mice: role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab. 2005;288:E125–E132. doi: 10.1152/ajpendo.00224.2004. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium independent and 1,25(OH)2D3-dependent regulation of the renin angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74:170–179. doi: 10.1038/ki.2008.101. [DOI] [PubMed] [Google Scholar]
- 10.Rahman A, Hershey S, Ahmed S, Nibbelink K, Simpson RU. Heart extracellular matrix gene expression profile in the vitamin D receptor knockout mice. J Steroid Biochem Mol Biol. 2007;103:416–419. doi: 10.1016/j.jsbmb.2006.12.081. [DOI] [PubMed] [Google Scholar]
- 11.Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 12.Bodyak N, Ayus JC, Achinger S, Shivalingappa V, Ke Q, Chen YS, Rigor DL, Stillman I, Tamez H, Kroeger PE, Wu-Wong RR, Karumanchi SA, Thadhani R, Kang PM. Activated vitamin D attenuates left ventricular abnormalities induced by dietary sodium in Dahl salt-sensitive animals. Proc Natl Acad Sci USA. 2007;104:16810–16815. doi: 10.1073/pnas.0611202104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mancuso P, Rahman A, Hershey SD, Dandu L, Nibbelink KA, Simpson RU. 1,25-Dihydroxyvitamin-D3 treatment reduces cardiac hypertrophy and left ventricular diameter in spontaneously hypertensive heart failure-prone (cp/+) rats independent of changes in serum leptin. J Cardiovasc Pharmacol. 2008;51:559–564. doi: 10.1097/FJC.0b013e3181761906. [DOI] [PubMed] [Google Scholar]
- 14.Rostand SG, Drueke TB. Parathyroid hormone, vitamin D, and cardiovascular disease in chronic renal failure. Kidney International. 1999;56:383–392. doi: 10.1046/j.1523-1755.1999.00575.x. [DOI] [PubMed] [Google Scholar]
- 15.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y. Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrology, Dialysis, Transplantation. 2004;19:179–184. doi: 10.1093/ndt/gfg513. [DOI] [PubMed] [Google Scholar]
- 16.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, Thadhani R. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. Journal of the American Society of Nephrology. 2005;16:1115–1125. doi: 10.1681/ASN.2004070573. [DOI] [PubMed] [Google Scholar]
- 17.Gafter U, Battler A, Eldar M, Zevin D, Neufeld HN, Levi J. Effect of hyperparathyroidism on cardiac function in patients with end-stage renal disease. Nephron. 1985;41:30–33. doi: 10.1159/000183542. [DOI] [PubMed] [Google Scholar]
- 18.Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 19.Wu-Wong JR. Potential for vitamin D receptor agonists in the treatment of cardiovascular disease. British Journal of Pharmacology. 2009;158:395–412. doi: 10.1111/j.1476-5381.2009.00171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elamin MB, Abu Elnour NO, Elamin KB, Fatourechi MM, Alkatib AA, Almandoz JP, Liu H, Lane MA, Mullan RJ, Hazem A, Erwin PJ, Hensrud DD, Murad MH, Montori VM. Vitamin D and cardiovascular outcomes: a systematic review and meta-analysis. J Clin Endocrinol Metab. 2011;96:1931–1942. doi: 10.1210/jc.2011-0398. [DOI] [PubMed] [Google Scholar]
- 21.Nemerovski CW, Dorsch MP, Simpson RU, Bone HG, Aaronson KD, Bleske BE. Vitamin D and cardiovascular disease. Pharmacotherapy. 2009;29:691–708. doi: 10.1592/phco.29.6.691. [DOI] [PubMed] [Google Scholar]