A fundamental objective of cardiovascular medicine is the early detection of structural changes within the heart and vasculature which would be harbingers of disease. For example, a much greater emphasis has been placed upon identifying patients with underlying changes in LV myocardial structure before the onset of occult LV dysfunction and symptomatic heart failure.[1]These changes in LV myocardial form and function have been generically termed LV remodeling, and an important research direction is to identify critical pathways which contribute to this adverse remodeling process. While significant past research efforts have been placed upon identifying changes in cardiocyte structure and function, LV remodeling is a multi-factorial process involving both cellular and extracellular pathways. An evolving concept is that a significant interplay exists between biological signaling molecules, transmembrane proteins and proteases within the myocardial interstitial space which in turn promulgates LV remodeling. In this current issue of Circulation, a pair of studies is presented which further demonstrate the diverse functionality of proteases which exist within the myocardial interstitial space and how genetically altering these proteolytic pathways can result in unexpected consequences on the LV remodeling process.[2,3]
Diversity of Proteolytic Interactions within the Myocardial Interstitium
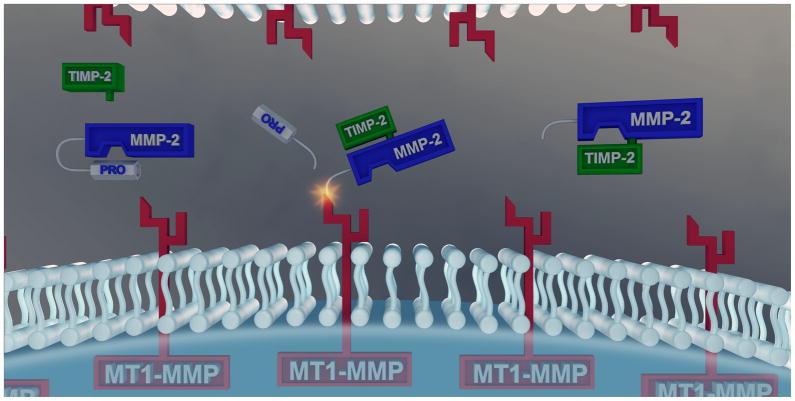
The matrix metalloproteinases (MMPs) were once considered a small set of proteases with a rather limited role in terms of degrading structural proteins within the interstitium. However, as the number of MMPs and the portfolio of proteolytic substrates continue to grow, so does the recognized functionality of these proteases in the myocardial remodeling process. The generalized nomenclature for the MMPs was initially established upon what was considered to be the fundamental proteolytic targets for each MMP class- hence the use of collagenases for the MMP types MMP-1, -8 and -13 and the gelatinases (processing of denatured collagen) for MMP-2 and MMP-9. However, this MMP nomenclature should be considered one of convenience and not one of proteolytic relevance. These MMP types such as the collagenases and the gelatinases, are synthesized and released from all myocardial cell types into the interstitial space in a non-active pro-enzyme, which in turn requires proteolysis and removal of the pro-domain at a specific sequence to yield a functional catalytic domain and a fully active MMP. Through the use of in-vitro mapping techniques and proteolytic assays, as well as the use of in-vivo substrate measurements, it has become clear that active MMPs process a large number of both structural and signaling proteins within the myocardial interstitial space.[4,5] A class of MMPs which is uniquely different is the membrane type-MMPs, such as MT1-MMP which is a transmembrane protease with both an intracellular domain and an extracellular domain, and is fully functional once trafficked to the cell membrane. All myocardial cell types express MT1-MMP including robust levels in cardiocytes and fibroblasts.[6,7] A large number of structural proteins, transmembrane adhesion molecules, and signaling moieties are processed by MT1-MMP. The wide range of substrates and biological import of MT1-MMP is further exemplified in that this is the predominant pathway by which proMMP-2 is processed to fully active MMP-2.[5,8,9] This proMMP-2 activational cascade highlights the complexity and tightly orchestrated interactions between MMPs and TIMPs. Specifically, pro-MMP-2 and TIMP-2 form a complex that results in removal of the pro-domain of MMP-2 by MT1-MMP yielding active MMP-2 (Figure). This form of MMP activational cascade can then yield a very potent and focused, pericellular proteolysis. In particular, MT1-MMP and MMP-2 can alter cell-matrix and cell-cell interactions through degrading the cell-matrix binding substrate or by directly processing transmembrane binding proteins- the integrins. While TIMP-2 facilitates MMP-2 activation, TIMP-2 can in turn bind to the active form of MMP-2 at a different site and cause MMP-2 inhibition (Figure). Thus, the activation and inhibition of MMP-2, one of the more ubiquitous and abundant MMP types, is facilitated by TIMP-2 and demonstrates how differences in location and context of specific members of the proteolytic cascade can yield distinctly different and opposing events within the interstitial space. The studies presented by Westermann et al (gene deletion of MMP-2) and Kandalam et al (gene deletion of TIMP-2)[2,3] underscore that a critical balance and set of essential interactions exist within the interstitial space between MMPs and TIMPs with respect to the myocardial biological response following a pathophysiological stimulus.
Figure A.
generic depiction of the cellular compartment, membrane and interstitial space in relation to both the transmembrane matrix metalloproteinase (MMP), MT1-MMP and the soluble MMP type, MMP-2. While MT1-MMP is constitutively active once inserted into the membrane, MMP-2 is released predominantly in a proform, proMMP-2. Through specific sequence recognition of proMMP-2, a complex forms with a specific tissue inhibitor of MMP; TIMP-2. This proMMP-2/TIMP-2 moiety then forms a trimeric complex with MT1-MMP and resultant proteolysis of the MMP-2 prodomain, yielding fully active MMP-2. TIMP-2 dissociates from this activational complex, and depending upon a number of spatial and biochemical events, can also interact with the catalytic domain of MMP-2 and result in proteolytic inhibition. Thus, a duality of function exists between MMP-2 and TIMP-2, whereby MT1-MMP provides for more spatial and targeted localization of proteolytic events within the myocardial interstitium. Study results reported in this issue of Circulation underscore the diversity of proteolytic substrates which exist for MMPs and the unexpected consequences of disturbing the balance between MMP-2/TIMP-2 in models of myocarditis and pressure overload hypertrophy.[2,3]
MMP-TIMP Interactions; New Insights and Targets
It has become abundantly clear that MMPs process a large portfolio of structural and signaling proteins critical for the maintenance of normal tissue structure and function, and dysregulation of this diverse family of MMPs can initiate and promulgate pathological remodeling in disease states such as inflammation, cancer and cardiovascular disease. The initial development of pharmacological MMP inhibitors was based upon the concept that MMPs were a relatively generic family of proteases that degraded matrix, and thereby inhibition would cause favorable effects in the context of pathological remodeling processes such as myocardial infarction.[8,10] However, any LV remodeling process particularly that of myocardial infarction, has an inflammatory component necessary for facilitating the wound healing response and MMPs likely play an important role in this early inflammatory/wound healing response. MMPs such as MT1-MMP and MMP-2 have been shown to process cytokines such as tumor necrosis factor alpha and growth factors such as transforming growth factor beta, that are operative in the LV remodeling process.[4,7,8,11] Using a murine model of myocarditis and a transgenic MMP-2 null mouse (MMP-2−/−), Westermann and colleagues demonstrated that following viral infection, LV function and survival worsened in MMP-2−/− mice.[2] Moreover, the degree of myocardial inflammation and injury was amplified with MMP-2 gene deletion and that this was likely due to an interruption in the normal processing and turnover of critical chemokines, such as monocyte chemoattractant protein-3(MCP-3). Through both in-vitro enzymatic assays and in-vivo neutralizing antibody studies, results are provided to suggest that MMP-2 plays a critical role in MCP-3 proteolysis and inactivation, and thereby is necessary to quell the inflammatory storm induced in this viral myocarditis model. The present study underscores how induction and activation of MMP-2 may actually play an anti-inflammatory role. There are several conclusions that can be drawn from this study. First, due to the ubiquitous expression profiles, high abundance and importance in the maintenance of normal tissue structure and function, suppression of MMP-2 through either pharmacological or genetic approaches in the context of LV remodeling may not yield beneficial effects. Second, integrated examination of LV remodeling and MMP substrates yielded an important protease-signaling interaction between MMP-2 and MCP-3, and that facilitating MCP-3 proteolysis may yield a novel target in myocardial wound healing and inflammation.
In the study by Kandalam and colleagues, the complementary end of the MMP-2/TIMP-2 activation/inhibition cascade was examined.[3] In this study, pressure overload induced LV hypertrophy (POH) was created in mice with TIMP-2 gene deletion (TIMP-2−/−). Based upon the classical role of TIMPs to inhibit MMPs, one would predict that removal of a specific TIMP would result in enhanced matrix proteolysis, reduced matrix accumulation and yield beneficial effects in the context of POH. In marked contrast to conventional expectations, the study by Kandalam et al demonstrated that in this murine POH model, LV mass, fibrosis and dysfunction were more severe in the TIMP-2−/− mice.[3] Moreover, MT1-MMP activity was higher and cardiocyte integrin mediated adhesion lower in the TIMP-2 null construct. The mechanisms by which ablation of TIMP-2 accelerated adverse LV remodeling in this murine model of POH are likely multifactorial. First, the proMMP-2/TIMP-2 complex will not be formed and thereby removes a potentially abundant substrate for MT1-MMP. Second, unlike TIMP-1 which is a poor inhibitor of MT1-MMP,[9] TIMP-2 effectively inhibits MT1-MMP catalytic activity. Thus, TIMP-2 deletion likely caused unbridled MT1-MMP activity within the myocardial interstitial space, particularly with a superimposition of a pathological stimulus, and the results from Kandalam and colleagues would support this postulate. MT1-MMP is a highly potent and diverse MMP type and with respect to LV growth and fibrosis, and likely plays an important role in pro-fibrotic signaling pathways.[5,7,8] Thus, increased MT1-MMP activity, which was a consequence of TIMP-2 deletion, may actually result in accelerated myocardial fibrosis in POH. The results from this study also underscore that a critical balance exists not only between MMP/TIMP binding, but also likely between TIMP types. The 4 TIMPs arise from distinctly different gene products and transduction pathways, and as such can have uniquely different effects on tissue growth and function.[8,9] In addition to different affinities to different active MMPs, TIMPs modulate cell growth, differentiation and viability. Thus, shifts in the relative balance of the different TIMP types within the myocardial interstitium, particularly in the context of relevant stimuli such as POH, would likely alter cardiocyte as well as fibroblast biological behavior. A differential increase in plasma levels of TIMPs occurs in clinical forms of POH, where by the changes in plasma TIMP-2 and TIMP-3 were relatively modest when compared to TIMP-1 and TIMP-4.[12] The results from the present murine study and this past clinical observations would suggest the intriguing possibility that an imbalance in the relative TIMP types with POH may actually facilitate adverse LV remodeling such as fibrosis. There are several potential targets and directions which arise from the study regarding POH and TIMP-2 deletion. First, MT1-MMP through both proMMP activation and profibrotic signaling, may present as a more specific target in the context of adverse LV remodeling. Second, targeted TIMP augmentation in order to normalize the relative balance between TIMP types in cardiac disease states such as POH, may yield a favorable response such as reducing matrix accumulation.
Diversity of Myocardial Interstitial Proteolytic Pathways-Conclusions
Using murine models of myocarditis and POH coupled with transgenic deletion of MMP-2 or TIMP-2, respectively, the studies by Westermann et al and Kandalam et al [2,3] underscore the diversity and complex interactions of proteolytic pathways which exist within the myocardial interstitium. MMP-2 deletion exacerbated inflammation and LV failure in a myocarditis model, revealing that chemokines serve as proteolytic substrates for MMPs. TIMP-2 deletion in POH accelerated LV hypertrophy and fibrosis, likely by altering relative MT1-MMP activity and increasing matrix accumulation through profibrotic effects. As with any basic studies utilizing murine systems, translational and clinical interpretation should be done with appropriate caution. First, global MMP-2 or TIMP-2 deletion are likely to cause a number of biological effects, some compensatory, and thus evaluating the effects of superimposition of a pathological stimulus within these transgenic constructs can be difficult. Second, the acute viral load to induce myocarditis or the abrupt and severe aortic constriction to induce POH in the mouse do not necessarily recapitulate the LV remodeling process and disease course in humans. Nevertheless, the findings from these studies continue to challenge dogma regarding MMP/TIMP functionality and interactions within the dynamic entity-the myocardial interstitium. These findings provide further evidence to suggest that our expectations from initial MMP pharmacological inhibition studies would yield clinical benefit,[8,10] without recognition as to timing, context, specificity and the diversity of proteolytic pathways affected, were extremely optimistic. The recognition and identification of the complex and diverse proteolytic interactions between MMPs and TIMPs within the myocardial interstitium will likely yield relevant targets for both the detection and treatment for early structural events in the LV remodeling process.
Acknowledgments
Sources of Funding: This work was supported by NIH grants HL057952, HL059165 and the Research Service of the Department of Veterans Affairs. The author is grateful to Shaun Riffle, M.F.A. -Office of Media Resources, University of SC School of Medicine for creation of the figure.
Footnotes
Disclosures: none.
References
- 1.ACC/AHA. 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult. Summary Article. Circulation. 2005;112:1825–1852. [Google Scholar]
- 2.Westermann D, Savvatis Kostantinos, Lindner Diana, Zietsch Christin, Becher Moritz, Hammer Elke, Heimesaat MM, Bereswill S, Volker U, Escher F, Plendl J, Riad R, Klingel K, Poller W, Schultheiss HP, Tschope C. Reduced degradation of the chemokine MCP-3 by matrix metalloproteinase-2 exacerbates myocardial inflammation in experimental viral myocarditis. Circulation. doi: 10.1161/CIRCULATIONAHA.111.035964. this issue- Publisher please update. [DOI] [PubMed] [Google Scholar]
- 3.Kandalam V, Basu R, Moore L, Fan D, Wang X, Jaworski D, Oudit GY, Kassiri ZK. Lack of Timp-2 leads to exacerbated left ventricular dysfunction and adverse extracellular matrix remodeling in response to biomechanical stress. Circulation. doi: 10.1161/CIRCULATIONAHA.111.030338. this issue- Publisher please update. [DOI] [PubMed] [Google Scholar]
- 4.Rodriquez D, Morrison CH, Overall CM. Matrix metalloproteinases: what do they not do? New substrates and biological roles identified by murine models and proteomics. Biochem Biophys Acta. 2010;1803:39–54. doi: 10.1016/j.bbamcr.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Dixon JA, Spinale FG. Myocardial remodeling: cellular and extracellular events and targets. Annu Rev Physiol. 2011;73:47–68. doi: 10.1146/annurev-physiol-012110-142230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Spinale FG, Coker ML, Heung LJ, Bond BR, Gunasinghe HR, Etoh T, Goldberg AT, Zellner JL, Crumbley AJ. A matrix metalloproteinase induction/activation system exists in the human left ventricular myocardium and is upregulated in heart failure. Circulation. 2000;102:1944–1949. doi: 10.1161/01.cir.102.16.1944. [DOI] [PubMed] [Google Scholar]
- 7.Spinale FG, Mukherjee R, Zavadzkas JA, Koval CN, Bouges S, Stroud RE, Dobrucki LW, Sinusas AJ. Cardiac restricted over-expression of membrane type-1 matrix metalloproteinase causes adverse myocardial remodeling following myocardial infarction. J Biol Chem. 2010 Sep 24;285:30316–27. doi: 10.1074/jbc.M110.158196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spinale FG. Matrix remodeling and the matrix metalloproteinases: Influence on cardiac form and function. Physiol Rev. 2007 Oct;87:1285–342. doi: 10.1152/physrev.00012.2007. [DOI] [PubMed] [Google Scholar]
- 9.Brew K, Nagase H. The tissue inhibitors of metalloproteinases (TIMPs): An ancient family with structural and functional diversity. Biochim et Biophys Acta. 2010;1803:55–71. doi: 10.1016/j.bbamcr.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hudson MP, Armstrong PW, Ruzyllo W, Brum J, Cusmano L, Krzeski P, Lyon R, Quinones M, Theroux P, Sydlowski D, Kim HE, Garcia MJ, Jaber WA, Weaver WD. Effects of selective matrix metalloproteinase inhibitor (PG-116800) to prevent ventricular remodeling after myocardial infarction: results of the PREMIER (Prevention of Myocardial Infarction Early Remodeling) trial. J Am Coll Cardiol. 2006;48:15–20. doi: 10.1016/j.jacc.2006.02.055. [DOI] [PubMed] [Google Scholar]
- 11.Dean RA, Cox JH, Bellac CL, Doucet A, Starr AE, Overall CM. Macrophage-specific metalloelastase (MMP-12) truncates and inactivates ELR+ CXC chemokines and generates CCL2,-7,-8 and -13 antagonists: Potential role of the macrophage in terminating polymorphonuclear leukocyte influx. Blood. 2008;112:3455–3464. doi: 10.1182/blood-2007-12-129080. [DOI] [PubMed] [Google Scholar]
- 12.Zile MR, DeSantis SM, Baicu CF, Stroud RE, Thompson SB, McClure CD, Mehurg SM, Spinale FG. Plasma biomarkers that reflect determinants of matrix composition identify the presence of left ventricular hypertrophy and diastolic heart failure. Circulation Heart Failure. 2011 May 1;4:246–56. doi: 10.1161/CIRCHEARTFAILURE.110.958199. [DOI] [PMC free article] [PubMed] [Google Scholar]