Abstract
Background
The benefits of exercise for reducing disability in people with Parkinson disease (PD) are becoming more evident. Optimal benefit, however, requires regular and sustained participation. Factors associated with engaging in regular exercise have received little scientific scrutiny in people with PD.
Objective
The purpose of this study was to explore factors associated with exercise behavior in patients with PD using the International Classification of Functioning, Disability and Health (ICF) as a guiding framework.
Design
This was a cross-sectional study.
Methods
The participants in this study were 260 patients with PD from 4 institutions. Participants were designated as “exercisers” or “nonexercisers” based on responses to the Stages of Readiness to Exercise Questionnaire. Exercise status was validated using the Physical Activity Scale for the Elderly and an activity monitor. Factors potentially associated with exercise behavior included measures of body structure and function, activity, participation, environmental factors, and personal factors. Their relative contributions were analyzed using logistic regression and quantified with odds ratios.
Results
One hundred sixty-four participants (63%) were designated as exercisers. Participants with high self-efficacy were more than twice as likely to engage in regular exercise than those with low self-efficacy (adjusted odds ratio=2.34, 95% confidence interval=1.30–4.23). College educated and older participants also were more likely to exercise. Disabling influences of impairments, activity limitations, and participation restrictions were not associated with exercise behavior.
Limitations
The cross-sectional nature of the study limited the ability to make causal inferences.
Conclusions
Self-efficacy, rather than disability, appears to be strongly associated with whether ambulatory, community-dwelling people with PD exercise regularly. The results of this study suggest that physical therapists should include strategies to increase exercise self-efficacy when designing patient intervention programs for patients with PD.
The benefits of exercise for people with Parkinson disease (PD) are well documented.1,2 People with PD who participate in exercise programs have reported better quality of life and demonstrated better walking ability, balance, strength, flexibility, and cardiovascular fitness compared with those who did not exercise.3 Exercise-based interventions that specifically target disability have increasingly been recognized as an important component of a multidisciplinary approach to long-term management of PD.4–6
Less is known about factors that lead to routine exercise among people with PD. Indeed, few factors associated with exercise behavior in people with PD (eg, social support, desire to control disease progression)7 have been identified. Consequently, physical therapists have limited knowledge of intervention strategies that might be used to help their patients with PD sustain the benefits of a prescribed program of exercise following an episode of care. Complicating the picture is the fact that people with PD, unlike individuals with other types of disabilities or older adults in general, continue to deteriorate in function over the course of the disease.8,9
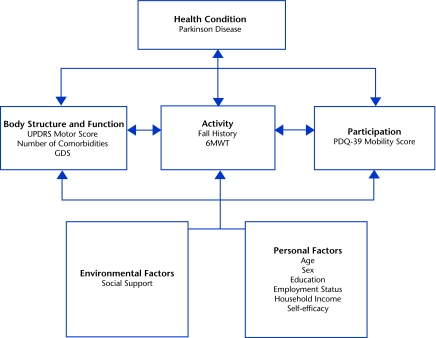
The International Classification of Functioning, Disability and Health (ICF) model10 provides a useful framework for organizing factors associated with exercise behavior (Figure). The model portrays human functioning and disability in 3 domains (ie, body functions and structures, activity, and participation) as the product of a dynamic interaction of health conditions, personal factors, and environmental factors.11 When viewed broadly in the participation domain, exercise behavior can be understood as a life situation in which an individual seeks to preserve, promote, or improve his or her personal health through structured physical activity. Likewise, using the ICF framework as a guide, previously reported determinants of physical activity among individuals with disabilities and older adults who were healthy can be categorized as impairments of body functions and structures (eg, weakness, pain), environmental factors (eg, transportation, cost, accessibility, social support), and personal factors (eg, motivation, exercise knowledge, self-efficacy).12–19 Self-efficacy, in the context of exercise behavior, is defined specifically as a person's belief in his or her capabilities to overcome personal, social, and environmental barriers to exercising.20
Figure.
Potential determinants of exercise behavior in people with Parkinson disease are categorized using the International Classification of Functioning, Disability and Health framework. UPDRS=Unified Parkinson Disease Rating Scale, GDS=Geriatric Depression Scale, 6MWT=Six-Minute Walk Test, PDQ-39=Parkinson's Disease Questionnaire-39.
The purpose of this study was to examine factors that may be associated with exercise behavior in a relatively large cohort of ambulatory people with PD. The study was part of a 2-year multicenter investigation designed to examine the development of disability in these individuals.21 Using the ICF model as a guiding framework, we were interested specifically in comparing the relative contributions of factors, categorized according to body function and structure, activity, participation, and contextual domains, with whether participants engaged in regular exercise. Our goal was to identify potentially modifiable factors, thereby helping to guide clinicians in the development of sustainable interventions for the growing population of people with PD. Based on the progressive nature of this disease as well as our understanding of the impairments, activity limitations, and participation restrictions common among people with PD, we hypothesized that exercise behavior among study participants would be associated with disability level and, to a lesser extent, with personal and environmental factors.
Method
Study Design and Sample
We conducted a cross-sectional analysis of baseline data collected from 260 people with PD who were participating in a parent longitudinal investigation. Participants were recruited from movement disorders clinics and local support groups at Boston University, the University of Utah, Washington University in St Louis, Missouri, and the University of Alabama at Birmingham. Inclusion criteria were: a diagnosis of idiopathic PD according to the UK Brain Bank Criteria,22 classification at modified Hoehn and Yahr (H&Y) stages 1 through 4,23 age ≥40 years, living in the community (not institutionalized), able to attend assessment sessions, and provide consent. Participants were excluded if they had a diagnosis of atypical parkinsonism, were classified at H&Y stage 5, or had previous surgical management of their PD. The institutional review board at each institution approved the study protocol. All participants provided informed consent. Participants were examined in an outpatient clinic between July 2009 and July 2010 over a 2½-hour period and were tested while on medication. All testers were provided with a standard operating procedures manual and an instructional video that described the protocol for administering and scoring each test on 2 patients with PD. Each evaluator rated both patients on 2 occasions separated by 1 week. Within-site and between-sites coefficients of variation were calculated for all measures and ranged from 0.08% (9-hole peg test) to 5% (Berg Balance Scale).
Outcome Variable
Regular exercise was determined using the Stages of Readiness to Exercise Questionnaire.24 This instrument contains 5 statements describing level of involvement in exercise, and participants were instructed to mark one statement that best described their exercise behavior. Participants were classified as “exercisers” if they endorsed the statement that described exercising at least 3 days per week for 20 minutes on a regular basis, either within the previous 6 months or for longer than 6 months. “Nonexercisers” reported not engaging in exercise on a regular basis or not engaging in exercise at all.
To validate the exercise behavior designation, we analyzed 2 sets of physical activity data: survey data collected using the Physical Activity Scale for the Elderly (PASE) and performance data collected with the StepWatch 3 Activity Monitor (SAM, Orthocare Innovations LLC, Mountlake Terrace, Washington). The PASE is a validated self-administered survey instrument that measures physical activity levels in individuals aged 65 years or older.25–27 It consists of 10 questions regarding the frequency and duration of leisure activity (eg, sports, jogging, swimming, strengthening and endurance exercise), household activity, and work-related activity during the previous 7-day period. The total PASE score is computed by: (1) multiplying either the time spent in each activity (hours per week) or participation (ie, yes/no) in an activity by empirically derived item weights and (2) summing over all activities.
The StepWatch 3 Activity Monitor is an unobtrusive, user-worn device that directly captures free-living ambulatory activity. It is the size of a pager, weighs 38 g, is attached at the ankle using self-adhesive closures, and requires no maintenance by the user. It uses a combination of acceleration, position, and timing to detect strides taken by the leg of attachment. Data subsequently are downloaded to a personal computer using the manufacturer's software. The monitor has good test-retest reliability (r=.84) and 96% accuracy with older adults and can accurately quantify steps in populations characterized by slow or shuffling gait.28–31
The SAM data were collected from a subset of study participants (n=100) based primarily on the availability of monitors at the Boston University, University of Utah, and University of Alabama at Birmingham sites. Monitors were not used at the Washington University site. Participants selected to wear a monitor were instructed to wear it during customary activity, including exercise, 24 hours per day for 7 consecutive days, except when bathing, showering, or swimming. The decision to give an individual participant a monitor was made by study personnel according to: (1) availability of a monitor that day, (2) the participant's willingness to wear a monitor, and (3) the presence of cognitive or integumentary impairments that might interfere with the wearing protocol.
Using manufacturer software, study personnel configured monitors to record stride counts in 1-minute intervals, such that each 24-hour period produced a time series of 1,440 values. Optimal accuracy was verified during the first minutes of recording by comparing monitor step counts, identified via a flashing indicator light, with visual observation. During data processing, stride counts were doubled to reflect steps accumulated by both legs. Overall activity was calculated as total number of steps per day.
Independent Variables
Measures quantifying potential factors associated with exercise behavior were selected based on their common use in PD clinical settings, their strong psychometric properties, and their representation of relevant ICF domains shown to be related to exercise adherence in previous studies23,32–34 (Figure).
Body Structure and Function
Unified Parkinson Disease Rating Scale.
The Movement Disorder Society (MDS) revised version of the Unified Parkinson Disease Rating Scale (UPDRS) motor examination (part III) was administered by trained evaluators to quantify disease severity. Validity and high internal consistency of the UPDRS have been demonstrated previously.35 Scores can range from 0 to 132; higher scores designate relatively greater impairment related to bradykinesia, tremor, rigidity, freezing, and postural control. For our analysis, we dichotomized the sample into “low-severity” (ie, score <40) and “high-severity” (ie, score ≥40) groups. This cutpoint was chosen because scores over 40 typically are associated with higher disease severity.36 Participants classified in H&Y stage 4 had UPDRS motor scores that exceeded 40. Sixty-eight percent of our sample had scores below 40, indicating lower disease severity, whereas 32% had scores of 40 or higher, indicating higher disease severity.
Comorbidities.
Participants were asked whether they had the following conditions (yes/no): cardiac disease, peripheral vascular disease, cerebrovascular disease, pulmonary disease, cancer, diabetes, nephropathy, neuropathy, diseases affecting vision, arthritis, or Alzheimer disease or other forms of dementia. The number of endorsed comorbidities was summed for use in subsequent analyses. The sum did not include a diagnosis of PD.
Geriatric Depression Scale.
Participants were screened for depressive symptoms using the Geriatric Depression Scale (GDS).32,37,38 The GDS is a self-report instrument in which participants respond “yes” or “no” to 30 questions, for a possible score of 0 to 30. Scores ranging from 0 to 9 generally are considered normal, scores from 10 to 19 suggest mild depression, and scores from 20 to 30 suggest severe depression. We treated GDS score as a continuous variable.
Activity
Six-Minute Walk Test.
The Six-Minute Walk Test (6MWT) was used to measure the distance participants could walk in 6 minutes. Participants were instructed to “cover as much ground as possible.” Validity and high test-retest reliability have been demonstrated for the 6MWT in patients with cardiopulmonary disease and in patients with neurological diseases, including those with PD.33,39,40 We treated distance walked as a continuous variable.
Fall history.
Participants were asked the number of times they had fallen within the previous 6 months. A fall was defined as an unexpected event where the person inadvertently came to rest on the ground.41 Participants were classified as “fallers” if they reported falling one or more times within the 6-month period or as “nonfallers” if they reported no falls during this period. Fall status was treated as a dichotomous variable.
Participation
The Parkinson's Disease Questionnaire-39 (PDQ-39) is a self-administered instrument made up of 8 domains.34,42 We used the mobility domain to capture participation in life situations related to physical mobility. This subscale contains 10 items related to the frequency of mobility problems or difficulty encountered while engaging in chores and leisure activities around the household and in the community. Each item is rated using a 5-point Likert scale containing response categories ranging from “never” to “always.” Total scores are scaled from 0 to 100; higher scores reflect more frequent mobility problems and, therefore, a lower health-related quality of life.43 Adequate internal consistency, test-retest reliability, and responsiveness of the mobility domain score have been demonstrated.44,45 We treated the PDQ-39 mobility score as a continuous variable.
Personal and Environmental Factors
Demographic data were collected by self-report and included age (in years), sex, ethnicity (white or nonwhite), educational level (college graduate or higher versus less than a bachelor's degree), employment status (working full-time or part-time versus not working), household income (6 categories listed in increments of $10,000 and ranging from $0–10,000 to $70,000 and over), and social support (living alone or not living alone).
We used the Self-Efficacy for Exercise (SEE) scale to capture participants' confidence in their ability to continue exercising in the face of barriers to exercise. The SEE consists of 9 items describing potential barriers to participation in exercise (eg, “too busy with other activities,” “did not enjoy exercise,” “felt pain with exercise,” “bored by the exercise”). For each item, participants circled a number from 0 (“not confident”) to 10 (“very confident”) that best described their belief that they could exercise 3 times a week for 20 minutes. Reliability, validity, and internal consistency (0.92) have been established for the SEE.46 We dichotomized the sample into participants with “high self-efficacy” (total score ≥5) or those with “low self-efficacy” (total score <5). Sixty-six percent of the sample had high self-efficacy, and 34 percent had low self-efficacy.
Statistical Analysis
Descriptive statistics.
The means and standard deviations of continuous variables and frequencies (ie, percentage of occurrence) of categorical variables were calculated to describe characteristics for the total sample and separately for exercisers and nonexercisers. Two-tailed independent t tests or chi-square tests, as appropriate, were used to assess differences between the 2 groups.
Validation of exercise behavior designation.
To evaluate the validity of the exercise outcome variable, we performed t tests to compare means (SD) between exercisers and nonexercisers for variables related to exercise activity: total PASE score, the individual score for each of the 10 PASE items, and the mean daily number of steps calculated from SAM data.
Determinants of exercise behavior.
We first conducted a series of univariate logistic regression analyses to investigate the contribution of each independent variable to exercise behavior. We subsequently conducted a multivariate logistic regression to assess the association between each independent variable and exercise behavior, controlling for the presence of confounders and other important variables. In the multivariate regression, independent variables were entered systematically in 5 separate blocks according to the domains in the ICF model. Body structure and function domain measures were entered first (block 1), the activity domain measures were entered next (block 2), followed by participation domain measures (block 3), environmental factors (block 4), and personal factors (block 5). Odds ratios (ORs) and 95% confidence intervals (CIs) were calculated for each independent variable.
The baseline multivariate model included all variables that had been evaluated in the univariate analyses. We then used a manual backward selection process to systematically remove those factors that were not statistically significant (P≤.10). We excluded the covariable that contributed the least to the model (highest P values) to arrive at the most parsimonious model. We retained the UPDRS motor score to determine the additional influence of high self-efficacy on exercise behavior, adjusting for disease severity. Study data were managed using Research Electronic Data Capture (REDCap) tools hosted at the University of Utah.47 All data were analyzed using the SPSS statistical software program, version 16.0 (SPSS Inc, Chicago, Illinois).
Role of the Funding Source
Funding for this project was provided by the Davis Phinney Foundation, the Parkinson Disease Foundation, and an NIH K12 Building Interdisciplinary Research in Women's Health grant (HD43444).
Results
Sample Characteristics
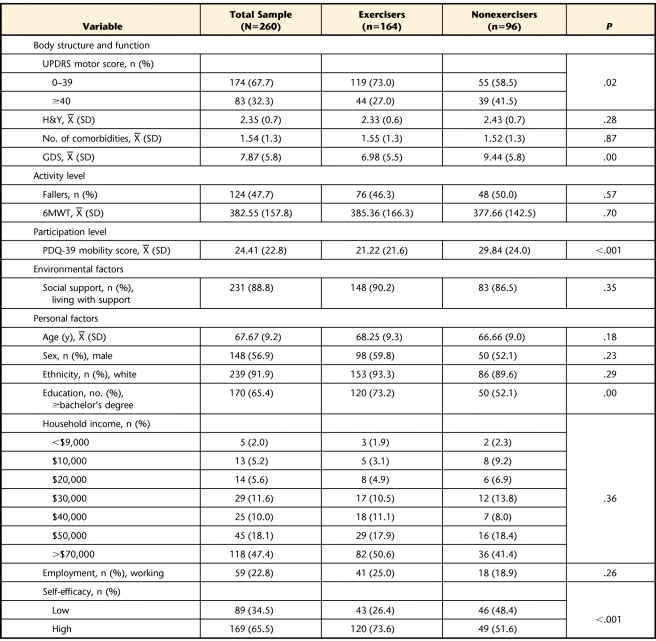
Sample characteristics, organized by ICF categories, are presented in Table 1. Measures in the body structure and function domain revealed that most participants had mild to moderate disease severity: 7.7% were categorized into H&Y stages 1 and 1.5, 66.4% into stages 2 and 2.5, 20.1% into stage 3, and 5.8% into stage 4, and 67.7% of the sample had UPDRS motor scores under 40. Seventy-eight percent of the participants reported only 1 or 2 non-PD comorbidities. Symptoms of clinical depression were relatively few (mean GDS score=7.9, SD=5.8). Measures from the activity domain revealed that 47.7% of the participants were fallers reporting between 1 and 4 falls (mean=1.68, SD=0.71) over the 6-month period. The 6MWT scores ranged from 29 to 744 m, indicating a wide range of limitation. In the participation domain, only 14% of PDQ-39 mobility scores were higher than 50, indicating that most participants had mild to moderate problems participating in mobility-related life situations in their home and community. Most participants (88.8%) reported having social support in the home. With regard to personal factors, the mean age of the sample was 67.7 (years (SD=9.2), and most participants were men (56.9%), white (91.9%), college educated (65.4%), and not working (77.2%). About two thirds of the sample reported high exercise self-efficacy (65.5%).
Table 1.
Participant Characteristicsa
UPDRS=Unified Parkinson Disease Rating Scale, H&Y=Hoehn and Yahr stage, GDS=Geriatric Depression Scale, 6MWT=Six-Minute Walk Test, PDQ-39=Parkinson's Disease Questionnaire-39.
Validation of Exercise Status
A total of 164 participants (63%) were classified as exercisers using the Stages of Readiness to Exercise Questionnaire. Exercise behavior determined by the questionnaire was validated using PASE and SAM data. Total PASE scores were significantly higher (P=.02) among exercisers (mean score=142.6, SD=79.4) than among nonexercisers (mean score=118.9, SD=85.7). An item analysis revealed that exercisers reported significantly greater frequency and amount of time spent in strenuous sports and in strength and endurance exercises than nonexercisers (P<.001). The SAM data from a subset of 100 participants revealed that exercisers (n=73) accumulated significantly more (P=.02) steps per day on average (mean=9,202, SD=4,096) than nonexercisers (n=27) (mean=7,360, SD=3,114).
Comparison of Exercisers and Nonexercisers
Exercisers had significantly lower UPDRS motor scores than nonexercisers, indicating a lower severity of motor impairments (Tab. 1). Exercisers were significantly less depressed, had significantly fewer restrictions in participation, had higher self-efficacy, and were more highly educated compared with nonexercisers. However, the 2 groups did not differ significantly on number of comorbidities, distance walked, number of falls, or other personal or environmental characteristics.
Factors Associated With Exercise Behavior
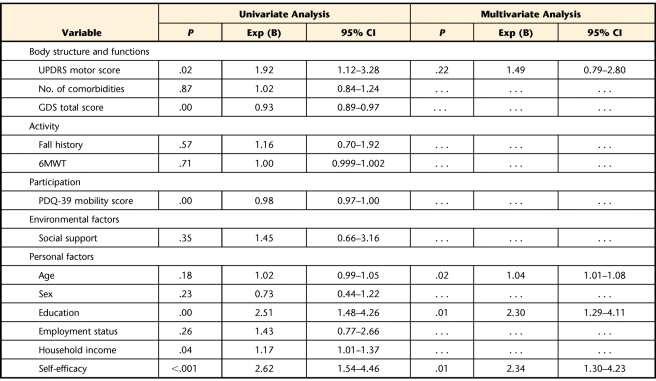
Factors from the body structure and function, participation, and personal factor domains were significantly associated with engaging in regular exercise (Tab. 2). These factors included lower UPDRS score (ie, less severe motor impairment), lower GDS score (ie, fewer depressive symptoms), lower PDQ-39 mobility score (ie, fewer participation restrictions), higher SEE score (ie, higher self-efficacy), and higher education and income levels. No factors in the activity or environmental domains were associated with exercise behavior.
Table 2.
Factors Associated With Exercise Behaviora
UPDRS=Unified Parkinson Disease Rating Scale, GDS=Geriatric Depression Scale, 6MWT=Six-Minute Walk Test, PDQ-39=Parkinson's Disease Questionnaire-39, Exp (B)=odds ratio, CI=confidence interval. Ellipsis indicates variable did not contribute to the multivariate model.
In the baseline multivariate logistic regression that contained all independent variables, only self-efficacy, education, and age were significantly associated with exercise behavior (P≤.05). Following backward elimination procedures, the final model confirmed the results of the initial baseline model, while adjusting for disease severity (Tab. 2). Respondents with high self-efficacy were more than twice as likely to engage in exercise as those with low self-efficacy (adjusted OR=2.34, 95% CI=1.30–4.23). Respondents who were college educated and older also were more likely to exercise. Although lower disease severity (UPDRS motor score) was significantly associated with exercise in univariate analyses, as shown in Table 2, it did not contribute significantly to exercise behavior when adjusted for other significant covariables. We obtained similar results when these analyses were repeated using continuous measures for self-efficacy (adjusted OR=1.31, 95% CI=1.15–1.49) and the UPDRS motor score (adjusted OR=0.99, 95% CI=0.97–1.01). The shared variance between disease severity and self-efficacy likely explains why the effect of disease severity on exercise was diminished in the multivariate analysis. To further investigate whether disease severity level influenced the relationship between self-efficacy and exercise behavior, we performed stratified analyses by UPDRS motor score. The resulting ORs associated with self-efficacy were 2.48 (P=.046) in the high disease severity group and 2.42 (P=.014) in the low disease severity group, confirming that level of disease severity did not modify the association between self-efficacy and exercise.
Discussion
Using the ICF framework as a guide, this cross-sectional study revealed that personal factors rather than impairments, activity limitations, and participation restrictions were most strongly associated with regular exercise among ambulatory people with PD. Specifically, participants with higher exercise self-efficacy were more likely to engage in exercise compared with those with low exercise self-efficacy, regardless of disease severity. In addition, higher education level and older age were associated with being more likely to exercise, although the age difference between exercisers and nonexercisers was not statistically significant.
Based on previous studies,19,48,49 we had hypothesized that self-efficacy would influence exercise behavior; however, it played a stronger role than we had expected. Because the disablement for PD is a progressive, complex experience dominated by motor symptoms, we proposed that exercise would be influenced by disease severity as well as self-efficacy. Indeed, participants in our sample had moderate impairment, reflected by relatively high UPDRS scores, bilateral limb involvement, comorbidities, and a history of recent falling. Despite these widespread limitations, disease severity was not the primary factor influencing participation. Rather, self-efficacy, or beliefs about capabilities to exercise, most influenced participation in exercise. These results are consistent with studies in adults who were healthy and other populations with disabilities.19,48,49 Moreover, our findings are particularly important in a population with a progressive, degenerative neurological disease in which the multisystem impairments can interfere substantially with a patient's ability to participate in exercise.
Self-efficacy generally is defined as people's beliefs about their capabilities to produce designated levels of performance, which determine how they feel, think, motivate themselves, and behave.50 In the context of exercise behavior, self-efficacy typically is defined more specifically as a person's belief in capabilities to overcome personal, social, and environmental barriers to exercising.20 Self-efficacy is one of the most frequently reported personal factors among all studies explicitly using the ICF framework.51 It influences the success of interventions and is frequently targeted in interventions with a person-centered approach to managing health conditions.51 Among other cognitive-behavioral factors, self-efficacy appears as an independent factor in a theoretical model of physical activity behavior that has been proposed to guide health promotion interventions for people with disabilities.52
Although self-efficacy has been recognized as an important determinant of exercise behavior, the extent to which it represents a modifiable target of intervention is unclear. Several recent reviews, for example, indicated that only limited evidence exists in support of interventions designed to improve various aspects of exercise adherence in individuals with musculoskeletal problems.53–55 Cognitive-behavioral training (CBT), although promoted as a potentially useful method for helping patients overcome personal barriers to exercise, has met with limited effectiveness.55–57 According to Rauch et al, “there is no evidence in how physiotherapists manage lack of confidence.”58(p249)
In contrast, several studies suggest that self-management approaches targeting self-efficacy show promise for improving exercise adherence. In a self-management approach, specific strategies to increase self-efficacy are incorporated into the overall management of a patient's condition. These strategies may include weekly action planning and feedback, modeling of behaviors, problem solving by participants, and guided decision making.20 Patients are guided by health care professionals in making choices and achieving success in reaching self-selected goals.59 Among older adults with a variety of chronic illnesses (eg, stroke, arthritis, heart disease), a group education program targeting the development of self-management skills, including maintaining adequate exercise, resulted in more weekly minutes of exercise as well as significantly improved health behaviors, health status, and quality of life and reduced health service utilization compared with a control condition.59
Self-management programs also are effective in patients with PD.60 A 6-week rehabilitation program based on a self-management approach revealed significant improvements in quality of life that were sustained for 6 months. Participants were guided in a problem-solving approach that focused on developing strategies to improve daily function and participation in self-identified roles in society. They were instructed in exercises that were linked to functional goals, thereby establishing relevance of each exercise. Participants identified strategies to overcome barriers to exercise, sustain the exercise program over the long term, resume exercise if lapses occurred, and alter the exercises with changes in health status.
We contend that the physical therapist, although well suited to promote long-term participation in exercise and physical activity, requires a specialized skill set to help patients recognize and overcome personal and environmental exercise barriers.61,62 Too often, neither personal nor environmental factors are adequately addressed in physical therapy intervention programs.63 Physical therapy sessions generally focus on the essential task of instructing patients in exercises with an emphasis on technique and dosing. Our results suggest the added importance of addressing self-efficacy for regularly engaging in and sustaining exercise behavior. Self-management approaches to exercise adherence show promise, not only as a means of improving exercise self-efficacy but also as a hallmark of person-centered care for individuals with chronic health conditions or disability.62
Our study had several limitations. First, participants may have overestimated their level of regular exercise. If participants with higher self-efficacy were more likely than those with low self-efficacy to overestimate their amount of regular exercise, our results regarding self-efficacy and exercise would have been exaggerated. However, the study participants were unaware of the study hypothesis, and it is more likely that overestimation of regular exercise was not associated with self-efficacy. Thus, our findings probably underestimate the true association between self-efficacy and exercise. Second, although 78% of exercisers reported exercising regularly for 6 months or more, we did not control for the possibility that factors associated with exercise may have been influenced by length of time engaged in regular exercise. Third, we did not identify whether participants were prescribed an exercise program, engaged independently in a self-directed program, or participated as part of a group—all of which may have influenced exercise behavior.
The cross-sectional nature of the study also limits our ability to make causal inferences. For example, although participants with greater levels of self-efficacy may have been more likely to engage in exercise, it also is possible that engagement in exercise preceded the higher levels of self-efficacy. Longitudinal studies are necessary to address this issue. Other potential limitations included our decision to dichotomize both the UPDRS motor scores and self-efficacy scores. Because the choice of cutoff scores could influence the results, we conducted additional analyses using continuous measures for both these variables and obtained similar results. Furthermore, generalizability may be limited to mainly white, highly educated people with high socioeconomic status. In addition, 63% of our participants were categorized as exercisers, potentially reflecting a highly motivated sample.
This study also had several strengths. To our knowledge, it is the first study to examine the relative contributions of a broad array of factors that may influence exercise behavior in a relatively large sample of patients with PD. Both self-report (ie, PASE) and physical performance (ie, SAM) data provided validity for the self-reported exercise data. Our results were further confirmed by multiple analyses that were conducted using both continuous and dichotomized variables, as well as analyses stratified by UPDRS score. The ICF provided a useful framework for organizing a diverse list of potential determinants into distinct domains and examining their individual and collective influences on exercise behavior. Finally, the study was conducted across 4 institutions, potentially increasing the generalizability of our findings from a geographical perspective.
Conclusion
Self-efficacy, education level, and, to a lesser extent, age appeared more strongly associated with exercise behavior in ambulatory, community-dwelling people with PD than disease severity, other disabling influences of activity limitations, or participation restrictions. The results highlight the importance of self-efficacy as a potential target for intervention by physical therapists for individuals with PD. Identifying successful methods of sustaining exercise is particularly important given the growing numbers of people living with PD, the long life span realized after diagnosis, and the growing body of literature revealing the benefits of exercise in reducing disability.
Footnotes
Dr Ellis, Dr Cavanaugh, Dr Ford, Dr Foreman, and Dr Dibble provided concept/idea/research design. Dr Ellis, Dr Cavanaugh, and Dr Fredman provided writing. Dr Ellis, Dr Earhart, Dr Ford, Dr Foreman, Dr Boudreau, and Dr Dibble provided data collection. Dr Ellis, Dr Cavanaugh, Dr Ford, Dr Fredman, and Dr Boudreau provided data analysis. Dr Ellis, Dr Ford, Dr Foreman, Dr Boudreau, and Dr Dibble provided project management. Dr Ellis, Dr Earhart, Dr Ford, Dr Foreman, and Dr Dibble provided fund procurement, participants, and facilities/equipment. Dr Ellis provided institutional liaisons. Dr Cavanaugh, Dr Earhart, Dr Ford, Dr Foreman, Dr Fredman, Dr Boudreau, and Dr Dibble provided consultation (including review of manuscript before submission).
Funding for this project was provided by the Davis Phinney Foundation, the Parkinson Disease Foundation, and an NIH K12 Building Interdisciplinary Research in Women's Health grant (HD43444).
References
- 1. Goodwin VA, Richards SH, Taylor RS, et al. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640 [DOI] [PubMed] [Google Scholar]
- 2. de Goede CJ, Keus SH, Kwakkel G, Wagenaar RC. The effects of physical therapy in Parkinson's disease: a research synthesis. Arch Phys Med Rehabil. 2001;82:509–515 [DOI] [PubMed] [Google Scholar]
- 3. Kwakkel G, de Goede CJ, van Wegen E. Impact of physical therapy for Parkinson's disease: a critical review of the literature. Parkinsonism Relat Disord. 2007;13:S478–S487 [DOI] [PubMed] [Google Scholar]
- 4. Horstink M, Tolosa E, Bonuccelli U, et al. Review of the therapeutic management of Parkinson's disease. Report of a joint task force of the European Federation of Neurological Societies and the Movement Disorder Society-European Section, part I: early (uncomplicated) Parkinson's disease. Eur J Neurol. 2006;13:1170–1185 [DOI] [PubMed] [Google Scholar]
- 5. Wade DT, Gage H, Owen C, et al. Multidisciplinary rehabilitation for people with Parkinson's disease: a randomised controlled study. J Neurol Neurosurg Psychiatry. 2003;74:158–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ellis T, Katz DI, White DK, et al. Effectiveness of an Inpatient multidisciplinary rehabilitation program for people with Parkinson disease. Phys Ther. 2008;88:812–819 [DOI] [PubMed] [Google Scholar]
- 7. Ravenek MJ, Schneider MA. Social support for physical activity and perceptions of control in early Parkinson's disease. Disabil Rehabil. 2009;31:1925–1936 [DOI] [PubMed] [Google Scholar]
- 8. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535 [DOI] [PubMed] [Google Scholar]
- 9. Poewe W. The need for neuroprotective therapies in Parkinson's disease: a clinical perspective. Neurology. 2006;66(10 suppl 4):S2–S9 [DOI] [PubMed] [Google Scholar]
- 10. International Classification of Functioning, Disability and Health: ICF. Geneva, Switzerland: World Health Organization; 2001 [Google Scholar]
- 11. Jette AM. Toward a common language for function, disability, and health. Phys Ther. 2006;86:726–734 [PubMed] [Google Scholar]
- 12. Damush TM, Plue L, Bakas T, et al. Barriers and facilitators to exercise among stroke survivors. Rehabil Nurs. 2007;32:253–260, 262 [DOI] [PubMed] [Google Scholar]
- 13. Motl RW, Snook EM, McAuley E, et al. Correlates of physical activity among individuals with multiple sclerosis. Ann Behav Med. 2006;32:154–161 [DOI] [PubMed] [Google Scholar]
- 14. Wilcox S, Der Ananian C, Abbott J, et al. Perceived exercise barriers, enablers, and benefits among exercising and nonexercising adults with arthritis: results from a qualitative study. Arthritis Rheum. 2006;55:616–627 [DOI] [PubMed] [Google Scholar]
- 15. Scelza WM, Kalpakjian CZ, Zemper ED, Tate DG. Perceived barriers to exercise in people with spinal cord injury. Am J Phys Med Rehabil. 2005;84:576–583 [DOI] [PubMed] [Google Scholar]
- 16. Rimmer JH, Riley B, Wang E, et al. Physical activity participation among persons with disabilities: barriers and facilitators. Am J Prev Med. 2004;26:419–425 [DOI] [PubMed] [Google Scholar]
- 17. King AC. Interventions to promote physical activity by older adults. J Gerontol A Biol Sci Med Sci. 2001;56 Spec. No. 2:36–46 [DOI] [PubMed] [Google Scholar]
- 18. Kinne S, Patrick DL, Maher EJ. Correlates of exercise maintenance among people with mobility impairments. Disabil Rehabil. 1999;21:15–22 [DOI] [PubMed] [Google Scholar]
- 19. Jette AM, Rooks D, Lachman M, et al. Home-based resistance training: predictors of participation and adherence. Gerontologist. 1998;38:412–421 [DOI] [PubMed] [Google Scholar]
- 20. Bandura A. Self-Efficacy: The Exercise of Control. New York, NY: Worth Publishers; 1997 [Google Scholar]
- 21. Dibble LE, Cavanaugh JT, Earhart GM, et al. Charting the progression of disability in Parkinson disease: study protocol for a prospective longitudinal cohort study. BMC Neurol. 2010;10:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Litvan I, Bhatia KP, Burn DJ, et al. Movement Disorders Society Scientific Issues Committee report: SIC Task Force appraisal of clinical diagnostic criteria for Parkinsonian disorders. Mov Disord. 2003;18:467–486 [DOI] [PubMed] [Google Scholar]
- 23. Goetz CG, Poewe W, Rascol O, et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: status and recommendations. Mov Disord. 2004;19:1020–1028 [DOI] [PubMed] [Google Scholar]
- 24. Courneya KS. Understanding readiness for regular physical activity in older individuals: an application of the theory of planned behavior. Health Psychol. 1995;14:80–87 [DOI] [PubMed] [Google Scholar]
- 25. Washburn RA, McAuley E, Katula J, et al. The Physical Activity Scale for the Elderly (PASE): evidence for validity. J Clin Epidemiol. 1999;52:643–651 [DOI] [PubMed] [Google Scholar]
- 26. Washburn RA, Smith KW, Jette AM, Janney CA. The Physical Activity Scale for the Elderly (PASE): development and evaluation. J Clin Epidemiol. 1993;46:153–162 [DOI] [PubMed] [Google Scholar]
- 27. Schuit AJ, Schouten EG, Westerterp KR, Saris WH. Validity of the Physical Activity Scale for the Elderly (PASE): according to energy expenditure assessed by the doubly labeled water method. J Clin Epidemiol. 1997;50:541–546 [DOI] [PubMed] [Google Scholar]
- 28. Resnick B, Nahm ES, Orwig D, et al. Measurement of activity in older adults: reliability and validity of the Step Activity Monitor. J Nurs Meas. 2001;9:275–290 [PubMed] [Google Scholar]
- 29. Bergman RJ, Bassett DR, Jr, Muthukrishnan S, Klein DA. Validity of 2 devices for measuring steps taken by older adults in assisted-living facilities. J Phys Act Health. 2008;5(suppl 1):S166–S175 [DOI] [PubMed] [Google Scholar]
- 30. Macko RF, Haeuber E, Shaughnessy M, et al. Microprocessor-based ambulatory activity monitoring in stroke patients. Med Sci Sports Exerc. 2002;34:394–399 [DOI] [PubMed] [Google Scholar]
- 31. Bowden MG, Behrman AL. Step Activity Monitor: accuracy and test-retest reliability in persons with incomplete spinal cord injury. J Rehabil Res Dev. 2007;44:355–362 [DOI] [PubMed] [Google Scholar]
- 32. Rojo A, Aguilar M, Garolera MT, et al. Depression in Parkinson's disease: clinical correlates and outcome. Parkinsonism Relat Disord. 2003;10:23–28 [DOI] [PubMed] [Google Scholar]
- 33. Steffen T, Seney M. Test-retest reliability and minimal detectable change on balance and ambulation tests, the 36-Item Short-Form Health Survey, and the Unified Parkinson Disease Rating Scale in people with parkinsonism. Phys Ther. 2008;88:733–746 [DOI] [PubMed] [Google Scholar]
- 34. Peto V, Jenkinson C, Fitzpatrick R. PDQ-39: a review of the development, validation and application of a Parkinson's disease quality of life questionnaire and its associated measures. J Neurol. 1998;245(suppl 1):S10–S14 [DOI] [PubMed] [Google Scholar]
- 35. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170 [DOI] [PubMed] [Google Scholar]
- 36. Tanji H, Gruber-Baldini AL, Anderson KE, et al. A comparative study of physical performance measures in Parkinson's disease. Mov Disord. 2008;23:1897–1905 [DOI] [PubMed] [Google Scholar]
- 37. Montorio I, Izal M. The Geriatric Depression Scale: a review of its development and utility. Int Psychogeriatr. 1996;8:103–112 [DOI] [PubMed] [Google Scholar]
- 38. Alexandre Tda S, Cordeiro RC, Ramos LR. Factors associated to quality of life in active elderly. Rev Saude Publica. 2009;43:613–621 [DOI] [PubMed] [Google Scholar]
- 39. Fulk GD, Echternach JL, Nof L, O'Sullivan S. Clinometric properties of the six-minute walk test in individuals undergoing rehabilitation poststroke. Physiother Theory Pract. 2008;24:195–204 [DOI] [PubMed] [Google Scholar]
- 40. Solway S, Brooks D, Lacasse Y, Thomas S. A qualitative systematic overview of the measurement properties of functional walk tests used in the cardiorespiratory domain. Chest. 2001;119:256–270 [DOI] [PubMed] [Google Scholar]
- 41. Wood BH, Bilclough JA, Bowron A, Walker RW. Incidence and prediction of falls in Parkinson's disease: a prospective multidisciplinary study. J Neurol Neurosurg Psychiatry. 2002;72:721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Den Oudsten BL, Van Heck GL, De Vries J. The suitability of patient-based measures in the field of Parkinson's disease: a systematic review. Mov Disord. 2007;22:1390–1401 [DOI] [PubMed] [Google Scholar]
- 43. Marinus J, Ramaker C, van Hilten JJ, Stiggelbout AM. Health related quality of life in Parkinson's disease: a systematic review of disease specific instruments. J Neurol Neurosurg Psychiatry. 2002;72:241–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jenkinson C, Fitzpatrick R, Peto V, et al. The Parkinson's Disease Questionnaire (PDQ-39): development and validation of a Parkinson's disease summary index score. Age Ageing. 1997;26:353–357 [DOI] [PubMed] [Google Scholar]
- 45. Peto V, Jenkinson C, Fitzpatrick R, Greenhall R. The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Qual Life Res. 1995;4:241–248 [DOI] [PubMed] [Google Scholar]
- 46. Resnick B, Jenkins LS. Testing the reliability and validity of the Self-Efficacy for Exercise Scale. Nurs Res. 2000;49:154–159 [DOI] [PubMed] [Google Scholar]
- 47. Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Resnick B, Palmer MH, Jenkins LS, Spellbring AM. Path analysis of efficacy expectations and exercise behaviour in older adults. J Adv Nurs. 2000;31:1309–1315 [DOI] [PubMed] [Google Scholar]
- 49. Wade DT. Personal context as a focus for rehabilitation. Clin Rehabil. 2000;14:115–118 [DOI] [PubMed] [Google Scholar]
- 50. Bandura A. Self-efficacy. In: Ramachaudran V, ed. Encyclopedia of Human Behavior. New York, NY: Academic Press; 1994:71–81 [Google Scholar]
- 51. Geyh S, Peter C, Müller R, et al. The personal factors of the International Classification of Functioning, Disability and Health in the literature: a systematic review and content analysis. Disabil Rehabil. 2010;33:1089–1102 [DOI] [PubMed] [Google Scholar]
- 52. van der Ploeg HP, van der Beek AJ, van der Woude LH, van Mechelen W. Physical activity for people with a disability: a conceptual model. Sports Med. 2004;34:639–649 [DOI] [PubMed] [Google Scholar]
- 53. McLean SM, Burton M, Bradley L, Littlewood C. Interventions for enhancing adherence with physiotherapy: a systematic review. Man Ther. 2010;15:514–521 [DOI] [PubMed] [Google Scholar]
- 54. Jordan JL, Holden MA, Mason EE, Foster NE. Interventions to improve adherence to exercise for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2010(1):CD005956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Rhodes RE, Fiala B. Building motivation and sustainability into the prescription and recommendations for physical activity and exercise therapy: the evidence. Physiother Theory Pract. 2009;25:424–441 [DOI] [PubMed] [Google Scholar]
- 56. Woodard CM, Berry MJ. Enhancing adherence to prescribed exercise: structured behavioral interventions in clinical exercise programs. J Cardiopulm Rehabil. 2001;21:201–209 [DOI] [PubMed] [Google Scholar]
- 57. Rundell SD, Davenport TE. Patient education based on principles of cognitive behavioral therapy for a patient with persistent low back pain: a case report. J Orthop Sports Phys Ther. 2010;40:494–501 [DOI] [PubMed] [Google Scholar]
- 58. Rauch A, Kirchberger I, Stucki G, Cieza A. Validation of the Comprehensive ICF Core Set for obstructive pulmonary diseases from the perspective of physiotherapists. Physiother Res Int. 2009;14:242–259 [DOI] [PubMed] [Google Scholar]
- 59. Lorig KR, Sobel DS, Stewart AL, et al. Evidence suggesting that a chronic disease self-management program can improve health status while reducing hospitalization: a randomized trial. Med Care. 1999;37:5–14 [DOI] [PubMed] [Google Scholar]
- 60. Tickle-Degnen L, Ellis T, Saint-Hilaire MH, et al. Self-management rehabilitation and health-related quality of life in Parkinson's disease: a randomized controlled trial. Mov Disord. 2010;25:194–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Shirley D, van der Ploeg HP, Bauman AE. Physical activity promotion in the physical therapy setting: perspectives from practitioners and students. Phys Ther. 2010;90:1311–1322 [DOI] [PubMed] [Google Scholar]
- 62. Bodenheimer T, Lorig K, Holman H, Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288:2469–2475 [DOI] [PubMed] [Google Scholar]
- 63. Scherer MJ, DiCowden MA. Organizing future research and intervention efforts on the impact and effects of gender differences on disability and rehabilitation: the usefulness of the International Classification of Functioning, Disability and Health (ICF). Disabil Rehabil. 2008;30:161–165 [DOI] [PubMed] [Google Scholar]