Abstract
We have previously reported smaller hippocampal volume and deficits in short-term memory in patients with combat-related posttraumatic stress disorder (PTSD) relative to comparison subjects. The purpose of this study was to compare hippocampal volume in adult survivors of childhood abuse to matched controls. Magnetic resonance imaging was used to measure volume of the hippocampus in adult survivors of childhood abuse (n = 17) and healthy subjects (n = 17) matched on a case-by-case basis for age, sex, race, handedness, years of education, body size, and years of alcohol abuse. All patients met criteria for PTSD secondary to childhood abuse. PTSD patients had a 12% smaller left hippocampal volume relative to the matched controls (p < .05), without smaller volumes of comparison regions (amygdala, caudate, and temporal lobe). The findings were significant after controlling for alcohol, age, and education, with multiple linear regression. These findings suggest that a decrease in left hippocampal volume is associated with abuse-related PTSD.
Keywords: Hippocampus, stress, posttraumatic stress disorder, cortisol, childhood abuse
Introduction
Childhood physical and sexual abuse is now recognized as a public health problem of enormous magnitude, with rates of sexual abuse estimated to be 11–62% in women and 3–39% in men (Finkelhor 1986). Estimates of the number of individuals who develop posttraumatic stress disorder (PTSD) following exposure to childhood abuse range from 25 to 62% (Albach and Everaerd 1993; Chu and Dill 1990; Kiser et al 1991; Palmer et al 1992). Although there has been considerable research into neurobiological correlates of other psychiatric disorders, such as schizophrenia, almost no research has been done in the area of the neurobiology of childhood abuse.
Preclinical and clinical evidence suggests that stress has long-term effects on memory function and brain regions involved in memory (Pitman 1989; Bremner et al 1995a). High levels of glucocorticoids seen during stress have been associated with a loss of neurons and a decrease in dendritic branching in the hippocampus (Uno et al 1989; Sapolsky et al 1990) with associated deficits in memory function (Arbel et al 1994; Luine et al 1994). Studies in human subjects with a history of exposure to traumatic stress related to being a prisoner of war in Korea (Sutker et al 1991), as well as studies in patients with PTSD related to Vietnam combat (Bremner et al 1993a; Uddo et al 1993; Yehuda et al 1995), have shown deficits in short-term verbal memory relative to controls. We have also found smaller right hippocampal volume as measured with magnetic resonance imaging (MRI) with associated deficits in verbal memory in Vietnam combat veterans with PTSD in comparison to matched healthy controls (Bremner et al 1995b). Some studies have shown a relationship between childhood abuse (Lewis et al 1979) or the stress of civil war (Saigh et al 1995) in children and adolescents and cognitive deficits measured with IQ (Lewis et al 1979) and academic achievement tests (Saigh et al in review). We have recently reported deficits in verbal short-term memory measured with the Wechsler Memory Scale in adult survivors of childhood abuse identified with the Early Trauma Inventory (Bremner et al 1995c). The purpose of this study was to use MRI to measure hippocampal volume and comparison brain structures in adult survivors of childhood abuse and healthy controls who were matched on a case-by–case basis for factors that could affect volume of the hippocampus. We hypothesized that patients with a history of childhood abuse would have smaller volume of the hippocampus, but not of other comparison brain structures, in comparison to their matched controls.
Methods
Subject Selection
The patient group included 17 adult survivors of severe childhood physical and/or sexual abuse who were admitted to the inpatient and outpatient psychiatric services of the West Haven Veterans Affairs Medical Center (VAMC) over a 10-month period. Screening was performed on all patients entering treatment for either inpatient or outpatient services, and individuals who met criteria for the study as outlined below were invited to participate. Twenty-two patients met criteria for the study, and 17 completed the study. Of the 5 patients who were not in the final study sample, 3 were dropped because of technical limitations in MRI acquisition, 1 patient could not be matched with a control, and 1 refused participation. Abuse patients were included who met DSM-III-R criteria for a psychiatric disorder based on the Schedule for Affective Disorders and Schizophrenia (SADS-L) or psychiatric interview, and who did not have a history of combat exposure. Childhood abuse was assessed with the Early Trauma Inventory (ETI), as described in detail in another publication (Bremner et al 1995c). We have developed specific criteria for severe abuse based on the ETI interview to identify a sample of subjects in which there is no question that abuse has occurred. Subjects were included who were exposed to physical abuse involving being hit with an object, burned, or locked in a closet, or penetrative sexual abuse, which occurred once a month or more for at least a year, which had an extremely negative effect on the individual at the time the event occurred, and which has an extremely negative effect currently emotionally or on social or occupational functioning. A composite index of abuse severity was determined based on the ETI as previously described (Bremner et al 1995c). Although only the presence of a psychiatric diagnosis was part of the inclusion criteria, all of the patients met criteria for PTSD based on the SADS-L or psychiatric interview.
All subjects in this study (both patients and controls) gave written informed consent for participation. Patients were excluded with a history of meningitis, traumatic brain injury, neurological disorder, loss of consciousness of greater than 10 min, HIV-positive status, current alcohol or substance abuse or lifetime schizophrenia based on the SADS-L, or shrapnel or other foreign bodies that would preclude MRI scanning. SADS-L data were available in 14/17 of the childhood abuse patients in the final sample (Table 1).
Table 1.
SADS-L-Based Diagnoses in Adult Survivors of Childhood Abuse
| Diagnosis | n (%) |
|---|---|
| Bipolar disorder | |
| Lifetime | 2/14(14%) |
| Major depression | |
| Lifetime | 12/14(86%) |
| Current | 4/14 (29%) |
| Dysthymia | |
| Lifetime | 1/14 (7%) |
| Posttraumatic stress disorder | |
| Lifetime | 14/14(100%) |
| Current | 14/14 (100%) |
| Panic disorder with agoraphobia | |
| Lifetime | 2/14(14%) |
| Current | 2/14 (14%) |
| Panic disorder without agoraphobia | |
| Lifetime | 5/14(36%) |
| Current | 2/14(14%) |
| Agoraphobia without panic disorder |
|
| Lifetime | 3/14(21%) |
| Current | 3/14(21%) |
| Social phobia | |
| Lifetime | 4/14 (29%) |
| Current | 3/14 (21%) |
| Simple phobia | |
| Lifetime | 3/14(21%) |
| Current | 3/14(21%) |
| Generalized anxiety disorder | |
| Lifetime | 1/14 (7%) |
| Current | 1/14 (7%) |
| Bulimia | |
| Lifetime | 1/14 (7%) |
| Current | 1/14 (7%) |
| Anorexia | |
| Lifetime | 1/14 (7%) |
| Alcohol dependence | |
| Lifetime | 10/14(71%) |
| Alcohol abuse | |
| Lifetime | 1/14 (7%) |
| Marijuana dependence | |
| Lifetime | 5/14 (36%) |
| Marijuana abuse | |
| Lifetime | 2/14(14%) |
| Stimulant dependence | |
| Lifetime | 4/14 (29%) |
| Stimulant abuse | |
| Lifetime | 2/14(14%) |
| Opiate dependence | |
| Lifetime | 4/14 (29%) |
| Cocaine dependence | |
| Lifetime | 8/14 (57%) |
| Hallucinogen abuse | |
| Lifetime | 1/14 (7%) |
There were no patients with a lifetime or current history of bipolar disorder not otherwise specified (NOS), psychosis NOS, schizophrenia, obsessive–compulsive disorder, somatization disorder, somatic pain disorder, hypochondriasis, sedative/hypnotic/anxiolytic dependence or abuse, hallucinogen dependence, or cocaine or opiate abuse. No patients had current anorexia, bipolar disorder, or dysthymia.
Comparison subjects were matched a priori on a case-by-case basis with the patients to be the same sex, race, and handedness, to be within 5 years of age, and within 2 years of education. The patient and control groups were also matched so that there would be no mean difference in height, weight, or years of alcohol abuse. Years of alcohol abuse were measured with the Addiction Severity Index (ASI) interview (McClellan et al 1985), using a procedure described in previous publications (Bremner et al 1993a, 1995b, 1995c). The rationale, procedure, and strategy for controlling for potential biases related to alcohol and substance abuse is described in detail in previous publications (Bremner et al 1995b). Demographic data for patients and controls are presented in Table 2, where it can be seen that there was perfect matching for sex, race, and handedness. Matching for age and education resulted in a close (but not perfect) proximity in the mean of these variables between the two groups, although the difference for alcohol abuse was greater. There were no statistically significant differences, however, between patients and controls in any of the demographic variables measured.
Table 2.
Demographic Variables in PTSD Patients and Controls
| Patients (n = 17) [mean (range), SD] |
Controls (n = 17) [mean (range), SD] |
Patients (n = 17) [No. (%)] |
Controls (n = 17) [No. (%)] |
Difference | SD | |
|---|---|---|---|---|---|---|
| Age (years) | 40.1 (30–50), 5.7 | 42.4 (25–52), 7.3 | 2.29 | 2.80 | ||
| Race | ||||||
| White | 14 (82%) | 14 (82%) | ||||
| Black | 2 (12%) | 2(12%) | ||||
| Hispanic | 1 (6%) | 1 (6%) | ||||
| Sex, n (%) | ||||||
| Male | 12(71%) | 12(71%) | ||||
| Female | 5 (29%) | 5 (29%) | ||||
| Education (years) | 13.2 (10–18), 2.0 | 14.5 (12–17), 1.8 | −1.29 | 2.36 | ||
| Height (inches) | 67.7 (60–73), 3.3 | 68.6 (60–75), 3.9 | −0.88 | 3.43 | ||
| Weight (lbs.) | 171 (125–250), 33 | 170 (120–240), 32 | 0.76 | 38.48 | ||
| Handedness | ||||||
| Right | 15 (88%) | 15 (88%) | ||||
| Nonright | 2(12%) | 2(12%) | ||||
| Alcohol abuse | 10.2 (0–27), 9.0 | 4.7 (0–22), 7.9 | 5.53 | 7.15 |
Comparison subjects were excluded with a history of psychiatric disorder or childhood abuse based on psychiatric interview as well as the other exclusion criteria outlined above for the patients. Urine toxicology and psychiatric history were utilized to exclude subjects with a history of substance abuse. Neuropsychological testing of IQ and memory was performed using the Wechsler Adult Intelligence Scale and the Wechsler Memory Scale as described in detail in previous publications (Bremner et al 1993a, 1995b, in press). These data have been reported in a previous publication (Bremner et al 1995c).
MRI Acquisition, Image Processing, and Analysis
Magnetic resonance images were obtained using a protocol of 3-mm contiguous slices on a 1.5-Tesla General Electric Signa device, with a spoiled GRASS (gradient recall acquisition in the steady state) sequence with repetition time = 25 msec, echo-time = 5 msec, NEX (number of excitations) = 2, matrix 256 × 256, and field of view = 16 cm. Images were transferred through computer network to a Sun Sparc 10 Workstation, where volumetric measurements were performed using methods previously described (Bronen and Cheung 1991; Bremner et al 1995b) and the ANALYZE program (Mayo Foundation, Rochester, MN).
Measurements of a mid-hippocampal segment were performed independently by two investigators (J.D.B. and E.V.) who were blinded to subject diagnosis using methods previously described in detail (Bremner et al 1995b). The mid-hippocampal segment included five coronal sections (15 mm) between the superior colliculus and the bifurcation of the basilar artery, with the first slice anterior to the superior colliculus (Bronen and Cheung 1991). Volume of mid-hippocampal body measured using this method has been shown to correlate with whole volume measurements (Kim et al 1994). The mean of the two raters was obtained for the final value of hippocampal volume. In 3 cases there was a greater than 20% discrepancy in measurements between the two operators, These scans were blindly reexamined and a consensus measurement was performed. Volumetric assessments were also made of three other regions for purposes of comparison, the temporal lobe, caudate, and amygdala. Methodology and rationale for measurement of temporal lobe and caudate have been described in detail previously (Bremner et al 1995b). Volume of the amygdala was determined by measuring cross-sectional area of the amygdala on the nonresliced MRI in all slices anterior to the bifurcation of the basilar artery (inclusive of the slice in which the bifurcation of the basilar artery was visualized) (Watson et al 1992), summing cross-sectional areas, and multiplying by the slice thickness. Volumes reported for temporal lobe, amygdala, and caudate are from measurements performed by a single rater (J.D.B.). We controlled for differences in brain size by matching patients with a comparison group of similar height and weight (Arndt et al 1991).
Interrater reliability determined for the measurements in this study using the intraclass correlation coefficient with one-way analysis of variance (ANOVA) (Bartko 1966) (with values of the coefficient approaching one representing a high level of agreement between two raters) showed a high level of agreement for the following regions: left hippocampus internal conversion coefficient (ICC) = .61 (F = 4.13; df = 33,34; p < .01), right hippocampus ICC = .79 (F = 8.54; df = 33,34; p < .01), left amygdala ICC = .56 (F = 3.56; df = 33,34; p < .01), right amygdala ICC = .56 (F = 3.55; df = 33,34; p < .01). We have previously reported interrater reliability data demonstrating a high level of agreement for hippocampus, temporal lobe, and caudate (Bremner et al 1995b).
Data Analysis
Repeated measures ANOVA with side (left vs. right) as the repeated measure was performed to compare left and right hippocampal volume (as well as left and right caudate and left and right temporal lobe) between patients and controls. Multiple linear regression was used to examine the relationship between hippocampal volume and diagnosis while controlling for other variables including alcohol abuse, education, and age. Two-tailed non-paired t tests were used to compare hippocampal volume in PTSD patients with and without comorbid depression and alcohol/substance abuse.
Results
Hippocampal Volume
Repeated measures ANOVA with hemisphere (left vs. right) as the repeated factor did not show a significant difference between patients and controls when left and right hippocampal volume were combined in a single model (Table 3). There was not a significant main effect for side (left vs. right hippocampal volume) (F = 2.15; df = 1,32; p = .15), although there was a significant interaction between side and diagnosis (F = 4.19; df = 1,32; p = .049). Childhood abuse patients had a 12% smaller left hippocampal volume than controls, which was statistically significant by univariate analysis (p < .01) (Figure 1). A 5% reduction in volume of the right hippocampus was not significant (Table 3).
Table 3.
Volume of the Hippocampus (mm3) in Patients and Controls
| Brain region | Patients (n = 17) (mean, SD) |
Controls (n = 17) (mean, SD) |
F | p value |
|---|---|---|---|---|
| Left hippocampus | 1050,152 | 1193,142 | 8.07 | .0077 |
| Right hippocampus | 1062,169 | 1116,190 | 0.74 | .40 |
| Mean hippocampus | 1056,154 | 1154,149 | 3.57 | .07 |
Figure 1.
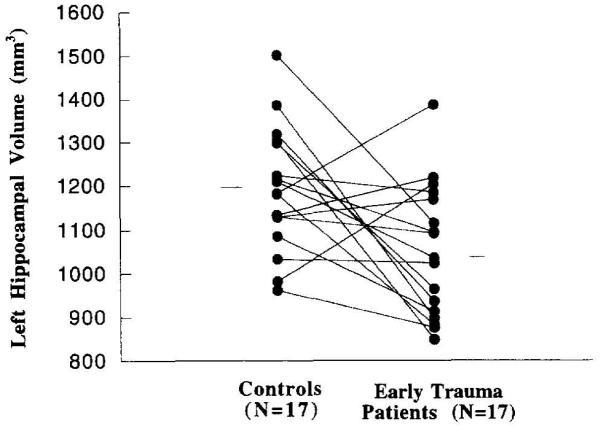
Left hippocampal volume in patients with childhood abuse-related PTSD and matched controls. Individual symbols represent left hippocampal volume in patients and controls, and the lines connect patients with the individual matches. There was a significant difference in volume of the left hippocampus between patients and controls (p < .05).
Volume of Comparison Regions
Repeated measures ANOVA with side (left vs. right temporal lobe volume) as the repeated factor did not show a difference in temporal lobe volume between patients and controls (i.e., no main effect for diagnosis) (Table 4). There was a significant main effect for side (left vs. right temporal lobe volume) (F = 19.53; df = 1,32; p = .0001) but no side by diagnosis interaction, which (consistent with our previous reports as well as reports from other groups) suggests a relative increase in right temporal volume in both groups relative to left. Univariate analyses showed that the childhood abuse patients had a greater volume of the left temporal lobe, but not right temporal lobe, in comparison to the matched controls (Table 4). Repeated measures ANOVA with side (left vs. right caudate volume) as the repeated factor showed no difference in caudate volume between patients and controls (i.e., no main effect for diagnosis), main effect for hemisphere or side by diagnosis interaction. Univariate analyses did not show a difference for left or right caudate between childhood abuse patients and comparison subjects when examined alone. Repeated measures ANOVA with side (left vs. right amygdala volume) as the repeated factor showed no difference in amygdala volume between patients and controls (i.e., no main effect for diagnosis), and no main effect for hemisphere. In addition, there was no side by diagnosis interaction (F = 3.66; df = 1,32; p = .06). Univariate analyses did not show a difference for left or right amygdala volume between childhood abuse patients and controls when examined alone (Table 4).
Table 4.
Volume of Comparison Brain Structures (mm3) in Patients and Controls
| Brain region | Patients (n = 17) (mean, SD) |
Controls (n = 17)a (mean, SD) |
F | p value |
|---|---|---|---|---|
| Left amygdala | 2012,454 | 2367,676 | 3.24 | .08 |
| Right amygdala | 2061,417 | 2286,629 | 1.51 | .23 |
| Mean amygdala | 2036,427 | 2326,643 | 2.40 | .13 |
| Left temporal lobe | 53,175,8264 | 46,839,6038 | 6.52 | .02 |
| Right temporal lobe | 56,908,9865 | 53,575,8792 | 1.08 | .31 |
| Mean temporal lobe | 55,041,8790 | 50,206,6211 | 3.43 | .07 |
| Left caudate | 2530,449 | 2388,593 | 0.62 | .44 |
| Right caudate | 2479,326 | 2378,509 | 0.47 | .50 |
| Mean caudate | 2504,373 | 2383,493 | 0.65 | .42 |
As described in the Methods section, the volume of the hippocampus and amygdala was subtracted from that of the temporal lobe.
We did not find a relationship between volume of the hippocampus and volumes of the caudate and temporal lobe in either the patients or the controls. There was a significant correlation within the childhood abuse patient group between mean temporal lobe and caudate (r = .79; df = 16; p = .0001), which was not seen within the controls. This correlation was seen on both the left and the right sides within the childhood abuse patient group.
Hippocampal Volume and Memory
We have previously reported specific deficits in short-term verbal memory (but not visual memory or IQ) as measured with the Wechsler Memory Scale–Logical component, for immediate and delayed recall and percent retention, in a slightly larger sample of childhood abuse patients that included all of the patients in this study (Bremner et al 1995c). Patients in the current sample also had significant deficits in verbal memory as measured by the Wechsler Memory Scale–Logical component relative to controls, for immediate recall [14.06 (5.92 SD) vs. 23.40 (5.89 SD)] (t = 3.92; df = 24; p = .0006), delayed recall [10.38 (5.92 SD) vs. 21.20 (5.14 SD)] (t = 4.76; df = 24; p = .0001), and percent retention [70.94 (25.93 SD) vs. 89.20 (12.36 SD)] (t = 2.07; df = 24; p = .024). In the current study there was no correlation between Wechsler Memory Scale immediate recall and left (r = .22; df = 14; p = .40) or right (r = .056; df = 14; p = .84) hippocampal volume, or between Wechsler Memory Scale delayed recall and left (r = .23; df = 14; p = .39) or right (r = .17; df = 14; p = .54) hippocampal volume in the patients with abuse-related PTSD.
Relationship between Hippocampal Volume and Demographic and Clinical Factors
Patients with abuse-related PTSD experienced an average of 14.9 years of physical abuse (4.0 SD), 7.4 years of sexual abuse (5.0 SD), and 15.1 years of emotional abuse (5.3 SD). Number of PTSD symptoms was correlated with duration (measured in years) of emotional abuse (r = .65; df = 13; p = .01), and physical abuse (r = .49; df = 13; p = .07), but not sexual abuse (r = .15; df = 10; p = .67). There was not a significant correlation between duration (measured in years) of childhood physical, sexual, or emotional abuse as measured with the ETI and left hippocampal volume in the childhood abuse patients. The strongest correlation was between years of sexual abuse and left hippocampal volume (r = −.23; df = 16; p = .38). There was no correlation between the number of years since the abuse stopped and left hippocampal volume for either physical, sexual, or emotional abuse. We also examined onset of abuse and hippocampal volume. When patients were divided by a median split into patients with early onset (less than age 8) and late onset (age 8 or greater) sexual abuse, there was no difference in left hippocampal volume between the two groups. Most patients had early onset physical and sexual abuse, which prevented a similar comparison between early and late onset. The correlation between number of PTSD symptoms and left hippocampal volume in the abuse patient group was not statistically significant (r = .29; df = 13; p = .31).
There was no difference in left hippocampal volume between PTSD patients with (n = 10) and without (n = 4) a lifetime history of alcohol dependence (mean = 1054, SD = 175 vs. mean = 1008, SD = 104), with (n = 5) and without (n = 9) a lifetime history of marijuana dependence (mean = 1007, SD = 144 vs. mean = 1060, SD = 167), with (n = 4) and without (n = 10) a lifetime histor of stimulant dependence (mean = 1014, SD = 149 vs. mean = 1052, SD = 165), with (n = 2) and without (n = 12) a lifetime history of sedate/hypnotic/anxiolytic dependence (mean = 1211, SD = 9.9 vs. mean = 1012, SD = 150), with (n = 4) and without (n = 10) a lifetime histor of opiate dependence (mean = 1102, SD = 128 vs. mean = 1016, SD = 165), or with (n = 8) and without (n = 6) a lifetime history of cocaine dependence (mean = 1003, SD = 133 vs. mean = 1093, SD = 181). There was no correlation within the childhood abuse patients between left or right hippocampal volume and years of alcohol, heroin, hallucinogen, cocaine, marijuana, or amphetamine abuse. There was no significant correlation between years of alcohol abuse and left or right hippocampal volume in the group as a whole.
We also examined the relationship between left hippocampal volume and comorbid depression in the abuse patients. There was no difference in left hippocampal volume between abuse patients with and without current major depression (mean = 1085, SD = 165 vs. mean = 1023, SD = 106 mm3). There was no correlation between years of depression, or number of hospitalizations for depression, and left hippocampal volume. None of the patients were treated with electroconvulsive therapy.
There was no significant correlation between age, years of education, height, or weight and left or right hippocampal volume in either patients or controls when examined separately. When analysis of covariance was performed using an index of body mass (height multiplied by weight) as a covariate, there continued to be a significant relationship between left hippocampal volume and abuse-related PTSD diagnosis. We did not find a difference in left hippocampal volume between men (mean = 1067, SD = 162) and women (mean = 1008, SD = 128) abuse patients (t = 0.72; df = 15; p = .48).
Assessment of Potential Confounders Related to the Findings of Smaller Left Hippocampal Volume in Abuse-Related PTSD
This study design involved matching of patients and controls for a number of factors that may affect hippocampal volume. As noted above, perfect matching was obtained for some factors (sex, race, and handedness), close but not perfect matching for others (age and education), and less precise matching for others (alcohol abuse). To assess the contribution of potential confounders to the finding of smaller hippocampal volume due to the lack of a perfect matching, we utilized multiple linear regression with factors that may affect hippocampal volume, such as age, years of education, and years of alcohol abuse, as covariates in a model examining the relationship between abuse-related PTSD diagnosis and left hippocampal volume. None of these covariates was significantly related to right hippocampal volume either individually, or when included simultaneously in the same model (p > .25 for all variables). Utilizing a backward selection procedure with a significance level to stay in the model of p < .05, all three variables were eliminated from the model, confirming their nonsignificance. When only diagnosis was in the model predicting left hippocampal volume, the effect size for the model (expressed as R2), was .20, and abuse-related PTSD diagnosis was significantly related to left hippocampal volume (t = 2.84; p = .0077). When years of alcohol abuse was added to the model, R2 was .21, and abuse-related PTSD diagnosis was related to left hippocampal volume (t = 2.52; p = .017), but alcohol was not (t = 0, 42; p = .68). With inclusion of years of education in the model, R2 was .20, and abuse-related PTSD diagnosis was related to left hippocampal volume (t = 2.62; p = .013), but years of education was not (t = .06; p = .95). With inclusion of age in the model, R2 was .22, and abuse-related PTSD diagnosis was related to left hippocampal volume (t = 2.61; p = .013), but age was not (t = 1.02; p = .31).
We also measured point estimates and confidence intervals for the difference in volume of left hippocampus between patients and controls before and after adjustment for covariates. The difference in left hippocampal volume between the two groups without adjusting for other factors was 12.0% [1050 vs. 1193 mm3; mean difference of 143 mm3, 95% confidence interval (CI) 40–245 mm3]. After adjustment for alcohol abuse the point estimate for the difference between the two groups was materially unchanged at 11.4% (1053 vs. 1189 mm3; mean difference of 133 mm3, 95% CI 39–247 mm3). After adjustment for alcohol, education, and age the estimated difference was materially unchanged at 10.6% (1058 vs. 1184 mm3; mean difference of 126 mm3, 95% CI 37–249 mm3). In summary, abuse-related PTSD diagnosis was related to small left hippocampal volume after controlling for potential confounders of alcohol, education, and age.
Discussion
Adult survivors of childhood abuse with the diagnosis of PTSD had a 12% smaller left hippocampal volume in relation to controls matched on a case-by-case basis for age, sex, race, handedness, years of education, socioeconomic status, body size, and years of alcohol abuse. Following application of multiple linear regression to control for differences in age, education, and alcohol abuse not completely addressed by the matching procedure, there continued to be a significant relationship between small left hippocampal volume and abuse-related PTSD. Furthermore, left hippocampal volume was correlated with duration of childhood abuse (measured in years). Right hippocampal volume was 5% smaller in the patients than in the controls, which was not statistically significant. None of the comparison regions measured in this study were significantly smaller in the patients in comparison to controls, including volume of the left or right caudate, left or right amygdala, or left or right temporal lobe volume (minus hippocampus and amygdala).
There is a considerable amount of evidence derived from research in animals that suggests that stress is associated with damage to hippocampal neurons (McEwen et al 1992). Most studies have focused on the role that glucocorticoids, which are released during stress, play in this hippocampal damage. The hippocampus, a major target organ for glucocorticoids in the brain (McEwen et al 1986), modulates the pituitary–adrenocortical response to stress (Sapolsky and McEwen 1988). Monkeys who died spontaneously following exposure to severe stress were found on autopsy to have multiple gastric ulcers, which suggested exposure to chronic stress, and hyperplastic adrenal cortices, consistent with sustained glucocorticoid release. These monkeys also had damage to the CA3 subfield of the hippocampus (Uno et al 1989). Follow-up studies suggested that hippocampal damage was associated with direct exposure of glucocorticoids to the hippocampus (Sapolsky et al 1990). Studies in a variety of animal species (Aus der Muhlen and Ockenfels 1969; Sapolsky et al 1985; Sapolsky et al 1988) suggest that direct glucocorticoid exposure results in decreased dendritic branching (Wooley et al 1990) and a loss of neurons (Uno et al 1990) that are steroid and tissue specific (Sapolsky 1985; Packan and Sapolsky 1990). Prenatal exposure to elevated levels of glucocorticoids also results in hippocampal damage (Uno et al 1990). Studies in patients with Gushing’s disease, a disease in which there are high levels of circulating glucocorticoids, show a negative correlation between plasma Cortisol level and volume of the hippocampus measured with MRI (Starkman et al 1992). Glucocorticoids appear to exert their effect through disruption of cellular metabolism (Lawrence and Sapolsky 1994) and by increasing the vulnerability of hippocampal neurons to a variety of insults, including endogenously released excitatory amino acids (Sapolsky and Pulsinelli 1985; Sapolsky 1986; Sapolsky 1990; Armanini et al 1990; Virgin et al 1991). Glucocorticoids have also been shown to augment extracellular glutamate accumulation (Stein-Behrens et al 1994). Furthermore, reduction of glucocorticoid exposure prevents the hippocampal cell loss associated with chronic stress (Landfield et al 1981; Stein and Sapolsky 1988; Meaney et al 1988).
Several studies have shown alterations in hypothalamic–pituitary–adrenal axis function in patients with PTSD. In reviewing this literature, it is important to differentiate between Cortisol levels at the time of traumatic stress and at some other time following exposure to the event. The fact that Cortisol is increased at the time of stressors is well established in the preclinical literature (Schatzberg and Nemeroff 1988). Studies in humans corroborate this: for example, Korean war veterans had increases in Cortisol at the time of combat stress (Howard et al 1955). Patients with chronic PTSD probably experience long-standing changes in Cortisol function that are different than the acute response to the initial stressor. Studies have shown that exposure to a previous stressor increases the risk of developing PTSD following exposure to a subsequent stressor, a phenomenon known as “sensitization.” For example, we found that exposure to childhood physical abuse increased the risk for developing combat-related PTSD in individuals who went to Vietnam (Bremner et al 1993b). Prior exposure to stress may lead to adaptive changes in the Cortisol system, resulting in a relative blunting of responsiveness of the Cortisol system to stress, as has been described in recent emerging work with rape victims who later develop PTSD. Studies of combat veterans showing low Cortisol (Mason et al 1986) may be explainable by long-term chronic adaptive changes in the system, which is to be differentiated from the acute response to stress. Other studies in combat veterans, however, have not replicated the initial findings of hypocortisolism in combat veterans with PTSD (Pitman and Orr 1990). The only published study we are aware of in sexually abused women showed hypercortisolism (Lemieux and Coe 1995), as did studies of individuals in the aftermath of natural disaster (Baum et al 1993). Based on these preclinical and clinical findings, one explanation for the findings of the current study is that a surge of Cortisol at the time of the childhood abuse led to damage to the hippocampus, which persisted until the present and is detectable by MRI. An alternative explanation is that patients who were born with smaller hippocampal volumes were more vulnerable to develop psychopathology in response to childhood abuse. Consistent with this idea, a recent report has found IQ to be predictive of combatrelated PTSD symptomatology, although again without premorbid IQ it is not possible to determine whether this is causal or an outcome of stress (McNally and Shin 1995).
Several questions remain unanswered by our study findings. We cannot conclude with certainty that small left hippocampal volume is specific to PTSD, rather than a nonspecific outcome of exposure to extreme trauma. Our study also did not answer whether small hippocampal volume is associated with psychiatric disorders other than PTSD. In fact, a number of studies have found small left hippocampal volume in patients with schizophrenia. We previously found a correlation between deficits in short-term verbal memory and smaller right hippocampal volume in patients with combat-related PTSD. There is a idea, based largely on lesion studies, that the hippocampus mediates short-term verbal memory. We found deficits in short-term verbal memory in patients with PTSD related to childhood abuse; however, in the current study these deficits were not correlated with smaller left hippocampal volume. There are several possible explanations for these discrepant results. It may be that trauma at different stages of development has different effects on the relationship between the hippocampus and memory function. For instance, one might speculate that early trauma results in hippocampal damage with a volume reduction, but that neuronal plasticity in the very young has the effect that short-term memory functions normally mediated by the hippocampus are partially taken over by other brain regions. Following this line of reasoning, trauma later in life, when there is less neuronal plasticity, results in a tighter relationship between hippocampal damage and loss of memory function.
In addition to smaller left hippocampal volume, childhood abuse patients with PTSD also had a larger volume of the left temporal lobe in comparison to controls. We have previously reported that childhood abuse patients with PTSD perform better than controls on visual memory tasks, although verbal memory is significantly worse (Bremner et al 1995c). This is a pattern that is divergent from our results in combat veterans with PTSD, in which we found significant reductions in hippocampal volume and verbal memory function, while temporal lobe volume and visual memory were only slightly (and not significantly) reduced. Again, these discrepant results between combat veterans and abuse patients may be explainable by the time of development at which the trauma occurred. Traumatic stress early in development may have an impact on brain development that results in a reduction in size of some brain regions with associated deficits in function mediated by that region, and an opposite pattern for other regions. The correlation between caudate volume and temporal lobe volume that we found within the abuse patients (but not the controls) may be consistent with a model in which abuse has specific effects on brain development that include deviations below and above normal region size, depending on the particular brain region.
We carefully considered the possible effects of comorbidity with alcohol and substance abuse on hippocampal volume in the childhood abuse patients. High levels of alcohol are known to be associated with hippocampal neuronal damage in rats (Bengoechea and Gonzalo 1991). Studies using MRI morphometries in alcoholic patients have shown volume losses in several brain regions, with the greatest magnitude of reduction in the caudate lobe, but also in dorsolateral prefrontal cortex, parietal cortex (Jernigan et al 1991), and mesial temporal lobe (which includes hippocampus) (Jernigan et al 1991; Sullivan et al 1995). We are not aware of any evidence of neuronal damage related to other substances, or a rationale for why exposure to these substances would be expected to be associated with hippocampal neuronal damage. We therefore elected to match for alcohol only. We continued to find a significant relationship between small hippocampal volume and abuse-related PTSD after controlling for differences in alcohol not addressed by the matching procedure with multiple linear regression, as noted above.
We have previously reported a statistically significant 8% smaller right hippocampal volume in patients with a history of combat-related PTSD, and a 4% smaller left hippocampal volume relative to controls, which was not statistically significant. This raises the question of why patients with abuse-related PTSD had smaller left hippocampal volume, whereas patients with combat-related PTSD had smaller right hippocampal volume, relative to comparison subjects. One might speculate that there is a difference in vulnerability of the hippocampus to stress-induced damage early in life in comparison to later stages of life. Administration of dexamethasone in utero to primates has been associated with toxicity to the hippocampus (Uno et al 1990). No studies to our knowledge, however, have compared the effects of glucocorticoid-mediated damage to the hippocampus during stress at different stages of development. Further work is needed to understand how stress at different points in the life cycle may affect the morphology of the hippocampus.
Acknowledgments
This study was supported by the National Center for PTSD grant and a Veterans Administration Career Development Award grant to Dr. Bremner.
We thank Valinda Ouellette, RN for assistance in patient assessments and Hedy Sarofin for expert assistance in MRI acquisition.
References
- Albach F, Everaerd W. Posttraumatic stress symptoms in victims of childhood incest. Psychother Psychosom. 1993;57:143–151. doi: 10.1159/000288591. [DOI] [PubMed] [Google Scholar]
- Arbel I, Kadar T, Silberman M, Levy A. The effects of long-term corticosterone administration on hippocampal morphology and cognitive performance of middle-aged rats. Brain Res. 1994;657:227–235. doi: 10.1016/0006-8993(94)90972-5. [DOI] [PubMed] [Google Scholar]
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:1–7. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Arndt S, Cohen G, Alliger RJ, Swayze VW, Andreasen NC. Problems with ratios and proportion measures of imaged cerebral structures. Psychiatr Res Neuroimaging. 1991;40:79–89. doi: 10.1016/0925-4927(91)90031-k. [DOI] [PubMed] [Google Scholar]
- Aus der Muhlen K, Ockenfels H. Morphologische veranderungen im diencephalon und telencephalon nach storngen des regelkreises adenohypophyse-nebennierenrinde: III. Ergebnisse beim meerschweinchen nach verabreichung von cortison und hydrocortison. Z Zellforsch. 1969;93:126–138. [PubMed] [Google Scholar]
- Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Baum A, Cohen L, Hall M. Control and intrusive memories as possible determinants of chronic stress. Psychosom Med. 1993;55:274–286. doi: 10.1097/00006842-199305000-00005. [DOI] [PubMed] [Google Scholar]
- Bengoechea O, Gonzalo LM. Effects of alcoholization on the rat hippocampus. Neurosci Lett. 1991;123:112–114. doi: 10.1016/0304-3940(91)90170-x. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Scott TM, Delaney RC, et al. Deficits in short-term memory in post-traumatic stress disorder. Am J Psychiatry. 1993a;150:1015–1019. doi: 10.1176/ajp.150.7.1015. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse in combat-related posttraumatic stress disorder. Am J Psychiatry. 1993b;150:235–239. doi: 10.1176/ajp.150.2.235. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Functional neuroanatomical correlates of the effects of stress on memory. J Traumatic Stress. 1995a;8:527–554. doi: 10.1007/BF02102888. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in combat-related post-traumatic stress disorder. Am J Psychiatry. 1995b;152:973–981. doi: 10.1176/ajp.152.7.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Randall PR, Capelli S, Scott T, McCarthy G, Charney DS. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Research. 1995c;59:97–107. doi: 10.1016/0165-1781(95)02800-5. [DOI] [PubMed] [Google Scholar]
- Bronen RA, Cheung G. Relationship of hippocampus and amygdala to coronal MRI landmarks. Mag Res Imag. 1991;9:449–457. doi: 10.1016/0730-725x(91)90434-n. [DOI] [PubMed] [Google Scholar]
- Chu JA, Dill DL. Dissociative symptoms in relation to childhood physical and sexual abuse. Am J Psychiatry. 1990;147:887–892. doi: 10.1176/ajp.147.7.887. [DOI] [PubMed] [Google Scholar]
- Finkelhor D. A Sourcebook on Childhood Sexual Abuse. California: 1986. [Google Scholar]
- Howard JM, Olney JM, Frawley JP, et al. Studies of adrenal function in combat and wounded soldiers. Ann Surg. 1955;141:314–320. doi: 10.1097/00000658-195503000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernigan TL, Butters N, DiTraglia G, et al. Reduced cerebral grey matter observed in alcoholics using magnetic resonance imaging. Alcohol Clin Exp Res. 1991;15:418–427. doi: 10.1111/j.1530-0277.1991.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Kim JH, Tien RD, Felsberg GJ, Osumi AK, Lee N. MR measurements of the hippocampus for lateralization of temporal lobe epilepsy: Value of measurements of the body versus the whole structure. Am J Radiology. 1994;163:1453–1457. doi: 10.2214/ajr.163.6.7992746. [DOI] [PubMed] [Google Scholar]
- Kiser LJ, Heston J, Millsap PA, Pruitt DB. Physical and sexual abuse in childhood: Relationship with posttraumatic stress disorder. J Am Acad Child Adolesc Psychiatry. 1991;30:776–783. [PubMed] [Google Scholar]
- Landfield P, Baskin R, Pitler T. Brain aging correlates: Retardation by hormonal-pharmacological treatments. Science. 1981;214:581–584. doi: 10.1126/science.6270791. [DOI] [PubMed] [Google Scholar]
- Lawrence MS, Sapolsky RM. Glucocorticoids accelerate ATP loss following metabolic insults in cultured hippocampal neurons. Brain Res. 1994;646:303–306. doi: 10.1016/0006-8993(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Lemieux AM, Coe CL. Abuse-related posttraumatic stress disorder: Evidence for chronic neuroendocrine activation in women. Psychosom Med. 1995;57:105–115. doi: 10.1097/00006842-199503000-00002. [DOI] [PubMed] [Google Scholar]
- Lewis DO, Shanok SS, Pinkus JH, Glaser GH. Violent juvenile delinquents: Psychiatric, neurological, psychological, and abuse factors. J Am Acad Child Psychiatry. 1979;18:307. doi: 10.1016/s0002-7138(09)61045-1. [DOI] [PubMed] [Google Scholar]
- Luine V, Villages M, Martinex C, McEwen BS. Repeated stress causes reversible impairments of spatial memory performance. Brain Res. 1994;639:167–170. doi: 10.1016/0006-8993(94)91778-7. [DOI] [PubMed] [Google Scholar]
- McClellan AT, Luborsky A, Cacciola J, et al. New data from the addiction severity index: Reliability and validity in three centers. J Nerv Mem Dis. 1985;73:412–423. doi: 10.1097/00005053-198507000-00005. [DOI] [PubMed] [Google Scholar]
- McEwen B, de Kloet E, Rostene W. Adrenal steroid receptors and actions in the nervous system. Physiol Rev. 1986;66:1121–1189. doi: 10.1152/physrev.1986.66.4.1121. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Angulo J, Cameron H, et al. Paradoxical effects of adrenal steroids on the brain: Protection versus degeneration. Biol Psychiatry. 1992;31:177–199. doi: 10.1016/0006-3223(92)90204-d. [DOI] [PubMed] [Google Scholar]
- McNally RJ, Shin LM. Association of intelligence with severity of posttraumatic stress disorder symptoms in Vietnam combat veterans. Am J Psychiatry. 1995;152:936–938. doi: 10.1176/ajp.152.6.936. [DOI] [PubMed] [Google Scholar]
- Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free Cortisol levels in post-traumatic stress disorder patients. J Nerv Ment Dis. 1986;174:145–149. doi: 10.1097/00005053-198603000-00003. [DOI] [PubMed] [Google Scholar]
- Meaney M, Aitken D, Bhatnager S, van Berkel C, Sapolsky R. Effect of neonatal handling on age-related impairments associated with the hippocampus. Science. 1988;239:766–769. doi: 10.1126/science.3340858. [DOI] [PubMed] [Google Scholar]
- Packan DR, Sapolsky RM. Glucocorticoid endangerment of the hippocampus: Tissue, steroid and receptor specificity. Neuroendocrinology. 1990;51:613–618. doi: 10.1159/000125400. [DOI] [PubMed] [Google Scholar]
- Palmer RL, Chaloner DA, Oppenheimer R. Childhood sexual experiences with adults reported by female psychiatric patients. Br J Psychiatry. 1992;160:261–265. doi: 10.1192/bjp.160.2.261. [DOI] [PubMed] [Google Scholar]
- Pitman RK. Posttraumatic stress disorder, hormones, and memory. Biol Psychiatry. 1989;26:221–223. doi: 10.1016/0006-3223(89)90033-4. editorial. [DOI] [PubMed] [Google Scholar]
- Pitman R, Orr S. Twenty-four hour urinary Cortisol and catecholamine excretion in combat-related posttraumatic stress disorder. Biol Psychiatry. 1990;27:245–247. doi: 10.1016/0006-3223(90)90654-k. [DOI] [PubMed] [Google Scholar]
- Saigh PA, Mroweh M, Bremner JD. Scholastic impairments among traumatized adolescents. J Abnorm Psychol. doi: 10.1016/s0005-7967(96)00111-8. in review. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. A mechanism for glucocorticoid toxicity in the hippocampus: Increased neuronal vulnerability to metabolic insults. J Neurosci. 1985;5:1228–1232. doi: 10.1523/JNEUROSCI.05-05-01228.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky R. Glucocorticoid toxicity in the hippocampus: Synergy with an excitotoxin. Neuroendocrinology. 1986;43:440–446. doi: 10.1159/000124561. [DOI] [PubMed] [Google Scholar]
- Sapolsky R. Glucocorticoids, hippocampal damage, and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, McEwen BS. Why dexamethasone resistance? Two possible neuroendocrine mechanisms. In: Schatzberg AF, Nemeroff CB, editors. The Hypothalamic-Pituitary-Adrenal Axis: Physiology, Pathophysiology, and Psychiatric Implications. Raven Press; New York: 1988. pp. 155–170. [Google Scholar]
- Sapolsky R, Pulsinelli W. Glucocorticoids potentiate ischemic injury to neurons: Therapeutic implications. Science. 1985;229:1397–1400. doi: 10.1126/science.4035356. [DOI] [PubMed] [Google Scholar]
- Sapolsky R, Krey L, McEwen B. Prolonged glucocorticoid exposure reduces hippocampal neuron number: Implications for aging. J Neurosci. 1985;5:1221–1226. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky RM, Packan DR, Vale WW. Glucocorticoid toxicity in the hippocampus: In vitro demonstration. Brain Res. 1988;453:367–371. doi: 10.1016/0006-8993(88)90180-1. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Uno H, Rebert CS, Finch CE. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatzberg AF, Nemeroff CB, editors. The Hypothalamic-Pituitary-Adrenal Axis: Physiology, Pathophysiology, and Psychiatric Implications. Raven Press; New York: 1988. [Google Scholar]
- Squire LR, Ojemann JG, Miezin FM, Petersen SE, Videen TO, Raichle ME. Activation of the hippocampus in normal humans: A functional anatomical study of memory. Proc Nat Acad Sci USA. 1992;89:1837–1841. doi: 10.1073/pnas.89.5.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkman MN, Gebarksi SS, Berent S, Schteingart DE. Hippocampal formation volume, memory dysfunction, and cortisol levels in patients with Cushing’s syndrome. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- Stein B, Sapolsky RM. Chemical adrenalectomy reduces hippocampal damage induced by kainic acid. Brain Res. 1988;473:175–181. doi: 10.1016/0006-8993(88)90332-0. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Lin WJ, Sapolsky RM. Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J Neurochem. 1994;63:596–602. doi: 10.1046/j.1471-4159.1994.63020596.x. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Marsh L, Mathalon DH, Lim KO, Pfefferbaum A. Anterior hippocampal volume deficits in nonamnesic, aging chronic alcoholics. Alcohol Clin Exp Res. 1995;19:110–122. doi: 10.1111/j.1530-0277.1995.tb01478.x. [DOI] [PubMed] [Google Scholar]
- Sutker PB, Winstead DK, Galina ZH, Allain AN. Cognitive deficits and psychopathology among former prisoners of war and combat veterans of the Korean conflict. Am J Psychiatry. 1991;148:67–72. doi: 10.1176/ajp.148.1.67. [DOI] [PubMed] [Google Scholar]
- Uddo M, Vasterling JT, Brailey K, Sutker PB. Memory and attention in posttraumatic stress disorder. J Psychopath Behav Assess. 1993;15:43–52. [Google Scholar]
- Uno H, Tarara R, Else JG, Suleman MA, Sapolsky RM. Hippocampal damage associated with prolonged and fatal stress in primates. J Neurosci. 1989;9:1705–1711. doi: 10.1523/JNEUROSCI.09-05-01705.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uno H, Lohmiller L, Thieme C, Kemnitz JW, Engle MJ, Roecker EB. Brain damage induced by prenatal exposure to dexamethasone in fetal rhesus monkeys: I. Hippocampus. Dev Brain Res. 1990;53:157–167. doi: 10.1016/0165-3806(90)90002-g. [DOI] [PubMed] [Google Scholar]
- Virgin CE, Taryn PTH, Packan DR, et al. Glucocorticoids inhibit glucose transport and glutamate uptake in hippocampal astrocytes: Implications for glucocorticoid neurotoxicity. J Neurochem. 1991;57:1422–1428. doi: 10.1111/j.1471-4159.1991.tb08309.x. [DOI] [PubMed] [Google Scholar]
- Watson C, Andermann F, Gloor P, et al. Anatomic basis of amygdaloid and hippocampal volume: Measurement by magnetic resonance imaging. Neurology. 1992;42:1743–1750. doi: 10.1212/wnl.42.9.1743. [DOI] [PubMed] [Google Scholar]
- Wooley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res. 1990;531:225–231. doi: 10.1016/0006-8993(90)90778-a. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Keefer RSE, Harvey PD, et al. Learning and memory in combat veterans with posttraumatic stress disorder. Am J Psychiatry. 1995;152:137–139. doi: 10.1176/ajp.152.1.137. [DOI] [PubMed] [Google Scholar]


