Abstract
Decreasing oxidative stress and increasing antioxidant defense has been hypothesized as one mechanism by which caloric restriction (CR) increases longevity in animals. A total of 46 moderately overweight volunteers (BMI: 25–30 kg/m2), ages 20–42 yr were randomized to either high glycemic (HG) or low glycemic (LG) dietary load CR regimen at either 10% (n=12) or 30% (n=34) of basal caloric intake. All food was provided to participants for 6 mo. Overall, after controlling for CR levels and dietary regimen for 6 mo, plasma glutathione peroxidase activity increased (p=0.04) and plasma protein carbonyl levels decreased (p=0.02) and a non-significant decrease in plasma 8-epi-prostaglandin F2α level was observed (p=0.09). No significant change was observed in other plasma antioxidants such as superoxide dismutase and catalase. These findings indicate that short term CR (10% or 30%) in moderately overweight subjects modulates some but not all measures of antioxidant defense and oxidative stress.
Keywords: Calorie restriction, antioxidants, oxidative stress, GPX, protein carbonyl, humans
Introduction
The oxidative stress theory of aging postulates that shifts in the antioxidant/prooxidant balance in response to oxidative stress lead to dysregulation of cellular function and aging. In the context of this theory, oxidative stress and antioxidants can influence both the primary “intrinsic” aging process and also several secondary age-associated pathological processes. This hypothesis is supported by the calorie restriction (CR) paradigm, which has been shown to increase medium and maximum lifespan in several animal species, to suppress oxidative stress and to increase the antioxidant defense system (1, 2). For these effects of CR, which is a highly regulated process, several cellular signaling proteins and energy–sensing pathways such as IGF-1, TOR and sirtuins are recruited and probably through modulation of oxidative stress and antioxidants counteract the age-associated pathologies, reduces the rate of aging, and increases longevity in several animal models (3, 4). Therefore, measures of oxidative stress and antioxidant status in the CR paradigm can be considered to be useful biological markers to determine the effectiveness of CR in animals and humans.
Measurement of lipid peroxidation has been used most frequently to support the involvement of free radical reactions in aging and pathological conditions. Among the several lipid peroxidation indices, isoprostanes, such as 8-epi-prostaglandin F2α (8-epi-PGF2α), nonenzymatic peroxidation products of lipids are considered to be good, sensitive markers of lipid peroxidation. These products, which circulate in plasma, are excreted in urine and appear to be chemically stable (5, 6). Recent reports indicate that urinary excretion of 8-epi-PGF2α is associated with oxidative stress and free radical production in vivo (7); thus, it may serve as a noninvasive index of in vivo lipid peroxidation and changes in the free radical status associated with CR (8).
CR also reduces the DNA oxidative damage associated with aging in animal models (9, 10). 8-Hydroxy-2′-deoxyguanosine (8-OHdG), a marker of oxidative DNA damage, has been shown to increase with age in the kidney, heart, and brain of rats (11). We have found that excretion of 8-OHdG is higher in 24-h urine of older human subjects compared to that of younger subjects and is higher in elderly patients with rheumatoid arthritis (a condition associated with oxidative stress) than in elderly controls (8).
Reactive oxygen intermediates are also damaging to proteins. Age-associated increases in the level of protein carbonyls have been reported in the human brain as well as in flies (12, 13) and in human muscle (14). CR has also been shown to reduce oxidative damage to proteins in the brain (15, 16) and in splenic lymphocytes and plasma (17).
Animal studies have demonstrated that CR significantly increases the activity of several antioxidant enzymes including catalase, superoxide dismutase (SOD), glutathione S-transferase, and glutathione peroxidase (GPx) (18–20). Thus, CR through up-regulation of endogenous antioxidant enzymes and repair mechanisms may reduce oxidative stress and damage to lipids, proteins, and DNA. Therefore, determination of changes in the in vivo markers of oxidative stress and antioxidant defense system can help us to assess the effectiveness of CR in humans. However, CR studies, even for the short term, are limited in humans. The present study was designed to study the effects of CR on measures of oxidative stress and antioxidant defense system in humans participating in the Comprehensive Assessment of Long-term Effects of Reducing Intake of Energy (CALERIE), the first human trial of CR conducted at Tufts University. We also examined the relationship between body weight, fatness, and markers of oxidative stress.
Materials and Methods
Study Subjects
This study was performed as part of the CALERIE trial conducted at the Jean Mayer, USDA Human Nutrition Research Center on Aging (HNRCA) at Tufts University with approval from the Tufts Medical Center, Institutional Review Board. The details of the study protocol and CR diets are described elsewhere (21, 22). Briefly, 46 overweight (BMI 25–29.9 kg/m2) but otherwise healthy men and women aged 24–42 years were recruited from the greater Boston area. Eligibility was determined by normal health history questionnaires, physical and psychological examinations, and blood and urine tests (routine blood chemistry including; lipid profile, blood sugar, complete blood counts, comprehensive metabolic panel, kidney, liver and heart functions, potassium, calcium, uric acid, electrolytes, iron and urine electrolytes and sugar). Exclusion criteria included high physical activity levels, smoking, alcoholism, weight fluctuations (>15lbs in the past year), inability to accurately complete a dietary record ( 70%< estimated energy requirements <130%), anticipated life-style changes (pregnancy, relocation), and any disease or medication known to affect mood, appetite, or metabolism (diabetes, cancer, cardiovascular disease, hypertension, endocrine disorders, psychiatric disorders, eating disorders). Subjects were also provided with daily multivitamin and calcium (500 mg/day) supplements and all subjects took the provided supplements for the entire duration of the study. Each subject gave written informed consent and was provided with a stipend.
Study Intervention
Subjects were asked to maintain their weight and to continue eating their usual diet for 7 weeks when baseline assessments were made of the usual energy requirements using the doubly-labeled water method (22) and outcome variables were determined. Of the 46 study subjects, 34 subjects were then randomized to 30% CR, and 12 subjects were randomized to 10% CR for 6 mo. Within each level of CR, subjects were also randomized to one of two diets: a high glycemic load diet (HG), (N=17 and N=6 for 30% and 10% CR, respectively) and low glycemic load diet (LG), (N=17 and N=6 for 30% and 10% CR, respectively). The HG load diet consisted of 60% carbohydrate, 20% protein, and 20% fat, 15 g fiber/1,000 kcal, mean estimated daily glycemic index of 86, and glycemic load of 118 g/1,000 kcal. The LG load diet consisted of 40% carbohydrate, 30% protein, 30% fat, 15 g fiber/1,000 kcal, mean estimated daily glycemic index of 52, and glycemic load of 45 g/1,000 kcals. The glycemic index and glycemic load of the diets were determined using the International Tables of Glycemic Index and Glycemic Load (23). Both diets and both levels of CR had levels of macronutrients and micronutrients consistent with current dietary recommendations for health.
During the 6-mo intervention period, all food was provided to the participants by the Metabolic Research Unit of the HNRCA at Tufts University. Subjects were asked to consume only this food, return any leftovers, and report additional foods eaten. To maximize adherence to the study diet, regular behavioral group meetings and individual sessions with a dietitian were held. From participants’ reports of leftover food and extra items, actual daily nutrient intake during the intervention period was calculated (22).
Outcome Measurements
All outcome assessment staff members were blinded to participant randomization. Height (± 0.1 cm) was measured at baseline, and body weight (± 50 g) was measured weekly at the HNRCA. Blood and urine samples were collected at baseline and after a 6 mo period of dietary intervention with provided food.
GPx enzyme activity (nmol/min/mL) was measured in EDTA plasma using the glutathione peroxidase assay kit (Cayman Chemical, Ann Arbor, MI). Each sample was assayed in triplicate in a 96-well plate. The absorbance was read once every minute at 340nm for five minutes using an ELx808 microplate reader (Bio-Tek Instruments, Inc., Winooski, VT).
Catalase (CAT) enzyme activity (nmol/min/mL) was measured in EDTA plasma using the Cayman Chemical catalase assay Kit. Each sample was assayed in duplicate in a 96-well plate and the absorbance was read at 540nm using an ELx808 microplate reader (Bio-Tek Instruments).
Plasma concentration of 8-epi-PGF2α (pg/mL) was measured in heparinized plasma samples using the Cayman Chemical EIA kit. Samples were purified using 8-Isoprostane affinity column (Cayman Chemical). Purified samples were assayed in duplicate in a coated 96-well plate. The absorbance was read at 412nm using a Spectra Max 340 microplate reader (Bio-Tek Instruments).
SOD activity (U/mL) was measured in heparinized plasma using the Cayman Chemical SOD assay kit. Each sample was assayed in duplicate in a 96-well plate and the absorbance was read at 450nm using an ELx808 microplate reader (Bio-Tek Instruments).
Protein carbonyl concentration (nmol/mL) was measured in EDTA plasma using the Cayman Chemical assay kit. The samples were derivatized with 2,4-dinitrophenylhydrazine. The derivatized carbonyl solution was assayed in duplicate in a 96-well plate and the absorbance was read at 370nm using a Spectra Max 340 microplate reader (Molecular Devices, Sunnyvale, CA).
Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) concentration (ng/mL) was measured using the DNA Damage ELISA Kit (Stessgen, Victoria, BC, Canada). Samples were assayed in duplicate in a coated 96 well plate. The absorbance was read at 450nm using an ELx808 microplate reader. Urinary 8-OHdG was normalized with urinary creatinine levels measured using an Olympus AU400e Chemistry Immuno Analyzer (Olympus America Inc., Melville, NJ) and expressed as 8-OHdG ng/mg creatinine.
Statistical Analysis
Statistical analyses were performed using SAS version 8. A mixed model, repeated measures analysis of variance was used to examine changes in the seven markers of oxidative stress/defense over the 6 mo period with time (baseline or 6 mo), degree of CR (10% or 30%), and their interactions with glycemic load (high or low) as covariates. Over the 6 mo period, changes in the oxidative markers for the entire study group as well as differences between groups were assessed. Furthermore, the relationships between changes in the oxidative markers and changes in BMI, percent fat, and fat mass were assessed while controlling for time, level of energy restriction, and glycemic load
Results
Change in body weight and BMI
Compliance of subjects with dietary regimens at the 6 month time point was good. The 30% CR group complied as prescribed, and those assigned to 10% CR group, restricted their diet by 20% on average (please see reference (22)). As reported elsewhere (21) CR decreased the body weight, however, there was no significant difference between the HG and LG diets or between 10% and 30% CR regimens in changes of body weight over time (21, 22). There was also no correlation between oxidative stress parameters and changes in body weight, BMI, and fat mass.
Glutathione Peroxidase
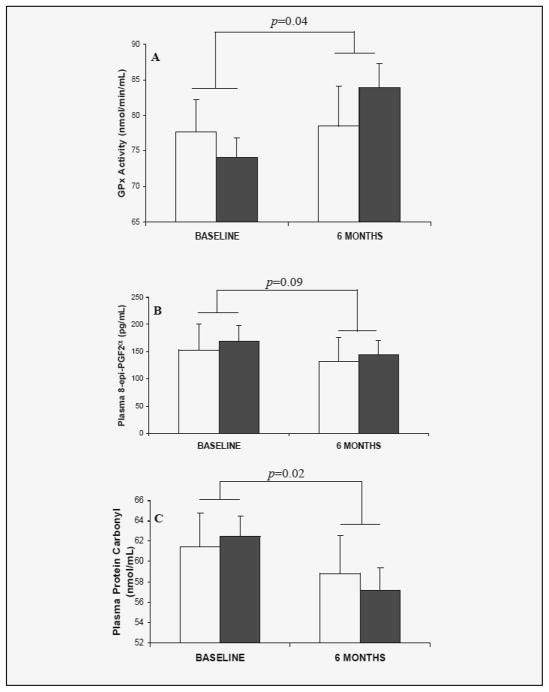
Overall, there was a significant (p=0.04) increase of 5.39nmol/min/mL in plasma glutathione peroxidase activity (Table 1 and Figure 1A) over the 6 month study period (95% CI: 0.25, 10.54). There was a non-significant trend towards an increase in enzyme activity in the 30% restricted group (9.86 nmol/min/mL, 95% CI: 4.08, 15.64) as compared to the 10% restricted group (0.93 nmol/min/mL, 95% CI: −5.81, 7.67; p=0.09) (Figure 1A). No significant effect of HG and LG diets was observed (p=0.52).
Table 1.
Overall changes in antioxidants and oxidative markers (Baseline – 6 Months)
| Antioxidant and Oxidative Markers | n | Mean Difference | p-valueb | |
|---|---|---|---|---|
| Unadjusted | Adjusteda | |||
| Glutathione Peroxidase (nmol/min/mL) | 42 | 7.52 | 5.39 | 0.04 |
| SOD (U/mL) | 42 | 0.10 | 0.06 | 0.49 |
| Catalase (nmol/min/mL) | 40 | 5.75 | 8.64 | 0.17 |
| 8-Isoprostane (pg/mL) | 42 | −23.91 | −22.88 | 0.09 |
| Protein Carbonyl (nmol/mL) | 42 | −4.61 | −3.98 | 0.02 |
| 8-Hydroxydeoxyguanosine (ng/mg creatinine) | 38 | −1.92 | −52.50 | 0.33 |
Means adjusted for degree of restriction and glycemic load;
Significance of change over the 6 month period.
Figure 1.
Overall change in plasma GPX activity (A), 8-epi-PGF2α (B) and protein carbonyl (C) from baseline after controlling for caloric restriction levels and dietary glycemic loads. Open bars: 10% CR; solid bars: 30% CR. Repeated measures, ANOVA. Data are Means ± SE
Catalase
Overall, there appeared to be an 8.64 nmol/min/mL increase in catalase activity over the 6 mo CR period. However, this did not reach to statistical significance (95% CI: −3.97, 21.24, p=0.17). This may be due in part to the random hemolysis of red blood cells, which contain high levels of catalase. There was no statistically significant difference in the change in catalase activity between the 10% and 30 % restricted groups (p=0.28) or between HG and LG groups (p=0.27).
8-epi-PGF2α
There was a non-significant decrease (p=0.09) in plasma 8-epi-PGF2α isoprostane levels (22.88 pg/mL; 95% CI: −48.44, 2.69,) over the 6 mo period (Table 1 and Figure 1B). However, there was no statistically significant difference in the level of change between the 10% and 30% CR groups (p=0.83), or between HG and LG groups (p=0.82).
SOD: an overall increase of 0.06 U/mL in plasma SOD activity over the 6 mo period. However, this increase in plasma SOD activity did not reach statistical significance (95% CI: −0.12, 0.24; p=0.49) (Table 1). There was also no significant difference in the degree of change in SOD activity between the 10% and 30% CR groups (p=0.38), or between HG and LG groups (p=0.96).
Protein Carbonyl
There was a significant (p=0.02) decrease in plasma protein carbonyl levels (3.98 nmol/mL 95% CI: −7.54, −0.43) over the 6 mo period (Table 1 and Figure 1C). The difference in the degree of decrease between the 10% and 30% CR groups did not reach statistical significance (p=0.51, Figure 1C), or between HG and LG groups (p=0.50).
8-Hydroxydeoxyguanosine
The overall decrease of 52.50 ng/mg creatinine in 8-OHdG (Table 1) over the 6 mo CR period was not statistically significant (95% CI: −166.71, 61.71; p=0.33). There was no statistically significant difference in the degree of change in 8-OHdG between the 10% and 30% restricted groups (p=0.07), or between HG and LG groups (p=0.13).
Over the 6 mo period, neither the change in BMI, percent fat, or fat mass was found to be associated with the change in any of the six markers of oxidative stress/defense.
Discussion
According to the free radical theory of aging, the cumulative damage from reactive oxygen species (ROS) and loss of protective systems to withstand the oxidative challenge intrinsically governs aging and longevity (24). The results of the present study showed that a CR regimen significantly altered two of the markers of oxidative stress in humans, which is consistent with the results previously reported for animal models. Since decrease of oxidative stress and increase of antioxidant defense systems associated with CR have been suggested to be one of the plausible mechanisms by which CR increases lifespan in laboratory animals, the results obtained here in humans are suggestive of some beneficial effects.
Previous data on the effect of CR on oxidative stress parameters in humans has been limited to an observational study of an Okinawan population and a few limited short-term studies mixing exercise and diet interventions (25–30). Since determination of human lifespan prolongation by CR is not practical, measurement of changes in the associated markers such as oxidative stress and endogenous antioxidant defense systems would provide useful predictive indices on the efficacy of short-term CR on age-associated chronic diseases and probably lifespan in humans. In the current study, we investigated the efficacy of 6-mo of CR at 30% and 10% restriction on the loss of body weight and on several associated biomarkers including oxidative stress and antioxidants indices. Six-months of CR in this study significantly reduced body weight (with no difference between HG and LG diets, or between 10% and 30% CR) and several other metabolic markers (21, 22, 31). This was partly due to the fact that the 10% CR group over-restricted themselves and the 30% CR group under restricted themselves (21).
We found that 6-mo CR significantly increased GPx activity and decreased protein carbonyl levels in plasma. Evidence suggests that CR attenuates oxidative stress through activation of NRF2/ARE pathway, inducing a variety of antioxidant proteins like GPx (32). In addition, CR can induce the expression of eNOS and nitric oxide production (33), which in turn up-regulates GPx (34). Protein carbonylation in animals has been shown to increase with age, and CR has been demonstrated to decrease this marker of protein oxidation in mice (15). Dandona et al. (35) reported that four wk of CR in nine obese subjects resulted in a significant decrease in plasma protein carbonyl levels. However, in a randomized clinical trial testing the effect of 6-mo CR on biomarkers of longevity conducted at the Pennington Biomedical Research Center (CALERIE, funded by National Institute on Aging), Heilbronn et al. (36) reported that the level of plasma protein carbonyl did not change over the 6-mo with 25% or more CR. While it could be argued that their study (36) did not alter the macronutrient composition of the diet and that the increase in protein carbonyls in the current study might have due to a compensatory increase in plasma GPx activity in response to oxidative stress induced by the high glycemic index. However, it must be noted that there was no significant difference in the decrease in protein carbonyls between the two glycemic load diets suggesting that the observed decrease may not be due to compensatory factors. Further, this increase in antioxidant activity was only observed with GPx, and not with SOD and catalase in both the CR and diet groups.
Another biomarker of oxidative stress that we tested was plasma 8-epi-PGF2α, which has been regarded as a specific and sensitive marker of lipid peroxidation (37, 38). While there was no statistically significant effect of CR on this biomarker, a trend of decrease in this marker was noted.
In a recent study, Heilbronn et al. (36) reported that 25% and higher CR in overweight subjects was effective in reducing oxidative DNA damage in lymphocytes as measured by comet assay in fresh samples. While this type of measurement may indicate the effect of CR on a specific cell type, it may not reflect CR effects on whole body status of oxidative DNA damage. The whole body DNA oxidative products measured in urine may better represent DNA damage products originating from several different tissues with differential sensitivity and adaptability to CR and oxidative stress. Therefore, measuring urinary excretion of the DNA-repair product 8OHdG has been suggested as a good biomarker of in vivo oxidative DNA damage to whole body cell DNA in humans (39). However, we found that this biomarker of oxidative DNA damage was not affected by either level of CR or glycemic load. Due to study design, the number of subjects in 10% CR was smaller than in 30% CR, and it is possible that we might not have observed level of CR effects on biomarkers of oxidative stress. Further studies with larger subject numbers are needed to establish levels of CR on these biomarkers. While CR in this study resulted in a significant decrease in body weight, BMI, and other indices of metabolic syndrome (22, 31), the absence of a strong effect on markers of oxidative stress/antioxidant defense may be attributed to the short period of CR for which the study measurements were obtained and the limited number of subjects in the 10% CR group. However, in concurrence with previous findings (36), our results suggest that CR in humans modulates some of the biomarkers of oxidative stress and antioxidant defense, which may be regarded as surrogate markers of aging. It is important to note that while changes in oxidative stress/antioxidants might be associated with CR, several energy sensing and nutrient sensing signaling pathways including IGF-1, TOR and sirtuins are believed to converge together with CR regimen leading to extension of lifespan (4, 40, 41). However, role and impact of these energy/nutrient-sensing signaling pathways need to be further elucidated in human CR.
Acknowledgments
This manuscript is based on work supported by grant from NIA/NIH grant NGA-3U01-AG20480 and the U.S. Department of Agriculture, under agreement No. 58-1950-7-707. Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the view of the U.S. Department of Agriculture. We would also like to thank Stephanie Marco for her assistance in the preparation of this manuscript.
References
- 1.Yu BP. Modulation of oxidative stress as a means of life prolonging action of dietary restriction. In: Cuttler RG, et al., editors. Oxidative stress and aging. Birkhauser Verlag; Basel: 1995. pp. 331–342. [Google Scholar]
- 2.Wanagat J, Allison DB, Weindruch R. Caloric intake and aging: mechanisms in rodents and a study in nonhuman primates. Toxicol Sci. 1999;52S:35–40. doi: 10.1093/toxsci/52.2.35. [DOI] [PubMed] [Google Scholar]
- 3.Merry BJ. Calorie restriction and age-related oxidative stress. Annal NY Acad Sci. 2000;908:180–198. doi: 10.1111/j.1749-6632.2000.tb06646.x. [DOI] [PubMed] [Google Scholar]
- 4.Narasimhan SD, Yen K, Tissenbaum HA. Converging pathways in lifespan regulation. Curr Biol. 2009;19:R657–66. doi: 10.1016/j.cub.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow JD. The isoprostanes: their quantification as an index of oxidant stress status in vivo. Drug Metab Rev. 2000;32:377–85. doi: 10.1081/dmr-100102340. [DOI] [PubMed] [Google Scholar]
- 6.Greco A, ML, Levi G. Isoprostanes, novel markers of oxidative injury, help understanding the pathogenesis of neurodegenerative diseases. Neurochem Res. 2000;25:1357–64. doi: 10.1023/a:1007608615682. [DOI] [PubMed] [Google Scholar]
- 7.Obata T, Tomaru K, Nagakura T, et al. Smoking and oxidant stress: assay of isoprostane in human urine by gas chromatography-mass spectrometry. J Chromato B, Biomed Sci Applic. 2000;746:11–5. doi: 10.1016/s0378-4347(00)00182-1. [DOI] [PubMed] [Google Scholar]
- 8.Rall LC, Roubenoff R, Meydani SN, et al. Urinary 8-hydroxy-2′-deoxyguanosine (8-OHdG) as a marker of oxidative stress in rheumatoid arthritis and aging: effect of progressive resistance training. J Nutr Biochem. 2000;11:581–584. doi: 10.1016/s0955-2863(00)00123-6. [DOI] [PubMed] [Google Scholar]
- 9.Sohal RS, Agarwal S, Candas M, et al. Effect of age and caloric restriction on DNA oxidative damage in different tissues of C57BL/6 mice. Mech Aging Develop. 1994;76:215–224. doi: 10.1016/0047-6374(94)91595-4. [DOI] [PubMed] [Google Scholar]
- 10.Ames BN, Shigenaga MK. Oxidants are a major contributor to aging. Annals of the New York Academy of Sciences. 1992;663:85–96. doi: 10.1111/j.1749-6632.1992.tb38652.x. [DOI] [PubMed] [Google Scholar]
- 11.Kaneko T, Tahara S, Matsuo M. Retarding effect of dietary restriction on the accumulation of 8-hydroxy-2′-deoxyguanosine in organs of Fischer 344 rats during aging. Free Rad Biol Med. 1997;23:76–81. doi: 10.1016/s0891-5849(96)00622-3. [DOI] [PubMed] [Google Scholar]
- 12.Smith CD, Carney JM, Starke-Reed PM, et al. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer’s disease. Proc Natl Acad Sci USA. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sohal RS, Gerwal SA, Dubey A, Orr WC. Protein oxidative damage is associated with life expectancy of houseflies. Proc Natl Acad Sci USA. 1993;90:7255–7259. doi: 10.1073/pnas.90.15.7255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mecocci P, Fano G, Fulle S, et al. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic Biol Med. 1999;26:303–8. doi: 10.1016/s0891-5849(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 15.Dubey A, Forster MJ, Lal H, Sohal RS. Effect of age and caloric intake on protein oxidation in different brain regions and on behavioral function of the mouse. Arch Biochem Biophys. 1996;333:189–197. doi: 10.1006/abbi.1996.0380. [DOI] [PubMed] [Google Scholar]
- 16.Aksenova MV, Aksenov MY, Carney JM, Butterfield DA. Protein oxidation and enzyme activity decline in old brown Norway rats are reduced by dietary restriction. Mech Ageing Dev. 1998;100:157–68. doi: 10.1016/s0047-6374(97)00133-4. [DOI] [PubMed] [Google Scholar]
- 17.Tian L, Ca IQ, Bowen R, Wei H. Effect of calorie restriction on age-related oxidative modifications of macromolecules and lymphocyte-proliferation in rats. Free Radic Biol Med. 1995;19:859–865. doi: 10.1016/0891-5849(95)00090-k. [DOI] [PubMed] [Google Scholar]
- 18.Koizumi A, Weindruch R, Walford RL. Influences of dietary restriction and age on liver enzyme activities and lipid peroxidation in mice. J Nutr. 1987;117:361–367. doi: 10.1093/jn/117.2.361. [DOI] [PubMed] [Google Scholar]
- 19.Rao G, Xia E, Nadakavukaren MJ, Richardson A. Effect of dietary restriction on the age-dependent changes in the expression of antioxidant enzymes in rat liver. J Nutr. 1990;120:602–609. doi: 10.1093/jn/120.6.602. [DOI] [PubMed] [Google Scholar]
- 20.Gomi F, Matsuo M. Effects of aging and food restriction on the antioxidant enzyme activity of rat livers. J Gerontol. 1998;53:B161–7. doi: 10.1093/gerona/53a.3.b161. [DOI] [PubMed] [Google Scholar]
- 21.Das SK, Saltzman E, Gilhooly CH, et al. Low or moderate dietary energy restriction for long-term weight loss: what works best? Obesity. 2009;17:2019–24. doi: 10.1038/oby.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Das SK, Gilhooly CH, Golden JK, et al. Long-term effects of 2 energy-restricted diets differing in glycemic load on dietary adherence, body composition, and metabolism in CALERIE: a 1-y randomized controlled trial. Am J Clin Nutr. 2007;84:1023. doi: 10.1093/ajcn/85.4.1023. [DOI] [PubMed] [Google Scholar]
- 23.Foster-Powell K, Holt SHA, Brand-Miller JC. International table of glycemic index and glycemic load values. Am J Clin Nutr. 2002;76:5–56. doi: 10.1093/ajcn/76.1.5. [DOI] [PubMed] [Google Scholar]
- 24.Finkel T, Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature. 2000;408:239–47. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- 25.Fontana L, Meyer TE, Klein S, Holloszy JO. Long-term calorie restriction is highly effective in reducing the risk for atherosclerosis in humans. Proc Natl Acad Sci U S A. 2004;101:6659–63. doi: 10.1073/pnas.0308291101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heilbronn LK, Ravussin E. Calorie restriction and aging: review of the literature and implications for studies in humans. Am J Clin Nutr. 2003;78:361–9. doi: 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- 27.Kagawa Y. Impact of Westernization on the nutrition of Japanese: changes in physique, cancer, longevity and centenarians. Prev Med. 1978;7:205–17. doi: 10.1016/0091-7435(78)90246-3. [DOI] [PubMed] [Google Scholar]
- 28.Walford RL, Harris SB, Gunion MW. The calorically restricted low-fat nutrient-dense diet in Biosphere 2 significantly lowers blood glucose, total leukocyte count, cholesterol, and blood pressure in humans. Proc Natl Acad Sci U S A. 1992;89:11533–7. doi: 10.1073/pnas.89.23.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walford RL, Mock D, MacCallum T, Laseter JL. Physiologic changes in humans subjected to severe, selective calorie restriction for two years in biosphere 2: health, aging, and toxicological perspectives. Toxicol Sci. 1999;52:61–5. [PubMed] [Google Scholar]
- 30.Walford RL, Mock D, Verdery R, MacCallum T. Calorie restriction in biosphere 2: alterations in physiologic, hematologic, hormonal, and biochemical parameters in humans restricted for a 2-year period. J Gerontol A Biol Sci Med Sci. 2002;57:B211–24. doi: 10.1093/gerona/57.6.b211. [DOI] [PubMed] [Google Scholar]
- 31.Pittas AG, Roberts SB, Das SK, et al. The effects of the dietary glycemic load on type 2 diabetes risk factors during weight loss. Obesity. 2006;14:2200–9. doi: 10.1038/oby.2006.258. [DOI] [PubMed] [Google Scholar]
- 32.Ungvari Z, Parrado-Fernandez C, Csiszar A, de Cabo R. Mechanisms underlying caloric restriction and lifespan regulation: implications for vascular aging. Circ Res. 2008;102:519–28. doi: 10.1161/CIRCRESAHA.107.168369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nisoli E, Tonello C, Cardile A, et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–7. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 34.Dobashi K, Asayama K, Nakane T, et al. Induction of glutathione peroxidase in response to inactivation by nitric oxide. Free Radic Res. 2001;35:319–27. doi: 10.1080/10715760100300851. [DOI] [PubMed] [Google Scholar]
- 35.Dandona P, Mohanty P, Ghanim H, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. doi: 10.1210/jcem.86.1.7150. [DOI] [PubMed] [Google Scholar]
- 36.Heilbronn LK, de Jonge L, Frisard MI, et al. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA. 2006;295:1539–48. doi: 10.1001/jama.295.13.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrow JD, Minton TA, Mukundan CR, et al. Free radical-induced generation of isoprostanes in vivo. J Biol Chem. 1994;269:4317–4326. [PubMed] [Google Scholar]
- 38.Meydani M. Isoprostanes as oxidant stress markers in coronary reperfusion. Nutr Rev. 1997;55:402–404. doi: 10.1111/j.1753-4887.1997.tb01583.x. [DOI] [PubMed] [Google Scholar]
- 39.Loft S, Fischer-Nielsen A, Jeding IB, et al. 8-Hydroxydeoxyguanosine as a urinary biomarker of oxidative DNA damage. J Toxicol Environ Health. 1993;40:391–404. doi: 10.1080/15287399309531806. [DOI] [PubMed] [Google Scholar]
- 40.Bordone L, Guarente L. Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol. 2005;6:298–305. doi: 10.1038/nrm1616. [DOI] [PubMed] [Google Scholar]
- 41.Ghosh HS, McBurney M, Robbins PD. SIRT1 negatively regulates the mammalian target of rapamycin. PLoS One. 2010;5:e9199. doi: 10.1371/journal.pone.0009199. [DOI] [PMC free article] [PubMed] [Google Scholar]