Abstract
Background
Little is known regarding how impairment-based exercises may improve performance of specific functional tasks in people with knee osteoarthritis (OA).
Objective
The purpose of this study was to compare the probability that participation in an impairment-based exercise program or the same impairment-based program supplemented with agility and perturbation training will improve patient-reported function on specific functional tasks.
Design
This study was a secondary analysis of data from a randomized clinical trial.
Setting
The study was conducted in the outpatient physical therapy clinic of a large, university-based health center.
Participants
One hundred eighty-three people with knee OA (122 women, 61 men) participated.
Interventions
Participants were randomly assigned to either a group that received agility and perturbation training with standard exercise therapy or a group that received only the standard exercise.
Measurements
Specific functional items were taken from the physical function subscale of the Western Ontario and McMasters Universities Osteoarthritis Index, the Lower Extremity Function Scale, and the Activities of Daily Living Scale of the Knee Outcome Survey.
Results
The probability of self-reported improvement in a variety of specific functional tasks was low following participation in either of the exercise programs. When only participants with moderate to severe difficulty performing specific functional tasks were considered in the analysis, the probability of success improved but was still limited.
Limitations
The results are generalizable only to self-reported assessment of performance of specific functional tasks.
Conclusions
Impairment-based exercise may not be enough to make substantial improvement in performance of specific functional tasks. Task-specific exercise approaches need to be explored for people with knee OA.
Therapeutic exercise is commonly recommended for people with knee osteoarthritis (OA). Published evidence-based guidelines advise its use for the management of pain and to lessen disability in people with knee OA.1–4 The recommended exercise programs are typically impairment based, designed to specifically address physical impairments associated with knee OA, such as lower-extremity muscle weakness, loss of joint motion, and aerobic deconditioning. Thus, an impairment-based program will include lower-extremity muscle strengthening and stretching, joint range of motion, and aerobic exercises. It is assumed that addressing these impairments will result in reduced pain and improved function. Studies have shown that impairment-based therapeutic exercise has been effective for reducing knee pain and improving physical function, muscle strength, and gait speed in individuals with knee OA.5–8 However, recent evidence suggests that it has yielded only fair improvements on general measures of physical function, with reported effect sizes in the moderate range (0.37, 95% confidence interval [95% CI]=0.25 to 0.49).9 In addition, the beneficial effects of exercise on physical function appear to decline within a 6-month period.10
Although the evidence suggests that impairment-based exercise programs are somewhat helpful in improving general function, knowledge of their impact on improving performance of specific functional tasks is lacking. There have been 2 studies that reported information on specific functional task difficulty in people with knee OA.11,12 Taken together, these studies indicate that people with knee OA generally report difficulty with distance walking, stair climbing, standing from a seated position, stooping and kneeling, carrying bundles, and performing heavy household chores.11,12 To our knowledge, there have been no studies reporting the extent to which an impairment-based exercise program can improve performance of these functional tasks.
Understanding how impairment-based exercises may influence specific functional tasks can help to improve exercise protocols for people with knee OA. For example, defining the functional tasks that are unlikely to improve with impairment-based exercises can reveal specific functional tasks that perhaps should be addressed differently than with a regular impairment-based approach. If impairment-based exercises do not appear to influence functional task performance, a refinement of exercise protocols may be needed to improve the overall effect of exercise on measures of physical function in people with knee OA.
Recently, there has been interest in enhancing traditional impairment-based exercise programs for people with knee OA by including agility training and techniques to challenge balance.13,14 Agility training techniques include activities that expose patients to quick stops and starts, sudden changes in direction, lateral movements, and negotiating obstacles. Balance training techniques may include balancing on a single limb or the use of wobble boards and roller boards to perturb the patient's base of support (perturbation training). It has been proposed that these additional training techniques may enhance the effects of exercise programs on improving function by exposing patients to various movement problems and challenges to balance and knee stability that would not otherwise occur with traditional impairment-based exercise programs.13,14
We recently conducted a randomized clinical trial to determine whether adding agility and perturbation training techniques to a standard impairment-based exercise program would enhance the effect of exercise therapy on general physical function in people with knee OA.15 We were unable to demonstrate an additive effect on general measures of physical function for the enhanced exercise program over a traditional impairment-based program.15 In reconsidering our results, we wondered whether, although the general measures of function were not really different between groups, it is possible that the 2 different exercise approaches had different effects on various specific functional tasks. We were curious whether the traditional impairment-based exercise approach might be more likely to improve different specific functional tasks than those that might be improved by the program that included the agility and perturbation training techniques.
We believe examining whether the 2 different exercise approaches may have differing effects on specific functional tasks could have important implications for clinical decision making. If we demonstrated that the different exercise approaches are more likely to improve different functional tasks, we could direct patients to receive the type of exercise program that would be more likely to improve their specific functional problem. This program would result in greater precision in exercise prescription. In contrast, if we were unable to demonstrate that either approach has a significant impact on specific functional tasks, it would mean that alternative strategies may need to be developed to enhance the overall impact of therapeutic exercise on physical function.
The main purpose of this study was to examine the effects of impairment-based exercise therapy programs on the specific functional tasks assessed in self-reported measures of function. Specifically, we compared the probability of improvement in various functional task items on self-reported measures of function between participants in a traditional impairment-based exercise program and those who received agility and perturbation training techniques in combination with the traditional impairment-based exercise program.
Method
Design Overview
The data reported here were taken from a randomized clinical trial of exercise therapy for people with knee OA.15 The current study used baseline and 2-month follow-up data from the randomized trial.
Setting and Participants
The study was conducted in the Department of Physical Therapy at the University of Pittsburgh Medical Center, Center for Sports Medicine, Pittsburgh, Pennsylvania. Participants were recruited from the greater Pittsburgh metropolitan area through physician offices, community flyers, newspaper advertisements, and the University of Pittsburgh Arthritis Institute Registry. All individuals provided written approval from their physician to participate in the study.
Inclusion criteria for the clinical trial were: ≥40 years of age, met the 1986 American College of Rheumatology clinical criteria for knee OA,16 and had grade 2 or greater Kellgren-Lawrence radiographic changes in the tibiofemoral joint.17 Exclusion criteria were presence of any conditions that would place the individual at risk for injury during the exercise training program (eg, required an assistive device for ambulation, had a history of ≥2 falls in the previous year), history of total knee arthroplasty, uncontrolled hypertension, cardiovascular disease, neurologic disorders that affected lower-extremity function (eg, stroke, peripheral neuropathy), and vision problems that affected performance of basic mobility tasks. All individuals signed an informed consent form approved by the University of Pittsburgh Institutional Review Board prior to participation in the study.
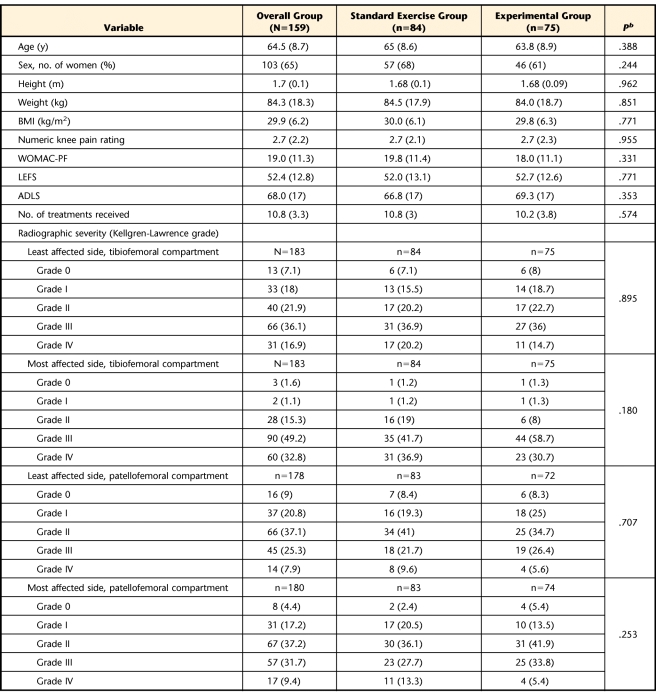
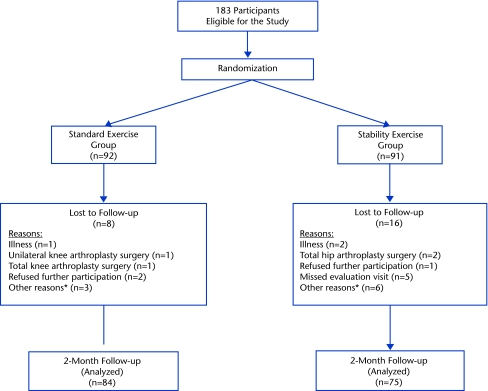
One hundred eighty-three people with knee OA (122 women, 61 men) participated in the study. Table 1 summarizes, by group, the descriptive statistics for the participants included in our analyses. There were no differences between groups on any of the descriptive variables. One hundred fifty-nine of these participants (103 women, 56 men) had complete baseline and 2-month follow-up data and were included in the analysis. Figure 1 is the CONSORT diagram of the study design, listing the reasons why participants were not included in this analysis.
Table 1.
Participant Characteristics by Group at Baselinea
BMI=body mass index, WOMAC-PF=Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale, LEFS=Lower Extremity Functional Scale, ADLS=Activities of Daily Living Scale of the Knee Outcome Survey. All measurements are expressed as mean (SD), except for measurements of radiographic severity, which are expressed as number (%) of participants.
b P value refers to the comparison between standard exercise and experimental groups.
Figure 1.
CONSORT diagram of flow of participants and loss to follow-up during the study. *Other reasons included: loss of contact with the participant, participant did not complete the questionnaires, and participant did not show up for the intervention sessions.
Randomization and Interventions
Participants were randomly assigned to 1 of 2 exercise intervention groups. To ensure a balanced assignment of participants to the 2 intervention groups, block randomization was used. To eliminate any potential biases that could occur from an investigator determining the next intervention assignment in the block sequence, the intervention assignments occurred in random block sizes of 2 and 4. A set of sequentially numbered, sealed envelopes containing participant intervention assignment were created by the study statistician. Following baseline testing, the trial coordinator assigned a participant to a group based on the instructions in the next sealed envelope in sequence. The trial coordinator did not take part in any of the follow-up testing or intervention procedures.
Participants assigned to the standard exercise group received an exercise program that included lower-extremity muscle stretching and strengthening, joint range of motion, and aerobic activities. This program was consistent with current published treatment recommendations for exercise programs for people with knee OA.1–4 Participants who were assigned to the experimental group received the same standard exercise program plus agility and perturbation training. The agility training techniques included side stepping, braiding (lateral stepping combined with forward and backward crossover steps), front crossover steps during forward ambulation, back crossover steps during backward ambulation, shuttle walking (forward and backward walking to and from designated markers), and a multiple change in direction drill in which the therapist provided hand signals at random to prompt the individual to change directions during walking (forward and backward, right and left lateral steps, diagonally backward and forward). The perturbation techniques incorporated the use of foam surfaces, tilt boards, and roller boards to expose the individual's lower limbs and body to potentially destabilizing forces. The participant attempted to maintain balance and control over the exercised lower extremity during the perturbations. Detailed descriptions of the exercises are provided in a previous report.18 The intervention was comprised of 12 supervised physical therapy sessions over 6 weeks.
Measures of Function
Data on self-reported physical function were collected at baseline and follow-up using the Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale (WOMAC-PF),19 the Lower Extremity Functional Scale (LEFS),20 and the Activities of Daily Living Scale of the Knee Outcome Survey (ADLS).21 The WOMAC-PF was used as a disease-specific measure of function. The index is designed to reflect the problems experienced by individuals with hip or knee OA.19 It consists of 17 questions about physical function. Individual items are assigned a score between 0 (“no difficulty”) and 4 (“extreme difficulty”). The reliability, validity, and responsiveness of the WOMAC have been established.19,22,23
The LEFS was used as a region-specific measure of lower-extremity function. The LEFS is a questionnaire that queries patients on their ability to perform general activities of daily living, general recreational activities, specific daily physical tasks, and specific recreational or occupational tasks.20 The scale consists of 20 items or functional tasks, and each item is scored between 0 (“extreme difficulty”) and 4 (“no difficulty”). The LEFS has been reported to be a reliable, valid, and responsive instrument for measuring physical function in a sample of people with lower-extremity musculoskeletal pathology that included individuals with knee OA.20
The ADLS is a knee-specific measure of physical function that assesses the effects of knee impairment on activities of daily living. The scale has 14 items that measure the full spectrum of symptoms and functional limitations that a person may experience during activities of daily living as a result of a variety of knee pathologies. Each item is scored on a 6-point scale from 0 (“I am unable to do the activity”) to 5 (“activity is not difficult”). Reliability, validity, and responsiveness of the ADLS were demonstrated in a sample of people with knee pathology that included individuals with knee OA.21
Data Analysis
We calculated the means (±SD) for continuous variables and frequency and percentages for discrete variables. Independent t tests (for continuous variables) and chi-square tests (for nominal variables) were used to determine differences between groups on demographic variables.
To determine the probability of improvement in specific functional tasks following the exercise interventions, only data from participants with complete baseline and 2-month follow-up data sets were used in the analysis. We used the relative position (RP) measure, as described by Svensson and Starmark.24,25 This method makes it possible to identify and measure the level of group change in ordered categorical responses.24,25 Changes in the categorical responses were evaluated by paired comparisons of individual changes in responses between the baseline and posttreatment assessments. The identification of the level of group change is clinically important in rehabilitation, as it reflects the efficacy of a common treatment or intervention program in the study group.26 More detail on the interpretation of the RP measure is provided in the “Results” section.
Role of the Funding Source
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1-R01-AR048760). The views contained in this publication are those of the grantees and do not necessarily reflect those of the funding agency.
Results
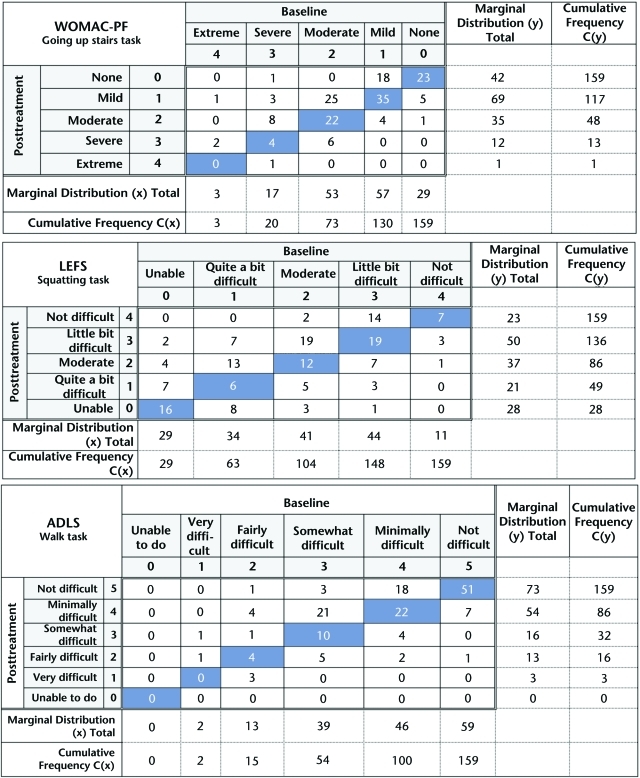
To calculate the RP values for each functional task, a contingency table was created for each functional task in each questionnaire. Although we do not provide the contingency table for each task in each self-report instrument in this report, Figure 2 provides examples of these contingency tables for a single task in each of the 3 self-report measures of function used in this study (ascending stairs task of the WOMAC-PF, squatting task of the LEFS, and walking task of the ADLS). The frequency of paired baseline and posttreatment responses is displayed in the contingency tables. The main diagonal of the contingency tables, which is oriented from the lower-left corner to the upper-right corner (blue cells in Fig. 2), represents the frequency of participants who did not change their ratings from baseline to posttreatment follow-up. The cells located above the main diagonal represent the frequency of participants who improved from baseline to the follow-up period. The cells located below the main diagonal represent the frequency of participants who worsened from baseline to follow-up.
Figure 2.
Representative examples of the contingency tables used for the Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale WOMAC-PF, Lower Extremity Functional Scale (LEFS), and Activities of Daily Living Scale of the Knee Outcome Survey (ADLS) questionnaires.
The formulas for calculating the RP measure are shown in the Appendix. Conceptually, the RP measure is based on the degree of systematic change in the marginal distribution from baseline to follow-up. The marginal distribution for baseline would be the sum of each column (marginal distribution [x] in Fig. 2), and the marginal distribution for follow-up would be the sum of each row (marginal distribution [y] in Fig. 2). When the marginal distributions differ between the baseline and follow-up periods, there is an indication of a systematic change in response between these 2 occasions for the study group.25 For example, if we examine the baseline marginal distribution for the WOMAC-PF ascending stairs task table in Figure 2, we see that 73 participants reported moderate to extreme difficulty for this task at baseline. However, when we examine the posttreatment marginal distribution, we see that only 48 participants reported moderate to extreme difficulty on this task, indicating that more of the group shifted to better scores at the posttreatment period. Examining the marginal distribution allows the calculation of the probability of the responses being shifted toward improvement and the probability of the responses being shifted toward worsening from baseline to the posttreatment follow-up period. The RP measure represents the difference between these 2 probabilities.
The RP value reflects the chances of improvement or worsening in the specific item or task after the treatment. Possible values of probabilities range between 0 and 1. As the RP measure is defined by the difference between 2 probabilities, its possible values range from −1 to 1. According to the configuration of the contingency tables in Figure 2, positive RP values would reflect a shift in the responses in the direction of improvement in function, and negative RP values would reflect a shift in the responses in the direction of a decline in function. In other words, negative values reflect the probability of getting worse in function, and positive values reflect the probability of getting better in function. Although no cutoff RP values have been reported for good, moderate, and poor effects, intuitively we would want the minimum responses to treatment to be above .50. An RP value greater than .50 would indicate that a person has a greater than 50% chance of improving with the treatment compared with getting worse with the treatment.
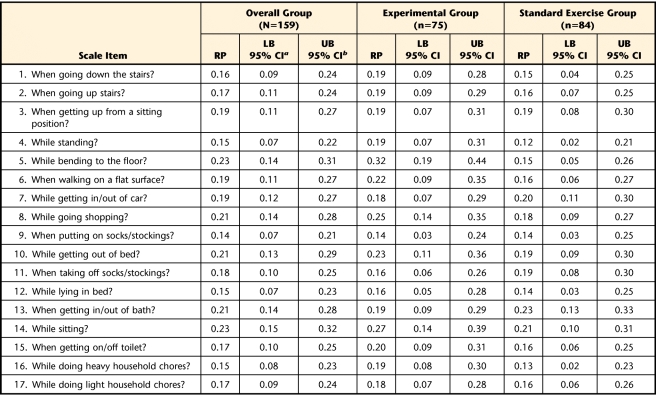
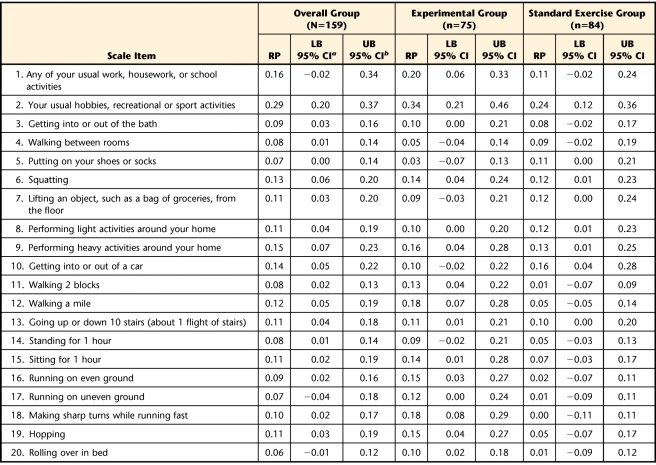
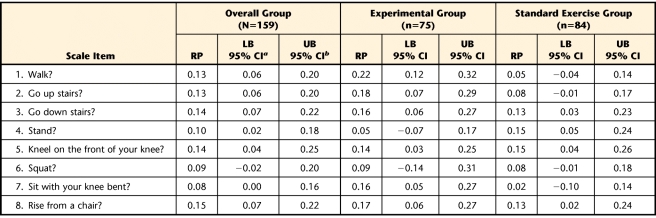
The calculated RP values for the change in item scores following the exercise intervention periods and respective 95% CI for the entire group of participants and for the standard exercise and experimental groups are listed according to each questionnaire in Tables 2, 3, and 4. In summary, for the overall group, the probability of an improvement in performance of specific functional tasks after treatment was never greater than 23% for the WOMAC-PF items, 29% for the LEFS items, and 15% for the ADLS items. For the experimental group, the probability of an improvement in performance of specific functional tasks after treatment was never greater than 32% for the WOMAC-PF items, 34% for the LEFS items, and 22% for the ADLS items. For the standard exercise group, chances of improvement in performance of specific functional tasks after treatment were no greater than 23% for the WOMAC-PF items, 24% for the LEFS items, and 15% for the ADLS items.
Table 2.
Relative Position (RP) Values for Items of the Western Ontario and McMasters University Osteoarthritis Index Physical Function Subscale
a LB 95% CI=lower bound of the 95% confidence interval.
b UB 95% CI=upper bound of the 95% confidence interval.
Table 3.
Relative Position (RP) Values for Items of the Lower Extremity Function Scale
a LB 95% CI=lower bound of the 95% confidence interval.
b UB 95% CI=upper bound of the 95% confidence interval.
Table 4.
Relative Position (RP) Values for Items of the Activities of Daily Living Scale of the Knee Outcome Survey
a LB 95% CI=lower bound of the 95% confidence interval.
b UB 95% CI=upper bound of the 95% confidence interval.
Discussion
The RP analyses indicated that the exercise interventions seemed to have a relatively low impact at best on improvement of performance of specific functional tasks. Across all 3 functional outcome measurements, the probabilities of improving ranged from 0% to slightly higher than 30% at best. There were a few activities where it appears that the experimental group may have had more improvement than the standard exercise group. In these instances, the RP value for the experimental group exceeded the upper limit of the RP 95% CI for the standard exercise group. Examples include bending to the floor (Tab. 2); walking, walking 2 blocks, or walking a mile (Tabs. 3 and 4); running on even or uneven surfaces or making sharp turns while running (Tab. 3); and ascending stairs or sitting with the knee bent (Tab. 4). However, the probability of improvement for these tasks in the experimental group ranged only from 12% to 32%. In our opinion, these values do not indicate a strong probability of improvement with the experimental exercise approach.
We believe that the results of our RP analyses indicate that an impairment-based exercise approach may not be enough to maximize overall improvement in physical function for people with knee OA. Although such programs can lessen the physical impairments associated with knee OA, they may not be sufficient to fully influence the performance of basic functional tasks, such as walking, or more complex tasks, such as participating in social role activities (work, recreation). Functional tasks such as rising from a seated position, stair climbing, or picking up objects from the floor can be considered complex physical tasks, where, in addition to minimum requirements of strength, joint range of motion, and endurance, other factors such as cognitive, perceptual, and motor skills interact to achieve best performance. Therefore, an impairment-based approach to therapeutic exercise may not be sufficient to address all factors required for successful performance of a given functional task. It may be that functional task-specific activities need to be incorporated into therapeutic exercise programs so that overall improvement in physical function can be maximized.
Task-specific exercise is not a new concept. There have been some promising reports in the literature that this approach can be beneficial in improving functional task performance in elderly people.27,28 Alexander et al27 described a task-specific training program for elderly people living in congregate housing that focused on rising from a bed and rising from a chair. The program consisted of breaking the functional tasks into components that were practiced by the participants. The difficulty of the training was altered by changing chair and bed heights, adding resistance to the performance of each component, practicing with and without the use of hands, or emphasizing greater speed of performance. In addition, impairments thought to influence performance of these tasks (trunk, hip, knee, and ankle mobility) were addressed with impairment-based exercises. The control group received a flexibility exercise program. Participants in the task-specific training program exhibited improvements in task performance ranging from 11% to 20% over those in the control group after 12 weeks of treatment. De Vreede et al28 also reported that a similar program was superior to resistance training in improving functional performance in elderly women.
We are not aware of any task-specific exercise programs that have been developed or reported specifically for people with knee OA. It would seem, based on our results and the results of Alexander et al27 and de Vreede et al,28 that such an approach may be worth pursuing for people with knee OA. A task-specific approach would provide patients with the opportunity to practice and learn problem-solving skills for the task that is giving them the most difficulty. It also might provide the therapist with a better evaluation tool to determine the types of impairments or physical deficits that might be influencing performance of the problematic task. This information, in turn, could help therapists to better design impairment-based exercise components in the rehabilitation program.
If a task-specific exercise approach is to be developed specifically for people with knee OA, it would be important to know which functional tasks are most problematic for those with knee OA so that the task-specific programs are designed to target these tasks. Guccione et al11 and Dillon et al12 reported that rising up from a chair, ascending and descending stairs, bending to the floor, kneeling, squatting, performing heavy household chores, and walking a mile were reported as most problematic in their cohorts of individuals with knee OA. It would seem reasonable that these tasks should be targeted in developing task-specific training programs for people with knee OA.
One potential limitation of our results is that our sample may have been less disabled than many people with knee OA because we excluded people who required assistive devices or were at risk for falls. It is likely that a significant proportion of participants did not have difficulty in performing many of the tasks prior to receiving treatment and, therefore, may not have had much room for improvement. This potential limitation could artificially lower the probability of improvement with impairment-based exercise approaches. To explore this possibility, in post hoc analyses, we recalculated RP values using only those participants who reported moderate to severe difficulty on the specific functional tasks known to be difficult for people with knee OA, as reported by Guccione et al11 and Dillon et al.12 The results of this post hoc analysis are reported in Table 5.
Table 5.
Relative Position (RP) Values for Participants With Moderate or Worse Difficulty in Performing Specific Functional Tasksa
WOMAC-PF=Western Ontario and McMaster Universities Osteoarthritis Index physical function subscale, LEFS=Lower Extremity Functional Scale, ADLS=Activities of Daily Living Scale of the Knee Outcome Survey.
b LB 95% CI=lower bound of the 95% confidence interval.
c UB 95% CI=upper bound of the 95% confidence interval.
The data in Table 5 indicate that the RP values improved when we examined only those participants with moderate or worse difficulty, but many of the RP values did not exceed 50% for the entire sample or for each of the exercise groups. For the overall sample, those tasks with RP values exceeding 50% (bending to the floor, getting on and off the toilet, getting into and out of a car, rising from a seated position) had values that remained in the 50% range. The experimental group appeared to do better in improving stair-climbing activities and standing for an hour (the RP value for the experimental group exceeded the upper bound of the 95% CI for the standard exercise group), but, again, most RP values for both groups were less than 50%, and most of those activities with RP values greater than 50% had RP values that remained in the 50% range. The task exhibiting the greatest probability of improvement (“getting into and out of a car” for the experimental group; RP=66%) also had the smallest sample size, which may have affected this result. We believe the bottom-line message here is that there is room for improvement in addressing performance of specific functional tasks, above and beyond what is achieved through impairment-based exercise programs.
Some may question whether 2 months is long enough for an exercise program to have significant effects on pain and function. Recent reports have indicated that people with knee OA have exhibited significant reduction in pain and improvement in function after 8 impairment-based treatment sessions administered in only 4 weeks.7,29 Our program provided 12 sessions in 6 to 8 weeks. The frequency and duration of our program were selected based on customary coverage for rehabilitation of people with knee OA in our area. Therefore, we believe our frequency and duration of treatment were consistent with what has been enough to exhibit relevant changes in the literature and what is relevant to day-to-day clinical practice.
Another limitation of our study is that data were taken from self-report measures of function that capture a person's perception of his or her ability to perform various functional tasks. Therefore, our report does not generalize to actual performance-based measures of these tasks. In our main randomized trial, we did compare the groups on performance of the Get “Up & Go” Test, which is a performance-based measure of function that combines rising from a chair and walking.30 Only the experimental group showed a statistically significant change in the time to complete this test from baseline (0.5 second faster, 95% CI=0.2 to 0.7 seconds), but this change was within the standard error of the measure of the test and may not be clinically relevant. There were no differences between groups in the change from baseline on this test. These results on our performance-based measure seem to indicate a low probability of improvement when using impairment-based exercise programs, corroborating our findings when using self-reported measures. Future studies that examine the effectiveness of task-specific exercise programs in people with knee OA probably should incorporate a combination of performance-based and self-report measures of function to obtain a more comprehensive assessment of treatment outcome.
Conclusion
The probability of self-reported improvement in a variety of specific functional tasks appears to be low following the impairment-based exercise programs described in this study. Although the probability of success improved when only participants with moderate to severe difficulty performing specific functional tasks were considered in the analysis, the results indicate the responses to training are still limited. The use of task-specific training activities in exercise programs for people with knee OA needs to be explored.
Appendix.
Appendix.
Formulas for Calculating the Measurements of Group Change: the Relative Position (RP) According to Svensson and Starmark25
Footnotes
All authors provided concept/idea/research design, writing, and data analysis. Mr Teixeira and Dr Piva provided data collection. Dr Piva and Dr Fitzgerald provided project management. Dr Fitzgerald provided fund procurement and facilities/equipment. Mr Teixeira provided clerical support. Dr Piva provided consultation (including review of manuscript before submission).
This study was approved by the University of Pittsburgh Institutional Review Board.
This research, in part, was presented at the Association for Rheumatological Health Professions Conference; October 17–21, 2009; Philadelphia, Pennsylvania.
This study was supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (grant 1-R01-AR048760). The views contained in this publication are those of the grantees and do not necessarily reflect those of the funding agency.
ClinicalTrials.gov registration: NCT00078624.
References
- 1. Zhang W, Moskowitz RW, Nuki G, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16:137–162 [DOI] [PubMed] [Google Scholar]
- 2. Ottawa Panel Evidence-Based Clinical Practice Guidelines for Therapeutic Exercises and Manual Therapy in the Management of Osteoarthritis. Phys Ther. 2005;85:907–971 [PubMed] [Google Scholar]
- 3. Jordan KM, Arden N, Doherty M, et al. EULAR Recommendations 2003; an evidence-based approach to the management of knee osteoarthritis: report of a task force of the Standing Committee for International Clinical Studies Including Therapeutic Trials. Ann Rheum Dis. 2003;62:1145–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. American College of Rheumatology Subcommittee on Osteoarthritis Guidelines Recommendations for the medical management of osteoarthritis of the hip and knee: 2000 update. Arthritis Rheum. 2000;43:1905–1915 [DOI] [PubMed] [Google Scholar]
- 5. Rogind H, Bibow-Nielsen B, Jensen B, et al. The effects of a physical training program on patients with osteoarthritis of the knees. Arch Phys Med Rehabil. 1998;79:1421–1427 [DOI] [PubMed] [Google Scholar]
- 6. Baker KR, Nelson ME, Felson DT, et al. The efficacy of home-based progressive strength training in older adults with knee osteoarthritis: a randomized controlled trial. J Rheumatol. 2001;28:1655–1665 [PubMed] [Google Scholar]
- 7. Deyle GD, Allison SC, Matekel RL, et al. Physical therapy treatment effectiveness for osteoarthritis of the knee: a randomized comparison of supervised clinical exercise and manual therapy procedures versus a home exercise program. Phys Ther. 2005;85:1301–1317 [PubMed] [Google Scholar]
- 8. O'Reilly SC, Muir KR, Doherty M. Effectiveness of home exercise on pain and disability from osteoarthritis of the knee: a randomised clinical trial. Ann Rheum Dis. 1999;58:15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fransen M, McConnell S. Exercise for osteoarthritis of the knee. Cochrane Database Syst Rev. 2008;4:CD004376 [DOI] [PubMed] [Google Scholar]
- 10. Pisters MF, Veenhof C, van Meeteren NL, et al. Long-term effectiveness of exercise therapy in patients with osteoarthritis of the hip or knee: a systematic review. Arthritis Rheum. 2007;57:1245–1253 [DOI] [PubMed] [Google Scholar]
- 11. Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Pub Health. 1994;84:351–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. J Rheumatol. 2006;33:2271–2279 [PubMed] [Google Scholar]
- 13. Fitzgerald GK, Childs JD, Ridge TM, Irrgang JJ. Agility and perturbation training for a physically active individual with knee osteoarthritis. Phys Ther. 2002;82:372–382 [PubMed] [Google Scholar]
- 14. Diracoglu D, Aydin R, Baskent A, Celik A. Effects of kinesthesia and balance exercises in knee osteoarthritis. J Clin Rheumatol. 2005;11:303–310 [DOI] [PubMed] [Google Scholar]
- 15. Fitzgerald GK, Piva SR, Gil AB, et al. Agility and perturbation training techniques in exercise therapy for reducing pain and improving function in people with knee osteoarthritis: a randomized clinical trial. Phys Ther. 2011;91:452–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Altman R, Asch E, Bloch D, et al. Development of criteria for the classification and reporting of osteoarthritis: classification of osteoarthritis of the knee. Arthritis Rheum. 1986;29:1039–1049 [DOI] [PubMed] [Google Scholar]
- 17. Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Scopaz KA, Piva SR, Gil AB, et al. Effect of baseline quadriceps activation on changes in quadriceps strength after exercise therapy in subjects with knee osteoarthritis. Arthritis Rheum. 2009;61:951–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellamy N, Buchanan WW, Goldsmith CH, et al. Validation study of WOMAC: a health status instrument for measuring clincically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–1840 [PubMed] [Google Scholar]
- 20. Binkley JM, Stratford PW, Lott SA, Riddle DL. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. Phys Ther. 1999;79:371–383 [PubMed] [Google Scholar]
- 21. Irrgang JJ, Snyder-Mackler L, Wainner RS, et al. Development of a patient-reported measure of function of the knee. J Bone Joint Surg Am. 1998;80:1132–1145 [DOI] [PubMed] [Google Scholar]
- 22. Bellamy N, Kean WF, Buchanan WW, et al. Double blind randomized controlled trial of sodium meclofenamate (Meclomen) and diclofenac sodium (Voltaren): post validation reapplication of the WOMAC osteoarthritis index. J Rheumatol. 1992;19:153–159 [PubMed] [Google Scholar]
- 23. Hawker G, Melfi C, Paul J, et al. Comparison of a generic (SF-36) and disease-specific (WOMAC) (Western Ontario and McMaster Universities Osteoarthritis Index) instrument in the measurement of outcomes after knee replacement surgery. J Rheumatol. 1995;22:1193–1196 [PubMed] [Google Scholar]
- 24. Svensson E. Ordinal invariant measures for individual and group changes in ordered categorical data. Stat Med. 1998;17:2923–2936 [DOI] [PubMed] [Google Scholar]
- 25. Svensson E, Starmark JE. Evaluation of individual and group changes in social outcome after aneurysmal subarachnoid haemorrhage: a long-term follow-up study. J Rehabil Med. 2002;34:251–259 [DOI] [PubMed] [Google Scholar]
- 26. Engman E, Andersson-Roswall L, Svensson E, Malmgren K. Non-parametric evaluation of memory changes at group and individual level following temporal lobe resection for pharmaco-resistant partial epilepsy. J Clin Exp Neuropsychol. 2004;26:943–954 [DOI] [PubMed] [Google Scholar]
- 27. Alexander NB, Galecki AT, Grenier ML, et al. Task-specific resistance training to improve the ability of activities of daily living-impaired older adults to rise from a bed and from a chair. J Am Geriatr Soc. 2001;49:1418–1427 [DOI] [PubMed] [Google Scholar]
- 28. de Vreede PL, Samson MM, van Meeteren NL, et al. Functional-task exercise versus resistance strength exercise to improve daily function in older women: a randomized, controlled trial. J Am Geriatr Soc. 2005;53:2–10 [DOI] [PubMed] [Google Scholar]
- 29. Deyle GD, Henderson NE, Matekel RL, et al. Effectiveness of manual physical therapy and exercise in osteoarthritis of the knee: a randomized, controlled trial. Ann Intern Med. 2000;132:173–181 [DOI] [PubMed] [Google Scholar]
- 30. Piva SR, Fitzgerald GK, Irrgang JJ, et al. Get up and go test in patients with knee osteoarthritis. Arch Phys Med Rehabil. 2004;85:284–289 [DOI] [PubMed] [Google Scholar]