Abstract
Steatocystoma multiplex (SM) is an uncommon disorder of the pilosebaceous unit characterized by the development of numerous sebum-containing dermal cysts which rarely involves the scalp. Here, we report a case of a 50-year-old man with multiple cystic nodules and alopecic patches on his scalp. On histopathological examination, the folded cyst was found to be lined by stratified squamous epithelium, while flattened sebaceous gland cells were identified in the cystic wall. Pigment casts were present in the hair papillae and perifollicular regions, suggesting trichotillomania as a possible cause of the observed alopecia. This case appears to represent an unusual clinical manifestation of SM.
Keywords: Alopecia, Scalp, Steatocystoma multiplex
INTRODUCTION
Steatocystoma multiplex (SM) is a hamartomatous malformation of the pilosebaceous duct junction1,2. Clinically, SM presents as multiple small skin-colored or yellowish cutaneous cystic nodules which contain a yellowish, creamy oily material. Empty cysts are located within the lower dermis or subcutaneous fat. Usually, sebaceous glands are associated with or located within the cystic wall3.
In typical cases of SM, cysts are distributed in areas where high numbers of sebaceous glands are found, most commonly the chest, arms, axillae, and neck. Several reports of localized SM limited to the scalp, face, retroauricular region, groin, and nasal region have been reported1,4-7.
Herein, we present a case of SM localized to the scalp with multiple alopecic patches.
CASE REPORT
A 50-year-old Korean man presented with alopecic patches and multiple cystic lesions on his scalp. These lesions had gradually grown larger and increased in number over a period of five years. On physical examination, asymptomatic smooth skin-colored cystic nodules were palpable on the whole scalp (Fig. 1.); however, no lesions were detected in other locations, including the trunk, extremities, and face. The diameters of the lesions ranged from 2 mm to 4 cm. Surrounding the larger nodules were irregular-shaped alopecic patches. The patient's past and family histories were non-contributory and the results of routine laboratory findings were within normal limits. The patient denied having any habit of pulling out his own hair. When we incised and squeezed a nodule, a homogenous, creamy, odorless, yellowish material was released. Subsequent histopathological examination revealed an empty cyst within the lower dermis and subcutaneous fat (Fig. 2A). The folded cystic wall was lined with stratified squamous epithelium and contained flattened sebaceous gland cells. The numbers of catagen and telogen hairs were increased, although there was no sign of fibrosis or inflammatory change. Some pigment casts were present in the hair papillae and perifollicular regions, as had been observed in histopathological investigations of trichotillomania (Fig. 2B). Some large lesions were surgically excised; however, the patient refused further treatment. At an 8-month follow-up, the cystic lesions were found to be stable, while partial hair re-growth was observed in alopecic regions.
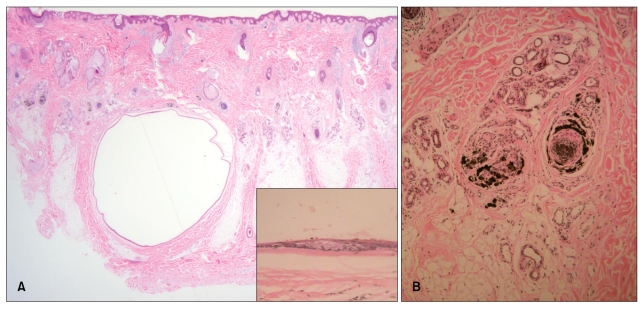
Fig. 1.
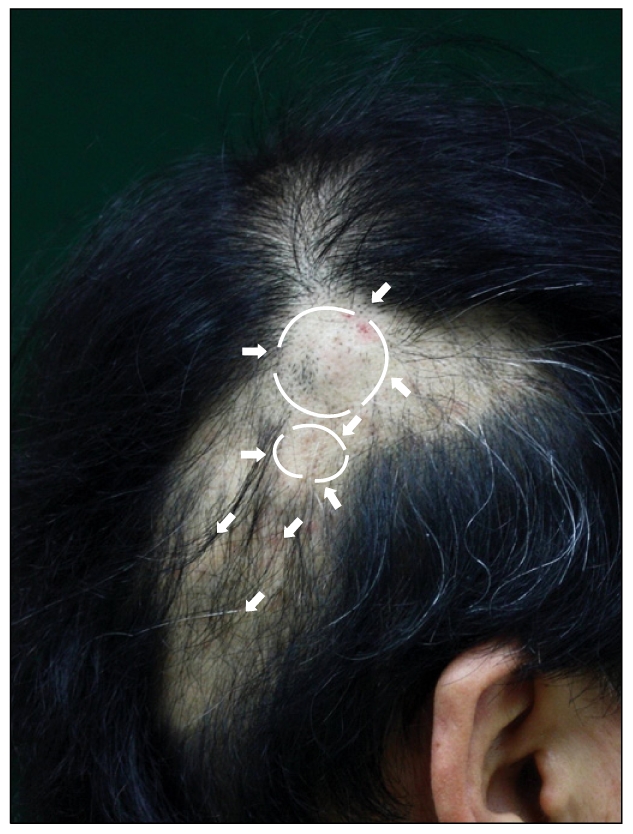
A 50-year-old man presented with asymptomatic multiple cystic nodules (ranging in size from 2 mm to 4 cm) and alopecic patches on his scalp.
Fig. 2.
(A) The cyst was located within the lower dermis and subcutaneous fat (H&E, ×12.5). The folded cystic wall was lined by stratified squamous epithelium and contained flattened sebaceous gland cells (inset) (H&E, ×400). (B) Pigment casts were present in the hair papillae and peribulbar connective tissue (H&E, ×100).
DISCUSSION
SM is most commonly found on the axillae, groin, trunk, and proximal extremities, and is rarely localized to the scalp. Since Marley et al. first described this defining feature in 1981, there have been eight reports of SM limited to the scalp1,4-7. There are key differences between typical SM and SM limited to the scalp1,4 in that typical SM is usually hereditary and is manifested from adolescence or early adult life. In contrast, SM limited to the scalp is typically nonhereditary and does not develop until later in life, as was the case with our patient.
Our case was interesting because of the presence of alopecic patches. In 1996, Lee et al.5 reported a case of SM confined to the forehead and frontal scalp, together with congenital alopecia in the frontal scalp. In the present case, the alopecic patches and cystic nodules appeared almost simultaneously, and irregular-shaped alopecic patches were found primarily around the large cystic lesions. There was no history of hair pulling. We performed a skin biopsy to differentiate between alopecia areata and trichotillomania with histopathological examination revealing no sign of inflammatory changes or fibrosis around the hair follicles. However, the anagen-to-telogen ratio was decreased and numerous pigment casts were identified. In light of these clinical and histopathological findings, self-removal of hair seemed to have been the most likely cause of the observed alopecia, although the patient denied a history of such behavior. It seems that the presence of large cysts on his scalp may have distressed him to the extent that he may have unconsciously pulled out his own hair.
In the present case, pachyonychia congenita (PC), a rare autosomal-dominant condition characterized by multiple ectodermal abnormalities was excluded8. In PC, SM and alopecia can present together; however, thickening of the nails and hyperkeratosis on the hands or feet, typical features of PC, were not seen in our patient, and he had no family history of ectodermal abnormalities.
Treatment of SM is largely symptomatic, and removal of the lesions can be successfully achieved through simple excision or drainage of the cystic material1,3,9. Inflamed lesions have been reported to respond to intralesional injection of steroids, carbon dioxide lasers, oral retinoid treatment, and cryotherapy1.
As previously mentioned, SM rarely localizes to the scalp. Our case was interesting for two reasons: the scattering of lesions over the entire scalp - a rare location in SM - and the presence of acquired alopecia (most likely caused by trichotillomania), which had not been previously described. In suspected cases of alopecia areata or trichotillomania, careful physical examination may be necessary to check for the presence of tumor lesions, which could be easily overlooked.
References
- 1.Kim CH, Kim DH, Jeon JS, Kang SG, Kim DW, Cho MK. A case of zosteriform Kaposi's sarcoma after prednisolon treatment. Korean J Dermatol. 2009;47:583–587. [Google Scholar]
- 2.Plewig G, Wolff HH, Braun-Falco O. Steatocystomamultiplex: anatomic reevaluation, electron microscopy, and autoradiography. Arch Dermatol Res. 1982;272:363–380. doi: 10.1007/BF00509068. [DOI] [PubMed] [Google Scholar]
- 3.Kim MB, Jang HS, Oh CK, Kwon KS. Clinical, histopathological, and immunohistochemical study of steatocystoma multiplex. Korean J Dermatol. 1999;37:1769–1776. [Google Scholar]
- 4.Jeong SY, Kim JH, Seo SH, Son SW, Kim IH. Giant steatocystoma multiplex limited to the scalp. Clin Exp Dermatol. 2009;34:e318–e319. doi: 10.1111/j.1365-2230.2009.03274.x. [DOI] [PubMed] [Google Scholar]
- 5.Lee YJ, Lee SH, Ahn SK. Sebocystomatosis: a clinical variant of steatocystoma multiplex. Int J Dermatol. 1996;35:734–735. doi: 10.1111/j.1365-4362.1996.tb00652.x. [DOI] [PubMed] [Google Scholar]
- 6.Lim SH, Ha JH, Chae KO, Park HJ, Baek SC, Byun DG. A case of eruptive steatocystoma multiplex on the scalp. Korean J Dermatol. 2000;38:1664–1667. [Google Scholar]
- 7.Marley WM, Buntin DM, Chesney TM. Steatocystoma multiplex limited to the scalp. Arch Dermatol. 1981;117:673–674. [PubMed] [Google Scholar]
- 8.Su WP, Chun SI, Hammond DE, Gordon H. Pachyonychia congenita: a clinical study of 12 cases and review of the literature. Pediatr Dermatol. 1990;7:33–38. doi: 10.1111/j.1525-1470.1990.tb01070.x. [DOI] [PubMed] [Google Scholar]
- 9.Lee HW, Oh SH, Chang SE, Choi JH, Moon KC, Koh JK. A case of steatocystoma multiplex: successful treatment with mini-incisions. Ann Dermatol. 2005;17:35–37. [Google Scholar]