Abstract
Melanoma with rhabdoid features is an uncommon variant of malignant melanoma. Here, we describe a rare case of primary rhabdoid malignant melanoma. A 54-year-old man presented with a black tumor measuring 3×4 cm on the right forearm. Histologic sections showed a tumor mass with rhabdoid features composed entirely of polygonal neoplastic cells with eccentric nuclei, prominent nucleoli, and large hyaline cytoplasmic inclusions. The tumor cells were immunoreactive with HMB-45, S100, Fontana-Masson silver and vimentin, and negative for smooth muscle actin, CD68, CD34, CD99, synaptophysin, desmin, and PAS. The differential diagnosis for this tumor included malignant peripheral nerve sheath tumor, malignant peripheral neuroectodermal tumor and rhabdomyosarcoma. The patient was treated with a wide excision and a local skin graft. The excised tumor was entirely composed of rhabdoid tumor cells. No recurrence or metastasis was evident 4 months after removal. This article is relevant to rare cases of primary malignant melanomas showing rhabdoid tumor cells over the entire excised lesion.
Keywords: Malignant melanoma, Rhabdoid
INTRODUCTION
The term "rhabdoid tumor" was originally coined by Haas et al.1 in 1981 to describe an aggressive childhood renal tumor, which was believed to represent a rhabdomyosarcomatous variant of nephroblastoma. However, it did not exhibit ultrastructural evidence of rhabdomyoblastic differentiation. Histologically, neoplastic cells were characterized by a rhabdoid appearance with the presence of eccentric nuclei, single prominent nucleoli, and large hyaline cytoplasmic inclusions. Ultrastructurally, the tumor cells had a cytoplasmic paranuclear whorl of intermediate filaments containing an entrapped rough endoplasmic reticulum, mitochondria, and lipids2. Since the original description of malignant rhabdoid tumors of the kidney, extrarenal rhabdoid tumors occurring in various sites such as the brain, spinal dura, lung, liver, colon, esophagus, ovary, uterus, vulva, bladder, skin, and soft tissue have been recognized and reported3.
Rhabdoid melanoma was first described in 1992 by Bittesini et al.4. Since then, out of approximately 40 cases of melanomas with rhabdoid features that have been reported, only five have involved rhabdoid features in primary melanomas. Here, we report for the first time in Korea, the case of a 54-year-old Korean man with a primary rhabdoid melanoma on the right forearm.
CASE REPORT
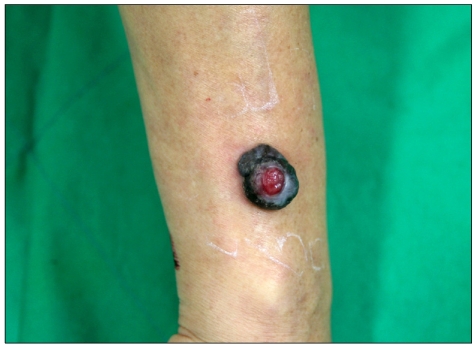
A 54-year-old male presented with a black mass on his right forearm that had first developed 1 month earlier. Cutaneous examination revealed a solitary, well-defined sessile black tumor measuring 3×4 cm with a granulating protrusion in the central area of the right forearm (Fig. 1). The remaining physical examination was unremarkable and the patient had no other significant health problems. Laboratory examinations, including blood and urine tests and chest X-ray, were within normal limits.
Fig. 1.
A solitary, well-defined sessile black tumor measuring 3×4 cm with a granulating protrusion in the central area of the right forearm.
A punch biopsy was performed and histopathology showed an ulcerated tumor nodule containing neoplastic proliferation of polygonal cells with rhabdoid features and scattered melanin pigments (Fig. 2). The tumor was composed entirely of neoplastic cells with eccentric nuclei, a single prominent nucleolus, and large hyaline cytoplasmic inclusions (Fig. 3). Immunohistochemistrically, the neoplastic cells were positive for HMB-45, S100, Fontana-Masson silver and vimentin, and negative for smooth muscle actin, CD68, CD34, CD99, synaptophysin, desmin, and PAS (Fig. 4). Vimentin staining showed diffuse and globular perinuclear accentuations (Fig. 4A). Therefore, we diagnosed the patient with rhabdoid melanoma, and performed PET-CT with no metastases. A wide excision with a 4 cm margin and split thickness skin graft was consequently performed. Histopathologic findings were similar to previous punch biopsy results. The tumor's Breslow thickness measured 9 mm, and it had a Clark level of 4. Four months after tumor removal, neither local recurrence nor metastasis occurred.
Fig. 2.
An ulcerated tumor nodule showed neoplastic proliferation of polygonal cells with rhabdoid features and scattered melanin pigments (H&E, ×40).
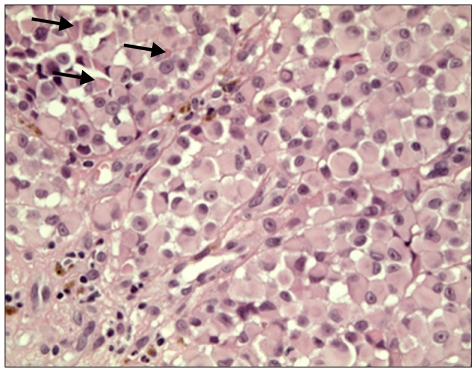
Fig. 3.
Tumor cells exhibited eosinophilic cytoplasm displacing a round eccentric vesicular nucleus with a prominent central nucleolus and a large intracytoplasmic hyalin inclusion (arrows) (H&E, ×400).
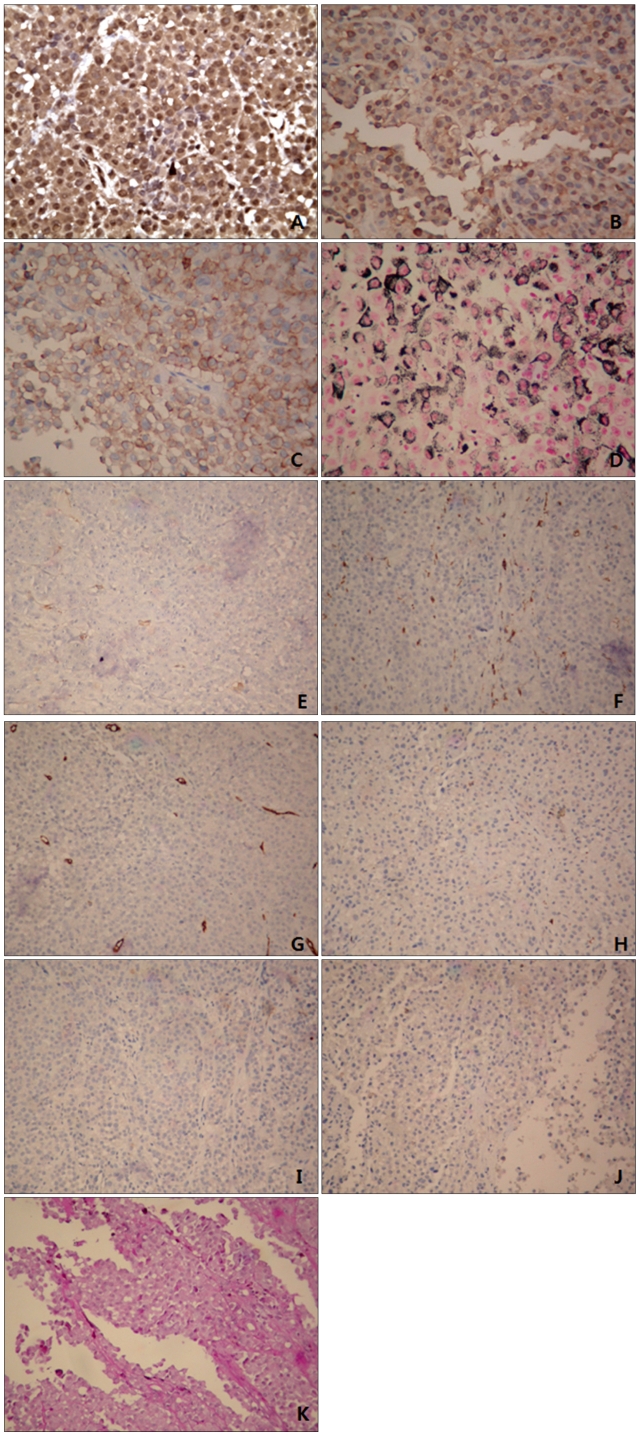
Fig. 4.
Immunohistochemically, the neoplastic cells were positive for vimentin (A), S100 (B), Fontana-Masson silver (C) and HMB-45 (D), and negative for smooth muscle actin (E), CD68 (F), CD34 (G), CD99 (H), synaptophysin (I), desmin (J), and PAS (K). Vimentin staining showed intense, diffuse, and globular perinuclear accentuations (A) (A~D: H&E, ×400, E~K: H&E, ×200).
DISCUSSION
Malignant melanoma is known for its wide range of histological patterns. Melanoma variants that may mimic the morphologic and immunohistochemical features of non-melanocytic neoplasms include balloon cell melanoma, signet-ring cell melanoma, myxoid melanoma, small cell melanoma, and rhabdoid melanoma5. Rhabdoid features are defined by morphologic characteristics such as eccentric nuclei, large nucleoli, and abundant cytoplasm containing hyaline filamentous inclusions, and are shaped like skeletal muscle cells. Rhabdoid melanoma exhibits significant histologic, immunohistochemical, and ultrastructural heterogeneity3.
The extent of rhabdoid change among neoplastic cells varies. In Chang's series6, 48% (15 out of 31) of samples were composed exclusively of rhabdoid cells. In 52% (16 out of 31) of samples, rhabdoid cells constituted less than 25% of the tumor. In our case, the entire excised lesion was composed almost entirely of rhabdoid cells. The results of immunohistochemistry were variable. In a previous case, there was strong staining with S100 and vimentin, but weak staining with neuron-specific enolase and epithelial membrane antigen7. All three of Borek's cases stained with S100 and vimentin, but none of them stained with HMB-45 and desmin8. Our case exhibited positive reactivity to antibodies against S100, HMB-45, Fontana-Masson silver and vimentin, but showed negative staining from smooth muscle actin, CD68, CD34, CD99, synaptophysin, desmin, and PAS (Fig. 4). The pattern of vimentin staining was a diffuse and globular perinuclear accentuation (Fig. 4A). This may be informative in rhabdoid malenoma. In other studies a similar vimentin staining patternwas displayed2,6,9. Further attention to this detail in future reports is required based on case studies to determine whether this type of vimentin staining pattern in rhabdoid malenoma is an unusual finding.
Because of the highly aggressive nature of some extrarenal rhabdoid and renal tumors, it may be expected that rhabdoid melanomas behave similarly. However, based on the cases reviewed here, rhabdoid melanoma appears to behave no more aggressively than conventional melanoma3. Our case showed a distinctively rapid progression within about one month, and has so far not had any metastasis or local recurrence. Since a four-month follow-up period is not enough to assure prognosis, long-term follow-up will be required. To date, the prognosis of malignant rhabdoid melanoma is unknown, primarily because malignant rhabdoid melanoma is rarely occurring and has been reported infrequently. Prognosis of this disease could be better understood through more case reports.
Differential diagnosis of a tumor with a rhabdoid phenotype is high. First, other possible clues to differential diagnosis in the tumor lesion besides rhabdoid appearance are investigated. Then, other phenotypes can be excluded via a broad panel of immunohistochemical stains. Rhabdoid tumors are often positive for vimentin, glial fibrillary acidic protein, desmin, actin, and AE1/AE37,10. Moreover, S100 protein expression is not observed in renal rhabdoid tumors. The malignant peripheral nerve sheath tumor, especially the epithelioid variant, is positive for epithelial membrane antigen and synaptophysin, and is only focally positive for S100 protein. Plasmacytoma or plasmablastic lymphoma and anaplastic large cell lymphoma are likely to be positive for CD138 and CD30, respectively. Rhabdoid morphology has been described in carcinoma, but lack of expression of both high- and low-molecular weight cytokeratins excludes an epithelial neoplasm, as well as epithelioid mesothelioma. Rhabdomyosarcoma may express muscle markers, such as desmin and myoglobin11. Even though the rhabdoid phenotype in malignant melanomas is rarely occurring and reported infrequently, it should be included in the differential diagnosis of neoplasms with rhabdoid morphology11.
Malignant rhabdoid melanoma appears mostly as a recurrent form, and only atypically as a primary lesion. Of the 40 cases of malignant rhabdoid melanomas reported, only five cases showed primary malignant rhabdoid melanomas. The histopathologic finding in most primary malignant rhabdoid melanoma rarely shows the tumor lesion wholly consisting of rhabdoid cells7,8,12,13. However, in this case whole excision of the lesion was composed of tumor cells with rhabdoid features. Our results are meaningful for understanding rare cases of primary malignant melanoma showing rhabdoid featured malignant tumor cells over whole excision lesions.
Footnotes
This study was supported by a grant of the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (No. A101550-1001-0000100).
References
- 1.Haas JE, Palmer NF, Weinberg AG, Beckwith JB. Ultrastructure of malignant rhabdoid tumor of the kidney. A distinctive renal tumor of children. Hum Pathol. 1981;12:646–657. doi: 10.1016/s0046-8177(81)80050-0. [DOI] [PubMed] [Google Scholar]
- 2.Schmidt D, Harms D, Zieger G. Malignant rhabdoid tumor of the kidney. Histopathology, ultrastructure and comments on differential diagnosis. Virchows Arch A Pathol Anat Histopathol. 1982;398:101–108. doi: 10.1007/BF00585617. [DOI] [PubMed] [Google Scholar]
- 3.Gavino AC, Gillies EM. Metastatic rhabdoid melanoma: report of a case with a comparative review of the literature. J Cutan Pathol. 2008;35:337–342. doi: 10.1111/j.1600-0560.2007.00811.x. [DOI] [PubMed] [Google Scholar]
- 4.Bittesini L, Dei Tos AP, Fletcher CD. Metastatic malignant melanoma showing a rhabdoid phenotype: further evidence of a non-specific histological pattern. Histopathology. 1992;20:167–170. doi: 10.1111/j.1365-2559.1992.tb00947.x. [DOI] [PubMed] [Google Scholar]
- 5.Magro CM, Crowson AN, Mihm MC. Unusual variants of malignant melanoma. Mod Pathol. 2006;19(Suppl 2):S41–S70. doi: 10.1038/modpathol.3800516. [DOI] [PubMed] [Google Scholar]
- 6.Chang ES, Wick MR, Swanson PE, Dehner LP. Metastatic malignant melanoma with "rhabdoid" features. Am J Clin Pathol. 1994;102:426–431. doi: 10.1093/ajcp/102.4.426. [DOI] [PubMed] [Google Scholar]
- 7.Parham DM, Weeks DA, Beckwith JB. The clinicopathologic spectrum of putative extrarenal rhabdoid tumors. An analysis of 42 cases studied with immunohistochemistry or electron microscopy. Am J Surg Pathol. 1994;18:1010–1029. doi: 10.1097/00000478-199410000-00005. [DOI] [PubMed] [Google Scholar]
- 8.Borek BT, McKee PH, Freeman JA, Maguire B, Brander WL, Calonje E. Primary malignant melanoma with rhabdoid features: a histologic and immunocytochemical study of three cases. Am J Dermatopathol. 1998;20:123–127. doi: 10.1097/00000372-199804000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Laskin WB, Weiss SW, Bratthauer GL. Epithelioid variant of malignant peripheral nerve sheath tumor (malignant epithelioid schwannoma) Am J Surg Pathol. 1991;15:1136–1145. doi: 10.1097/00000478-199112000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Weeks DA, Beckwith JB, Mierau GW. Rhabdoid tumor. An entity or a phenotype? Arch Pathol Lab Med. 1989;113:113–114. [PubMed] [Google Scholar]
- 11.Gattenlöhner S, Brocker EB, Muller-Hermelink HK. Malignant melanoma with metastatic rhabdomyosarcomatoid transdifferentiation. N Engl J Med. 2008;358:649–650. doi: 10.1056/NEJMc0707079. [DOI] [PubMed] [Google Scholar]
- 12.Tallon B, Bhawan J. Primary rhabdoid melanoma with clonal recurrence. Am J Dermatopathol. 2009;31:200–204. doi: 10.1097/DAD.0b013e3181986d1c. [DOI] [PubMed] [Google Scholar]
- 13.Abbott JJ, Amirkhan RH, Hoang MP. Malignant melanoma with a rhabdoid phenotype: histologic, immunohistochemical, and ultrastructural study of a case and review of the literature. Arch Pathol Lab Med. 2004;128:686–688. doi: 10.5858/2004-128-686-MMWARP. [DOI] [PubMed] [Google Scholar]