Abstract
Purpose
To characterize the angiogenic and inflammatory responses of human choroidal endothelial cells (HCECs) to stimulators and inhibitors of the ubiquitin proteasome pathway (UPP).
Methods
The regulation of the UPP by the inhibitor withaferin A and its congener, withanolide D, two natural products derived from the medicinal plant Withania somnifera was assessed in the three-dimensional endothelial cell sprouting assay (3D-ECSA), by using HCEC- and human umbilical vein endothelial cell (HUVEC)– derived spheroids embedded in a collagen I matrix. Western blot analysis was used to investigate the effect of withanolides on IκB-α, polyubiquitination, and heme oxygenase (HO)-1 regulation in HCEC and HUVEC cultures.
Results
HCECs, like HUVECs, responded to fibroblast growth factor-2, vascular endothelial growth factor, and tumor necrosis factor (TNF)-α stimulation and sprouted vessel-like structures in collagen I matrix. However, HCECs were slower to generate these sprouting vessels, when compared with HUVECs. The extent of inhibition of endothelial cell sprouting in 3D matrix, the blockade of TNF-α-induced IκB-α degradation, levels of global polyubiquitinated proteins, and induced production of HO-1 in response to treatment by the withanolides in cultured endothelial cells was similarly regulated between HCECs and HUVECs.
Conclusions
HCECs share with HUVECs a similar response to UPP inhibitors, suggesting that this well-conserved pathway that regulates angioinflammatory mechanisms could be exploited for drug-targeting in the development of novel agents for CNV treatment.
The leading cause of irreversible blindness in the Western hemisphere is age-related macular degeneration (AMD). Choroidal neovascularization (CNV), the pathogenic proliferation of blood vessels from the choriocapillaris, contributes to greater than 90% of visual loss in AMD.1,2 Thus, therapeutic strategies for treatment of wet AMD have focused on blocking choriocapillary growth.3,4 However, presently, it is largely unknown how choroidal endothelial cells compare with other commonly studied endothelial cell types in their biological responsiveness to angiogenesis stimulation and inhibition. This is important, not only for studying the cell biology of this tissue and its pathogenesis, but also for drug discovery.
There is now increasing evidence that inflammation plays a key role in the pathogenesis of AMD.5-7 The potent angioinflammatory cytokine tumor necrosis factor (TNF)-α is believed to be one of the major activators and regulators of CNV. For instance, TNF-α is overexpressed in patients with AMD who have a higher prevalence of CNV,8 and heightened expression of this cytokine has been localized to the neovascular membranes from patients with AMD.9 Evidence that TNF-α may contribute to the angiogenic cascade is revealed from studies showing that TNF-α can directly induce the expression of angiogenic growth factors in cultured choroidal endothelial cells (CECs).9 Moreover, the importance of TNF-α as a regulator of wet-AMD is demonstrated in a recent clinical study that showed that anti-TNF-α therapy can induce regression of CNV in patients with AMD10 Because the blunting of TNF-α by targeting its functions directly or by interference with its nuclear factor (NF)-κB-mediated autoregulatory transcriptional mechanisms has been shown to attenuate angioinflammatory processes in many nonocular disease models,11, 12 such an approach may also be therapeutically feasible for CNV treatment.1 Clearly, a better understanding of the angioinflammatory pathways of CECs could provide for many novel targets for drug discovery, which can increase the arsenal of therapeutics for CNV treatment.13
The ubiquitin proteasome pathway (UPP) is now widely recognized as an important target for drug discovery, because many important intracellular processes, such as cell cycle progression, NF-κB-driven expansion of inflammatory gene expression, and cell sprouting, are orchestrated through the orderly degradation of key regulatory protein factors.14 Because the 20S proteasome’s enzymatic functions control these processes, small molecule agents targeting the catalytic subunits of the 20S proteasome have been introduced as anticancer agents15: Bortezomib, an FDA-approved proteasome inhibitor potently blocks NF-κB activation, leading to arrest of tumor cell proliferation, metastasis, and angiogenesis.16 Proteasome inhibitors downregulate NF-κB and also exert potent anti-inflammatory activity in vivo in nonmalignant disease models as well17, 18; thus, there is very active interest in targeting the UPP for control of a wide range of angioinflammatory diseases.19,20
In a previous study, we used a three-dimensional endothelial cell-sprouting assay (3D-ECSA) of human umbilical vein endothelial cell (HUVEC)– derived spheroids in a collagen I matrix to screen Withania somnifera, a medicinal plant known to be highly effective in treatment of inflammatory diseases.21 The 3D-ECSA mimics the in vivo angiogenic tissue-remodeling cascade, representing endothelial cell migration, invasion, and tube formation as an integrated morphogenic sprouting process.22 Using this assay, we identified from W. somnifera the natural product withaferin A as one of its abundant and potent angiogenesis inhibitors and demonstrated that withaferin A targets the UPP and causes the inhibition of NF-κB.23 The doses of NF-κB-inhibitory activity of withaferin A were associated with inhibition of angiogenic sprouting, as demonstrated by potent blockade of vessel growth in the 3D-ECSA.23 With the isolation of human CECs in several laboratories24,25 and demonstration that HCECs differentiate into capillary-like structures in collagen I gels,24 we became interested in understanding the control of the UPP of angiogenic sprouting in HCECs.
Recently, we showed that withaferin A potently inhibits angiogenic sprouting from HCEC-derived spheroids in the 3D-ECSA (Bargagna-Mohan P, et al. IOVS 2005;46:ARVO E-Abstract 467). In that study, we showed that withanolide D, another major withanolide from W. somnifera, targets the UPP and exerts potent inhibition of angiogenic sprouting in HCECs. Withaferin A and withanolide D share similar mechanisms in that they inhibit inflammatory-induction of IκB-α degradation. In addition, we identified a novel cytoprotective mechanism of these withanolides that relates to induction of heme oxygenase (HO)-1 expression in endothelial cells. The induction of HO-1 by antioxidants is a key mechanism that is widely recognized in different classes of cytoprotective agents.26,27 Thus, upregulation of HO-1 expression by members of the withanolide class of natural products is consistent with their chemopreventive activity.28 Taken together, our findings identify the UPP as a potential target for CNV treatment and feature the withanolides as a new class of angiogenesis inhibitors that could have a potential application for treatment or prevention of wet AMD.
Materials and Methods
Cell Culture
HUVECs (Cascade Biologicals, Portland, OR) were cultured in M-199 medium (Invitrogen-Gibco, Grand Island, NY) supplemented with 2 mM glutamine (Invitrogen-Gibco), 20% fetal bovine serum (FBS; Invitrogen-Gibco), 0.5 mg/mL penicillin/streptomycin (Invitrogen-Gibco), and 50 μg/mL endothelial cell growth factor (Sigma-Aldrich, St. Louis, MO). Cells were used within the sixth passage. HCECs, given to us by Jayakrishna Ambati (University of Kentucky), were originally isolated and characterized in Steven Ryan’s laboratory (Doheny Eye Institute, Los Angeles, CA).24 HCECs were grown in EBM medium (Clonetics, San Diego, CA) supplemented with 20% FBS, 0.5 mg/mL penicillin-streptomycin and an endothelial growth supplement kit (Cascade Biologicals) containing hydrocortisone (1 μg/mL), human epidermal growth factor (10 ng/mL), b-FGF (3 ng/mL), and heparin (10 μg/mL). Medium was changed every 2 to 3 days, and confluent plates were split at a 1:3 ratio, using 0.05% trypsin-0.02% EDTA in Hanks’ balance salt solution (HBSS; Invitrogen-Gibco). Cells were received at passage 6 and were used before passage 10.
Three-Dimensional Endothelial Cell Sprouting Assay
For the 3D-ECSA assay, we adopted the published procedure of Korff and Augustin22 with some modifications to the method we previously reported.23 Briefly, 10–μL drops (containing 1000 cells) of HUVEC and HCEC suspension were seeded onto the lids of nonadhesive 100-mm tissue culture plates (Falcon; BD Biosciences, Bedford, MA) and inverted over the bottom plates containing 5 mL of sterile phosphate-buffered saline (PBS) solution. Endothelial cell spheroids were allowed to form under gravity by incubation for 24 hours at 37°C in 5% CO2. The spheroids were collected in PBS solution containing 10% FBS and centrifuged at 500 rpm for 5 minutes, the supernatant was removed, and spheroids were carefully resuspended in an ice-cold solution of neutralized collagen type I obtained from rat tails, as described.22 The clear collagen stock solution was collected from different rat tails, and these batches were stored at 4°C for at least 4 months to mature. To obtain a neutral pH collagen solution 5% FBS, 10× M-199 medium (Invitrogen-Gibco), 0.2 N NaOH, 50 mg/mL NaHCO3 and 1:1000 diluted solution of sterile acetic acid were added. A bed of 50 μL of neutralized collagen I solution was formed in separate set of 96 well-tissue culture plates (Falcon) and polymerized by incubation for 30 minutes at 37°C. Next, 50 μL neutralized collagen-I containing equal number of HUVECs or HCECs spheroids was then distributed over this matrix bed, and newly added collagen was polymerized. An aliquot of 50 μL of culture medium containing 5% FBS was added to each well. To induce sprouting, 10 ng/mL of vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), or TNF-α (Sigma-Aldrich) was added to the medium in the presence or absence of different concentrations of inhibitors. HUVEC-derived spheroids were incubated at 37°C in 5% CO2 for 24 hours, whereas HCEC-derived spheroids were incubated for 36 to 40 hours because of the slower sprouting growth rate. Sprouting was visualized with an inverted microscope at 10× objective, and digital images were imported to image-management software (Photoshop; Adobe Systems, Mountain View, CA) for evaluation and analysis. Vessel growth measurements (cumulative length of vessels) were performed with NIH ImageJ (available by ftp at zippy.nimh.nih.gov/ or at http://rsb.info.nih.gov/nih-imagej; developed by Wayne Rasband, National Institutes of Health, Bethesda, MD) and IC50 was derived as previously described.23
Natural Products
The withanolides were isolated from the root powder of Withania somnifera (Bazaar of India Imports, Menlo Park, CA) essentially as previously described,23 with the modification that an improved HPLC procedure was used to separate the individual natural products.29 Withanolide D was characterized and provided as a gift by Alberto Bargagna (Department of Pharmaceutical Sciences, University of Genova, Italy). Epoxomicin was synthesized by using a published procedure30 and given to us by Kyung Bo Kim (Department of Pharmaceutical Sciences, University of Kentucky).
Western Blot Analysis
We performed IκB-α and ubiquitin protein analyses by using our published procedure.23 For time-course and dose–response experiments, HUVECs and HCECs were growth arrested by contact inhibition in 60-mm tissue culture plates, trypsinized, and replated in 100-mm tissue culture plates at a density of 2.5 × 105 cells/mL in medium containing 2% FBS. Cells were left to rest for 1 hour and then treated with 10 ng/mL VEGF (Sigma-Aldrich) in the presence and absence of the inhibitors (withaferin A, withanolide D, or epoxomicin). For HO-1 protein analysis, total cell extracts from HUVEC and HCEC were immunoblotted with a rabbit polyclonal HO-1 antibody. All antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA) and used at a dilution of 1:500.
Results
Sensitivity of Angiogenic Sprouting of HCEC Spheroids to Proteasome Inhibition
Using a method we had established for production of HUVEC-derived spheroids,23 we initiated studies to generate spheroids from HCECs and investigated their growth responses to angiogenic stimulators and inhibitors. Initially, we were struck by the observation that HCEC-derived spheroids embedded in collagen I matrix were slower to respond to angiogenic stimulators. To determine whether this poorer growth performance was due to variations in collagen I, we tested different batches of collagen I obtained from different rat tails and found that the retarded growth was not due to differences in the matrix (data not shown). Therefore, to support robust angiogenic sprouting in HCECs, we incubated the spheroids for periods of 36 to 40 hours, a longer time than was found to be necessary to generate the sprouting arbor similar in density to those obtained from HUVECs (24 hours; Fig. 1A). We also did not observe any remarkable differences in the kinetics of vessel growth in response to VEGF, bFGF or TNF-α (Fig. 1B), although it was consistently observed that angiogenic sprouting after growth factor stimulation was slower in the HCECs than in the HUVECs. To next test the biological response of endothelial cell spheroids to agents that target the UPP, we investigated the inhibitory activity of epoxomicin, a well-known 20S proteasome inhibitor that binds to the β-catalytic subunits of the proteasome and blocks its enzymatic activities. Epoxomicin potently inhibited sprouting from both HUVEC- and HCEC-derived spheroids (Fig. 1C), which was observed to occur in a dose-dependent manner showing greater than 90% inhibition at 125 nM (data not shown). This finding revealed that the catalytic functions of the proteasome are critical for vessel formation in this in vitro model of angiogenesis.
Figure 1.
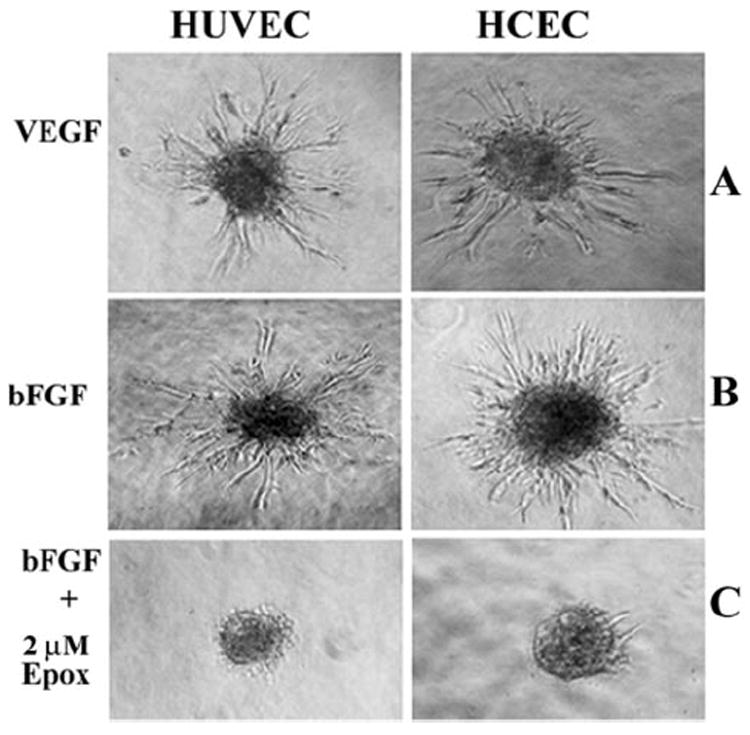
Growth factor induction of sprouting in HUVECs and HCECs. Angiogenic sprouting in HUVEC- and HCEC-derived spheroids was induced by 10 ng/mL of (A) VEGF or (B) bFGF. Incubation in the presence of growth factors was perforemd for 24 hours for HUVECs and for 36 hours for HCECs. Growth factor-induced spouting was abrogated by the co-incubation of spheroids with 2 μM of the proteasome inhibitor (C) epoxomicin.
We next assessed the angiogenic response of HUVEC- and HCEC-derived spheroids to withanolide D (Fig. 2), a congener of withaferin A, and also a known potent inhibitor of inflammation.31 As seen in Figure 3, withanolide D dose-dependently inhibited growth of angiogenic sprouts in response to VEGF in both HUVECs and HCECs. As previously observed with withaferin A, withanolide D also demonstrated an IC50 for angiogenic sprouting at 500 nM (data not shown), suggesting efficacy comparable with that of withaferin A as an angiogenesis inhibitor.23
Figure 2.
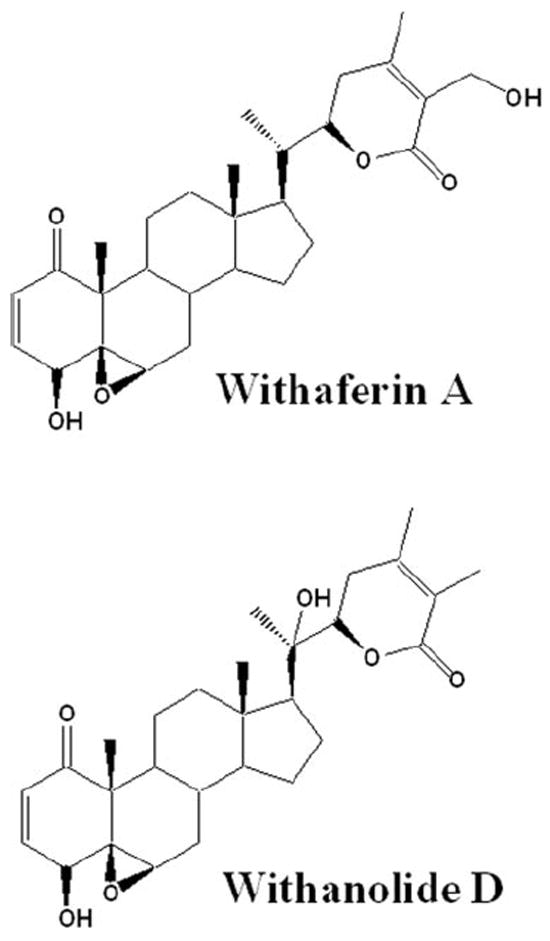
Structures of withaferin A and withanolide D, two major natural product inhibitors from Withania somnifera.
Figure 3.
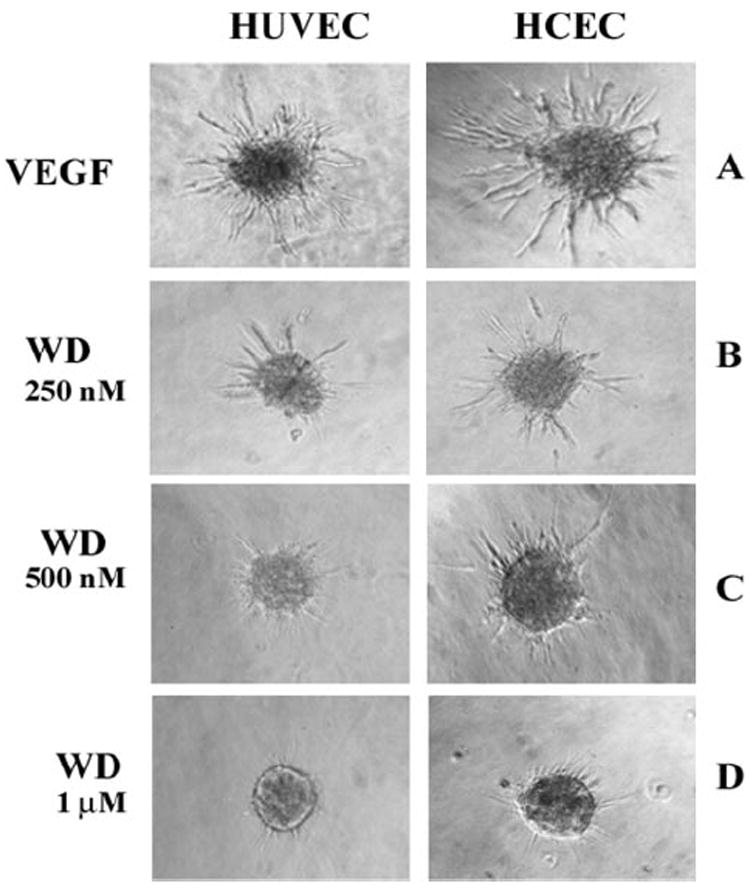
Withanolide D inhibited angiogenic sprouting in 3D-ECSA. Endothelial cell spheroids from HUVECs and HCECs were treated with 10 ng/mL VEGF in the presence and absence of different concentrations of withanolide D (WD). The HCEC-derived sprouts and HUVEC-derived sprouts were photographed after 36 and 24 hours, respectively.
Targeting of the Ubiquitin-Proteasome Pathway by Withanolide D
Given the structural similarity of withaferin A and withanolide D (see Fig. 2) and their known high abundance in methanolic and chloroform extracts from W. somnifera that we previously showed to possess antiangiogenic activity,23 we wanted next to ascertain whether withanolide D also shares a similar mechanism of action with withaferin A. We therefore assessed the effect of withanolide D on cultured HUVECs and HCECs that were treated with the inflammatory cytokine TNF-α to stimulate the UPP and activate NF-κB. As seen in Figure 4, addition of withanolide D to HUVECs potently blocked the degradation of phosphorylated IκB-α in a dose-dependent manner. In comparison, HCECs also showed a similar response to withanolide D, with doses above 500 nM being inhibitory. These findings suggest that the inhibitory activity of withanolide D on NF-κB signaling, which appears to be similar to that of withaferin A,23 occurs through prevention of degradation of phosphorylated IκB-α. Because IκB-α is ubiquitinated on activation by phosphorylation before its degradation by the 26S proteasome, we next wanted to determine the general status of protein ubiquitination in withanolide D-treated HUVECs and HCECs. In agreement with our hypothesis, the withanolide D-treated cells showed dose-dependent increased levels of ubiquitinated species (Fig. 5), mimicking the activity of withaferin A on HUVECs.23 Next, we investigated the action of TNF-α on HUVEC sprouting with the 3D-ECSA. TNF-α was found to activate angiogenic sprouting potently (Fig. 6A). To determine whether this TNF-α-induced sprouting was also dependent on the UPP, we cotreated the TNF-α-stimulated spheroids with the cell-permeable inhibitor ubiquitin aldehyde. Addition of 5 μM ubiquitin aldehyde to TNF-α treated spheroids significantly arrested the growth of angiogenic sprouts (Fig. 6B). Furthermore, TNF-α-induced sprouting was shown to be dependent on NF-κB, since the NF-κB-specific inhibitor parthenolide32 blocked sprouting completely (Fig. 6C).
Figure 4.
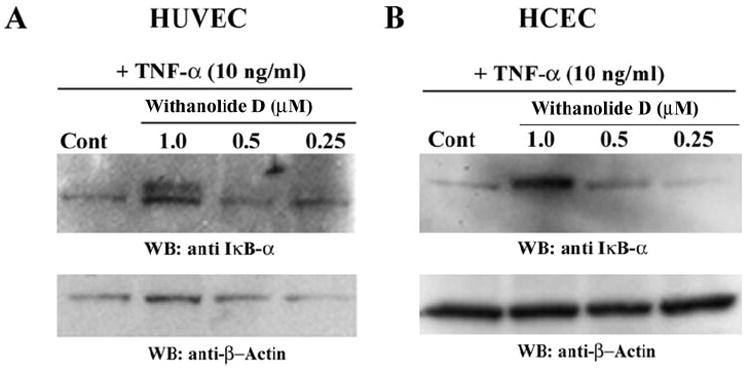
Withanolide D inhibited degradation of IκBα, the cytoplasmic inhibitor of NF-κB. HUVECs (A) and HCECs (B) were pretreated for 30 minutes with different concentrations of withanolide D and subsequently stimulated with 10 ng/mL TNF-α for 20 minutes. Cytoplasmic extracts were analyzed by Western blot analysis for IκBα The blots were reprobed for β-actin.
Figure 5.
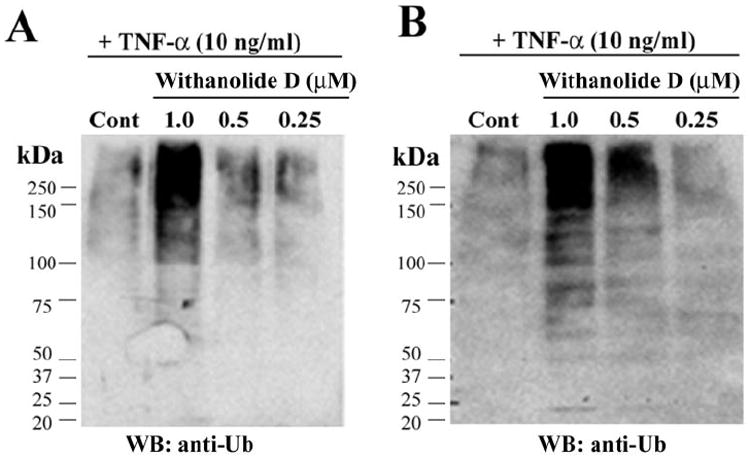
Withanolide D targets the UPP in HUVECs and HCECs. HUVECs (A) and HCECs (B) were pretreated for 30 minutes with different concentrations of withanolide D (WD) and subsequently stimulated with 10 ng/mL TNF-α for 20 minutes. Cytoplasmic extracts were analyzed by Western blot with a monoclonal antibody against ubiquitin.
Figure 6.
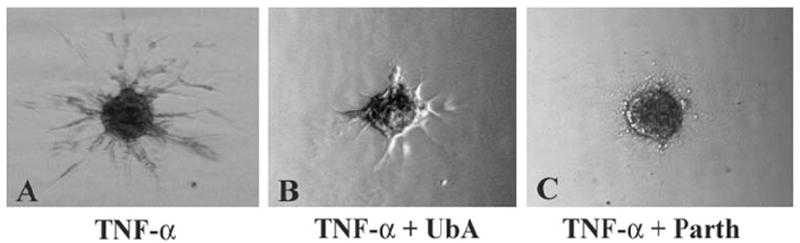
TNF-α-induced angiogenic sprouting is blocked by inhibitors of the UPP. Endothelial cell spheroids from HUVECs were treated with 10 ng/mL TNF-α in the presence and absence of 5 μM ubiquitin aldehyde (UbA) or 5 μM parthenolide (Parth). The spheroids were photographed after 24 hours.
Finally, we wanted to compare the efficacy of withanolide D with that of epoxomicin on UPP targeting activity. HCECs and HUVECs treated with different doses of epoxomicin caused a TNF-α-induced dose-dependent increase in ubiquitinated proteins, even at the lowest tested dose of 250 nM (Figs. 7A, 7B). Revealing the potency of this class of proteasome inhibitor in blocking inflammatory signaling, we found that IκB-αdegradation was also potently inhibited by epoxomicin in both HUVECs and HCECs at its lowest dose (Figs. 7C, 7D).
Figure 7.
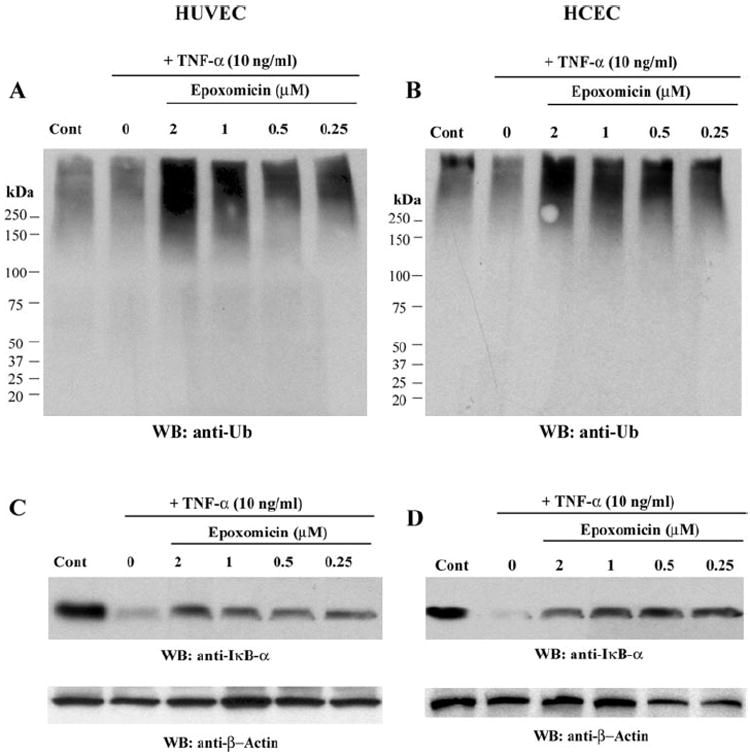
The 20S proteasome inhibitor epoxomicin targets the UPP in HUVECs and HCECs. HUVECs and HCECs were pretreated for 30 minutes with epoxomicin and then stimulated with TNF-α for 20 minutes. Cytoplasmic extracts were analyzed by Western blot for ubiquitin (A, B) and blots reprobed for IκBα expression (C, D). The blots were subsequently reprobed for β-actin to control for protein loading.
Induction of Heme Oxygenase-1 Expression in Vascular Endothelial Cells by Withanolides
Plant extracts from W. somnifera containing withanolides have been reported to exert a chemopreventive effect21 and also to demonstrate cardiovascular protection,33 mechanisms that imply that these natural products promote vascular homeostasis. One of the major mechanisms of cytoprotection exerted by the withanolides is known to involve the induction of antioxidant gene responses.28 We became specifically interested in testing the effects of withaferin A on the antioxidant enzyme HO-1, because this enzyme is known to be of critical importance in vascular homeostasis34 and HO-1 expression can be induced by low doses of proteasome inhibitors.35 Moreover, we had found that withaferin A treatment of HUVECs in the presence of TNF-α for 4 and 12 hours increases HO-1 message levels by three- and ninefold, respectively, as assessed by DNA microarray analysis (Bargagna-Mohan P, Mohan R, unpublished data, 2005). These findings led us to hypothesize that transcriptional upregulation of HO-1 by withanolides is a novel feature of their cytoprotective activity (Bargagna-Mohan P, et al. IOVS 2005;46:ARVO E-Abstract 467). Therefore, here we performed a time course experiment with HCECs and HUVECs to investigate whether HO-1 protein expression also occurred in response to withaferin A treatment in endothelial cells. We found that HO-1 protein became detectable as early as 4 hours after treatment (Fig. 8A), and this induced expression of HO-1 was found to be maintained over 24 hours. As seen in Figure 8B, HUVECs responded to doses as low as 200 nM of withaferin A and expressed HO-1 after a 12-hour treatment. In a manner paralleling the response of HUVECs, HCECs treated with withaferin A for 12 hours also displayed dose-dependent expression of HO-1 (Fig. 8C). Finally, we show that HUVECs treated with 10 ng/mL TNF-α in the presence of withaferin A strongly induced HO-1 expression by 24 hours (Fig. 8D). Taken together, these findings show a similarity between these two vascular endothelial cell types in their response to low doses of angioprotective agents.
Figure 8.
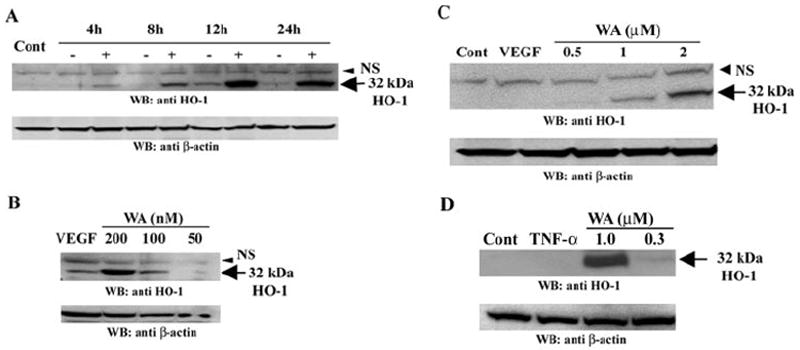
Withaferin A induces heme oxygenase-1 expression. HUVECs (A) were treated with 10 ng/mL VEGF for different times in the absence or presence of 1 μM withaferin A (WA). HUVECs (B) and HCECs (C) were treated with 10 ng/mL VEGF in the presence of different doses of WA for 12 hours. HUVECs (D) were treated with 10 ng/mL TNF-α in the absence or presence of different doses of WA for 24 hours Protein extracts were analyzed by Western blot for HO-1 expression, and the blots reprobed with a β-actin antibody to control for protein loading.
Discussion
The key findings of this study are that the UPP–NF-κB regulatory signaling network plays an important role in angiogenic sprouting and inflammatory signal transduction in HCECs, a signaling target we believe to be of importance for therapeutic development of novel treatments for CNV. We show that withanolide D, like its potent angiogenesis inhibitory congener withaferin A,23 targets the UPP and inhibits angiogenic sprouting in the in vitro tube formation assay. Both these withanolides block inflammatory signaling and exert cytoprotective action, through prevention of degradation of IκB-α and induction of HO-1 expression, respectively, which are two key mechanisms involved in vascular homeostasis.36-38
It is well known that NF-κB plays a seminal role in inflammation and drives expression of an expansive array of gene networks within the vasculature.39 In the NF-κB signaling pathway, IκB-α, the cytoplasmic inhibitor of NF-κB, on its ubiquitination and subsequent degradation by the 26S proteasome allows NF-κB to translocate to the nucleus and engage in inflammatory gene expression.40 Notably, recent evidence implicates NF-κB activation in the choroid with oxidative stress-induced injury. For instance, although not a model of AMD, light-induced photo-oxidative stress can also cause levels of lipid peroxides to become elevated in choroidal endothelial cells.41 Others have related these toxic effectors of injury to ensuing inflammatory responses via NF-κB activation in choroidal endothelial cells.42 Whereas these reports bear credence to oxidative stress pathways initiating inflammatory damage to the macula and contributing to AMD,43,44 an important role for NF-κB activation in response to age-related production of advanced glycation end-products has also been reported.45 Because many cytokines including the angioinflammatory protein TNF-α act through NF-κB signaling,46 it is logical to deduce that TNF-α gene expression in the choroidal vasculature may also be critically dependent on NF-κB. This implies TNF-α expression with angioinflammatory processes in the choroid. Corroborating evidence for such a role is revealed in the finding that TNF-α is localized to CNV membranes and overexpressed in patients with AMD, who have a higher prevalence of CNV.8 These findings, when taken together with those in our previous report that withaferin A potently attenuates TNF-α expression in vivo in a mouse model of inflammation at concentrations that are associated with its NF-κB inhibitory dose,23 underscore the importance of harnessing the potential of withanolide-based therapies for CNV treatment. This hypothesis is further corroborated now by our findings that withanolide D antagonizes TNF-α-induced activation of the UPP and suppress NF-κB through preventing IκB-α degradation in HCECs, paralleling the mechanism of action of the proteasome inhibitors.17,18 Finally, our investigations have revealed that these bioactive withanolides induce HO-1 protein expression in HUVECs and HCECs, a mechanism believed to relate to their known cytoprotective activity.20 Withaferin A treatment of HUVECs was also found to increase HO-1 message levels significantly in cultured HUVECs, as assessed in three independent DNA microarray analysis experiments (Bargagna-Mohan P, et al. IOVS 2005;46:ARVO E-Abstract 467). The upstream transcriptional mechanism by which withanolides induce HO-1 expression in endothelial cells has not been uncovered; we speculate that the UPP-targeting activity of withanolides may mimic the action of proteasome inhibitors, because the latter class of inhibitors are shown to induce the expression of HO-1 in cultured cells.35 Finally, it is intriguing that in humans HO-1 levels decline with age in the macula,47 an observation thought to relate to the general decline of protective mechanisms in the retina against oxidative stress. These collective data support the thesis that further investigation of UPP–NF-κB regulatory network in choroidal endothelial cells is warranted for better understanding of wet AMD.
We discovered a small difference in the kinetics of cell migratory–invasive behavior of endothelial cells in the 3D-ECSA. Whereas HUVECs displayed a well-orchestrated branched vessel-like network in a timely fashion, HCECs produced such organized branched tubule networks with delayed kinetics. It should be pointed out, however, that inhibitors of the UPP-NF-κB network, such as withaferin A, withanolide D, and epoxomicin, as well as NF-κB-specific inhibitor parthenolide, arrest sprouting in the 3D-ECSA potently, whether the assay is derived from HCECs or HUVECs. These findings suggest that the differences between HUVECs and HCECs in invasive growth in 3D-matrix lie in differential responsiveness to angiogenic signals, but not to growth inhibition. To our knowledge this is the first report on the slower angiogenic sprouting characteristics of HCECs compared to other endothelial cell types. The precise mechanism of this difference remains to be elucidated.
The development of assays to screen vast chemical libraries of synthetic compounds and natural products for antiangiogenic drug discovery has been hampered by the lack of suitable high-throughput screening (HTS) assays that adequately represent the sequential steps in angiogenic sprouting.22 We have used the 3D-ECSA from HUVEC to screen natural products23 and now further validate this approach by demonstrating the antiangiogenic activity of withaferin A also in a mouse model of corneal inflammatory angiogenesis (Mohan R, et al. IOVS 2005; 46:ARVO E-Abstract 4493). As demonstrated by this study that HCECs respond as effectively as HUVECs to UPP inhibition in the 3D-ECSA, we rationalize that the HUVEC 3D-ECSA is well suited as a primary HTS model because the tissue source of endothelial cells is not limited. In addition, because we used HUVECs that were pooled from different donors we can ameliorate any concerns regarding genetic biases in HTS data analysis. Improvements to the 3D-ECSA are currently being pursued in our laboratory to facilitate the adaptation to HTS platforms.
Withania somnifera, also known as “Indian ginseng” possesses many diverse pharmacological activities, including antiinflammatory, antitumor, brain cognition, immunostimulatory, and sedative effects.21 Based on its effective use in Ayurvedic medicine for a number of inflammatory and ageing problems, many health tonics comprising withanolide-containing herbal mixtures are gaining popular use in recent years in the United States and across Europe. Because of known natural variations in levels of these natural products due to differences in plant cultivars and geographic collection sites and because these extracts are neither quality controlled for their chemical composition or biological actions,48 caution must be used in unguided self-medication with such over-the-counter tonics. Hopefully, findings such as ours that help unveil the molecular mechanisms of the major bioactive withanolides will provide the scientific knowledge to assess the health benefits and potential side effects of natural medicines, and at the same time, such molecular studies will assist in the identification of new chemical classes of drugs that target the UPP, an important, validated target for angioinflammatory diseases.14
Acknowledgments
The authors thank Jayakrishna Ambati and Andrew Pearson (University of Kentucky) for helpful discussions during the development of this project.
Supported by a Fight for Sight Grant-in-Aid, Kentucky Tobacco Research and Development Center grants (RM), and a Research to Prevent Blindness Challenge Grant.
Footnotes
Disclosure: P. Bargagna-Mohan, P; P.P. Ravindranath, None; R. Mohan, P
References
- 1.Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48:257–293. doi: 10.1016/s0039-6257(03)00030-4. [DOI] [PubMed] [Google Scholar]
- 2.Fine SL. Age-related macular degeneration 1969–2004: a 35-year personal perspective. Am J Ophthalmol. 2005;139:405–420. doi: 10.1016/j.ajo.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 3.Grossniklaus HE, Green WR. Choroidal neovascularization. Am J Ophthalmol. 2004;137:496–503. doi: 10.1016/j.ajo.2003.09.042. [DOI] [PubMed] [Google Scholar]
- 4.Kijlstra A, La Heij E, Hendrikse F. Immunological factors in the pathogenesis and treatment of age-related macular degeneration. Ocul Immunol Inflamm. 2005;13:3–11. doi: 10.1080/09273940590909185. [DOI] [PubMed] [Google Scholar]
- 5.Ambati J, Anand A, Fernandez S, et al. An animal model of age-related macular degeneration in senescent Ccl-2- or Ccr-2-deficient mice. Nat Med. 2003;9:1390–1397. doi: 10.1038/nm950. [DOI] [PubMed] [Google Scholar]
- 6.Bok D. Evidence for an inflammatory process in age-related macular degeneration gains new support. Proc Natl Acad Sci USA. 2005;102:7053–7054. doi: 10.1073/pnas.0502819102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penfold PL, Madigan MC, Gillies MC, Provis JM. Immunological and aetiological aspects of macular degeneration. Prog Retin Eye Res. 2001;20:385–414. doi: 10.1016/s1350-9462(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 8.Cousins SW, Espinosa-Heidmann DG, Csaky KG. Monocyte activation in patients with age-related macular degeneration: a biomarker of risk for choroidal neovascularization? Arch Ophthalmol. 2004;122:1013–1018. doi: 10.1001/archopht.122.7.1013. [DOI] [PubMed] [Google Scholar]
- 9.Hangai M, He S, Hoffmann S, Lim JI, Ryan SJ, Hinton DR. Sequential induction of angiogenic growth factors by TNF-alpha in choroidal endothelial cells. J Neuroimmunol. 2006;171:45–56. doi: 10.1016/j.jneuroim.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 10.Markomichelakis NN, Theodossiadis PG, Sfikakis PP. Regression of neovascular age-related macular degeneration following infliximab therapy. Am J Ophthalmol. 2005;139:537–540. doi: 10.1016/j.ajo.2004.09.058. [DOI] [PubMed] [Google Scholar]
- 11.DeBusk LM, Chen Y, Nishishita T, Chen J, Thomas JW, Lin PC. Tie2 receptor tyrosine kinase, a major mediator of tumor necrosis factor alpha-induced angiogenesis in rheumatoid arthritis. Arthritis Rheum. 2003;48:2461–2471. doi: 10.1002/art.11213. [DOI] [PubMed] [Google Scholar]
- 12.Lizzul PF, Aphale A, Malaviya R, et al. Differential expression of phosphorylated NF-kappaB/RelA in normal and psoriatic epidermis and downregulation of NF-kappaB in response to treatment with etanercept. J Invest Dermatol. 2005;124:1275–1283. doi: 10.1111/j.0022-202X.2005.23735.x. [DOI] [PubMed] [Google Scholar]
- 13.van Wijngaarden P, Coster DJ, Williams KA. Inhibitors of ocular neovascularization: promises and potential problems. JAMA. 2005;293:1509–1513. doi: 10.1001/jama.293.12.1509. [DOI] [PubMed] [Google Scholar]
- 14.Ciechanover A. Intracellular protein degradation: from a vague idea thru the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Cell Death Differ. 2005;12:1178–1190. doi: 10.1038/sj.cdd.4401692. [DOI] [PubMed] [Google Scholar]
- 15.Mitsiades CS, Mitsiades N, Hideshima T, Richardson PG, Anderson KC. Proteasome inhibitors as therapeutics. Essays Biochem. 2005;41:205–218. doi: 10.1042/EB0410205. [DOI] [PubMed] [Google Scholar]
- 16.Rajkumar SV, Richardson PG, Hideshima T, Anderson KC. Proteasome inhibition as a novel therapeutic target in human cancer. J Clin Oncol. 2005;23:630–639. doi: 10.1200/JCO.2005.11.030. [DOI] [PubMed] [Google Scholar]
- 17.Meng L, Mohan R, Kwok BH, Elofsson M, Sin N, Crews CM. Epoxomicin, a potent and selective proteasome inhibitor, exhibits in vivo antiinflammatory activity. Proc Natl Acad Sci USA. 1999;96:10403–10408. doi: 10.1073/pnas.96.18.10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Palombella VJ, Conner EM, Fuseler JW, et al. Role of the proteasome and NF-kappaB in streptococcal cell wall-induced polyarthritis. Proc Natl Acad Sci USA. 1998;95:15671–15676. doi: 10.1073/pnas.95.26.15671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson PA, Ardley HC. Ubiquitin-protein ligases: novel therapeutic targets? Curr Protein Pept Sci. 2004;5:163–176. doi: 10.2174/1389203043379800. [DOI] [PubMed] [Google Scholar]
- 20.Amit S, Ben-Neriah Y. NF-kappaB activation in cancer: a challenge for ubiquitination- and proteasome-based therapeutic approach. Semin Cancer Biol. 2003;13:15–28. doi: 10.1016/s1044-579x(02)00096-2. [DOI] [PubMed] [Google Scholar]
- 21.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–346. [PubMed] [Google Scholar]
- 22.Korff T, Augustin HG. Integration of endothelial cells in multicellular spheroids prevents apoptosis and induces differentiation. J Cell Biol. 1998;143:1341–1352. doi: 10.1083/jcb.143.5.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mohan R, Hammers HJ, Bargagna-Mohan P, et al. Withaferin A is a potent inhibitor of angiogenesis. Angiogenesis. 2004;7:115–122. doi: 10.1007/s10456-004-1026-3. [DOI] [PubMed] [Google Scholar]
- 24.Sakamoto T, Sakamoto H, Hinton DR, Spee C, Ishibashi T, Ryan SJ. In vitro studies of human choroidal endothelial cells. Curr Eye Res. 1995;14:621–627. doi: 10.3109/02713689508998488. [DOI] [PubMed] [Google Scholar]
- 25.Browning AC, Gray T, Amoaku WM. Isolation, culture, and characterisation of human macular inner choroidal microvascular endothelial cells. Br J Ophthalmol. 2005;89:1343–1347. doi: 10.1136/bjo.2004.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leopold JA, Loscalzo J. Oxidative enzymopathies and vascular disease. Arterioscler Thromb Vasc Biol. 2005;25:1332–1340. doi: 10.1161/01.ATV.0000163846.51473.09. [DOI] [PubMed] [Google Scholar]
- 27.Prawan A, Kundu JK, Surh AJ. Molecular basis of heme oxygenase-1 induction: implications for chemoprevention and chemoprotection. Antioxid Redox Signal. 2005;7:1688–1703. doi: 10.1089/ars.2005.7.1688. [DOI] [PubMed] [Google Scholar]
- 28.Su B-Ng J-Q, Kang Y-H, Park E-J, Pezzuto JM, Kinghorn AD. Induction of the phase II enzyme, quinone reductase, by withanolides and norwithanolides from solanaceous species. Rev Organ Chem. 2004;1:115–123. [Google Scholar]
- 29.Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia. 2003;74:68–76. doi: 10.1016/s0367-326x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 30.Sin N, Kim KB, Elofsson M, et al. Total synthesis of the potent proteasome inhibitor epoxomicin: a useful tool for understanding proteasome biology. Bioorg Med Chem Lett. 1999;9:2283–2288. doi: 10.1016/s0960-894x(99)00376-5. [DOI] [PubMed] [Google Scholar]
- 31.Budhiraja RDK, Pawan SS. Biological activity of withanolides. J Sci Ind Res. 2000;59:904–911. [Google Scholar]
- 32.Kwok BH, Koh B, Ndubuisi MI, Elofsson M, Crews CM. The anti-inflammatory natural product parthenolide from the medicinal herb feverfew directly binds to and inhibits IkappaB kinase. Chem Biol. 2001;8:759–766. doi: 10.1016/s1074-5521(01)00049-7. [DOI] [PubMed] [Google Scholar]
- 33.Gupta SK, Mohanty I, Talwar KK, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 34.Ohta K, Yachie A. Development of vascular biology over the past 10 years: heme oxygenase-1 in cardiovascular homeostasis. J Endovasc Ther. 2004;11(suppl 2):140–150. doi: 10.1177/15266028040110S607. [DOI] [PubMed] [Google Scholar]
- 35.Chen J, Regan RF. Increasing expression of heme oxygenase-1 by proteasome inhibition protects astrocytes from heme-mediated oxidative injury. Curr Neurovasc Res. 2005;2:189–196. doi: 10.2174/1567202054368344. [DOI] [PubMed] [Google Scholar]
- 36.Hoekstra KA, Godin DV, Cheng KM. Protective role of heme oxygenase in the blood vessel wall during atherogenesis. Biochem Cell Biol. 2004;82:351–359. doi: 10.1139/o04-006. [DOI] [PubMed] [Google Scholar]
- 37.Denk A, Goebeler M, Schmid S, et al. Activation of NF-kappa B via the Ikappa B kinase complex is both essential and sufficient for proinflammatory gene expression in primary endothelial cells. J Biol Chem. 2001;276:28451–28458. doi: 10.1074/jbc.M102698200. [DOI] [PubMed] [Google Scholar]
- 38.Verma IM. Nuclear factor (NF)-kappaB proteins: therapeutic targets. Ann Rheum Dis. 2004;63(suppl 2):57–61. doi: 10.1136/ard.2004.028266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Madge LA, Pober JS. TNF signaling in vascular endothelial cells. Exp Mol Pathol. 2001;70:317–325. doi: 10.1006/exmp.2001.2368. [DOI] [PubMed] [Google Scholar]
- 40.Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kayatz P, Heimann K, Schraermeyer U. Ultrastructural localization of light-induced lipid peroxides in the rat retina. Invest Ophthalmol Vis Sci. 1999;40:2314–2321. [PubMed] [Google Scholar]
- 42.Wu T, Handa JT, Gottsch JD. Light-induced oxidative stress in choroidal endothelial cells in mice. Invest Ophthalmol Vis Sci. 2005;46:1117–1123. doi: 10.1167/iovs.04-0517. [DOI] [PubMed] [Google Scholar]
- 43.Taylor HR, West S, Munoz B, Rosenthal FS, Bressler SB, Bressler NM. The long-term effects of visible light on the eye. Arch Ophthalmol. 1992;110:99–104. doi: 10.1001/archopht.1992.01080130101035. [DOI] [PubMed] [Google Scholar]
- 44.Cruickshanks KJ, Klein R, Klein BE, Nondahl DM. Sunlight and the 5-year incidence of early age-related maculopathy: the Beaver Dam Eye Study. Arch Ophthalmol. 2001;119:246–250. [PubMed] [Google Scholar]
- 45.Hammes HP, Hoerauf H, Alt A, et al. N(epsilon)(carboxymethyl)lysin and the AGE receptor RAGE colocalize in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1999;40:1855–1859. [PubMed] [Google Scholar]
- 46.Kempe S, Kestler H, Lasar A, Wirth T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005;33:5308–5319. doi: 10.1093/nar/gki836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Frank RN, Amin RH, Puklin JE. Antioxidant enzymes in the macular retinal pigment epithelium of eyes with neovascular age-related macular degeneration. Am J Ophthalmol. 1999;127:694–709. doi: 10.1016/s0002-9394(99)00032-x. [DOI] [PubMed] [Google Scholar]
- 48.Sangwan RS, Chaurasiya ND, Misra LN, et al. Phytochemical variability in commercial herbal products and preparations of Withania somnifera (Ashwagandha) Current Science. 2004;86:461–465. [Google Scholar]


