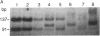Abstract
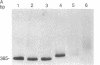
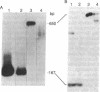
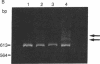
We have developed a method for distinguishing fragments of DNA that contain single-base mismatches from their perfectly paired homologues. Single-stranded regions within a duplex fragment are accessible to 1-cyclohexyl-3-(2-[4-(4-methyl)morpholinyl]ethyl)carbodiimide, which reacts with unpaired guanidylate and thymidylate residues in DNA. Intact linear duplex DNA molecules do not react with carbodiimide, whereas DNA molecules containing single-base mismatches react quantitatively. After carbodiimide reaction, the DNA molecules are electrophoresed in high-percentage polyacrylamide gels so that modified and unmodified fragments can be resolved. Application of this technique should make it possible to locate and purify DNA fragments that exhibit sequence differences from those that do not; these might be used to signal phenotypic variation as well as to diagnose inherited disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aboul-ela F., Koh D., Tinoco I., Jr, Martin F. H. Base-base mismatches. Thermodynamics of double helix formation for dCA3XA3G + dCT3YT3G (X, Y = A,C,G,T). Nucleic Acids Res. 1985 Jul 11;13(13):4811–4824. doi: 10.1093/nar/13.13.4811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown T., Kennard O., Kneale G., Rabinovich D. High-resolution structure of a DNA helix containing mismatched base pairs. Nature. 1985 Jun 13;315(6020):604–606. doi: 10.1038/315604a0. [DOI] [PubMed] [Google Scholar]
- Capon D. J., Chen E. Y., Levinson A. D., Seeburg P. H., Goeddel D. V. Complete nucleotide sequences of the T24 human bladder carcinoma oncogene and its normal homologue. Nature. 1983 Mar 3;302(5903):33–37. doi: 10.1038/302033a0. [DOI] [PubMed] [Google Scholar]
- Fasano O., Aldrich T., Tamanoi F., Taparowsky E., Furth M., Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4008–4012. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S. G., Lerman L. S. DNA fragments differing by single base-pair substitutions are separated in denaturing gradient gels: correspondence with melting theory. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1579–1583. doi: 10.1073/pnas.80.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Ho N. W., Gilham P. T. The reversible chemical modification of uracil, thymine, and guanine nucleotides and the modification of the action of ribonuclease on ribonucleic acid. Biochemistry. 1967 Dec;6(12):3632–3639. doi: 10.1021/bi00864a002. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Kelly J. M., Maden B. E. Chemical modification studies and the secondary structure of HeLa cell 5.8S rRNA. Nucleic Acids Res. 1980 Oct 10;8(19):4521–4534. doi: 10.1093/nar/8.19.4521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd V. J., Wallace R. B., Itakura K., Woo S. L. alpha 1-antitrypsin deficiency detection by direct analysis of the mutation in the gene. Nature. 1983 Jul 21;304(5923):230–234. doi: 10.1038/304230a0. [DOI] [PubMed] [Google Scholar]
- Kowalski D., Kroeker W. D., Laskowski M., Sr Mung bean nuclease I. Physical, chemical, and catalytic properties. Biochemistry. 1976 Oct 5;15(20):4457–4463. doi: 10.1021/bi00665a019. [DOI] [PubMed] [Google Scholar]
- Lebowitz J., Chaudhuri A. K., Gonenne A., Kitos G. Carbodiimide modification of superhelical PM2 DNA: considerations regarding reaction at unpaired bases and the unwinding of superhelical DNA with chemical probes. Nucleic Acids Res. 1977 Jun;4(6):1695–1711. doi: 10.1093/nar/4.6.1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebowitz J., Garon C. G., Chen M. C., Salzman N. P. Chemical modification of simian virus 40 DNA by reaction with a water-soluble carbodiimide. J Virol. 1976 Apr;18(1):205–210. doi: 10.1128/jvi.18.1.205-210.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman L. S., Fischer S. G., Hurley I., Silverstein K., Lumelsky N. Sequence-determined DNA separations. Annu Rev Biophys Bioeng. 1984;13:399–423. doi: 10.1146/annurev.bb.13.060184.002151. [DOI] [PubMed] [Google Scholar]
- Lu P., Cheung S., Arndt K. Possible molecular detent in the DNA structure at regulatory sequences. J Biomol Struct Dyn. 1983 Oct;1(2):509–521. doi: 10.1080/07391102.1983.10507458. [DOI] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Lerman L. S., Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers R. M., Fischer S. G., Maniatis T., Lerman L. S. Modification of the melting properties of duplex DNA by attachment of a GC-rich DNA sequence as determined by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985 May 10;13(9):3111–3129. doi: 10.1093/nar/13.9.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T. E., Rhodes C., Rigby P. W., Berg P. Biochemical method for mapping mutational alterations in DNA with S1 nuclease: the location of deletions and temperature-sensitive mutations in simian virus 40. Proc Natl Acad Sci U S A. 1975 Mar;72(3):989–993. doi: 10.1073/pnas.72.3.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Schold M., Johnson M. J., Dembek P., Itakura K. Oligonucleotide directed mutagenesis of the human beta-globin gene: a general method for producing specific point mutations in cloned DNA. Nucleic Acids Res. 1981 Aug 11;9(15):3647–3656. doi: 10.1093/nar/9.15.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]