Thioredoxins are small disulfide-containing proteins that participate in redox regulation in a wide variety of organisms. There are several different plant thioredoxins, which can act as antioxidants by activating enzymes that scavenge reactive oxygen species, such as H2O2. They also regulate a number of key enzymes in processes as diverse as deoxyribonucleotide synthesis, photosynthetic carbon fixation, and anther dehiscense (Meyer et al., 2008).
Thioredoxins activate their target enzymes by reduction of Cys disulfides and, in turn, must be kept in their reduced state via interaction with reduced ferredoxin or NADPH (Montrichard et al., 2009). However, the master regulators that maintain redox homeostasis in the cell remain largely unknown. Yoo et al. (pages 3577–3594) describe two Arabidopsis thaliana proteins, CBSX1 and CBSX2, that activate thioredoxin and in turn help to regulate H2O2 levels in the cell. These proteins contain the cystathionine β-synthase (CBS) domain, which contains a characteristic arrangement of three β sheets and two α helices that binds adenosine-containing ligands, such as AMP, ATP, or NADPH, and helps to regulate the enzymatic activity of adjacent domains (Ignoul and Eggermont, 2005).
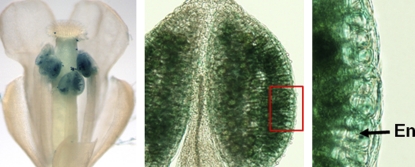
CBSX1 expression was strongest in anthers (see figure) and a CBSX1–green fluorescent protein fusion localized to the chloroplast. In the chloroplast, CBSX1 interacted with four distinct thioredoxins, as shown by yeast two-hybrid and in vitro pull-down studies. The interaction of CBSX1 and thioredoxin also occurred in living plant cells, as shown by bimolecular fluorescence complementation analysis in tobacco (Nicotiana tabacum) leaves.
A ProCBSX1:β-glucuronidase fusion is strongly expressed in Arabidopsis anther tissues. Panels show intact flowers (left), an anther cross section (middle), and a close-up of the endothecium (En) layer enclosed in the red box (right).
Based on these interactions, the authors hypothesize that CBSX1 and CBX2 positively regulate thioredoxins, which then reduce target proteins, such as peroxiredoxin, which in turn reduce cellular H2O2 and regulate its level. They show that both CBSX proteins increase the activity of all four thioredoxins, in some cases more effectively than the chemical reductant DTT. Addition of AMP further increased the activation of all four thioredoxins by CBSX1 and CBSX2, but ADP and ATP did not have this enhancing effect.
The authors also present a crystal structure of CBSX2 and describe a positively charged ligand binding pocket formed by Lys and Arg residues. This pocket is similar to those in other CBS domain proteins that contain adenosine-related ligands. In CBSX1, this pocket can accommodate AMP, but not the bulkier ADP or ATP, consistent with the above demonstration that CBSX1-induced activation of thioredoxin was enhanced only by AMP.
35S:CBSX1-overexpressing plants were sterile, and although the pollen and stigmas appeared normal, the anthers were indehiscent. This indehiscence was due to defective secondary wall thickening in the endothecium, which resulted from deficient deposition of lignin. This deficiency was due to the overactivation of thioredoxins leading to insufficient H2O2 needed for lignin polymerization.
To provide further physiological context, the authors also analyze the expression of known H2O2-regulated genes and thioredoxin-regulated Calvin cycle genes. Finally, they present a model describing how chloroplast CBSX1, ferredoxin, thioredoxins, adenosine-containing ligands, and target enzymes interact to regulate H2O2 levels and help to maintain cellular redox homeostasis and Calvin cycle activity.
References
- Ignoul S., Eggermont J. (2005). CBS domains: Structure, function, and pathology in human proteins. Am. J. Physiol. Cell Physiol. 289: C1369–C1378 [DOI] [PubMed] [Google Scholar]
- Meyer Y., Siala W., Bashandy T., Riondet C., Vignols F., Reichheld J.P. (2008). Glutaredoxins and thioredoxins in plants. Biochim. Biophys. Acta 1783: 589–600 [DOI] [PubMed] [Google Scholar]
- Montrichard F., Alkhalfioui F., Yano H., Vensel W.H., Hurkman W.J., Buchanan B.B. (2009). Thioredoxin targets in plants: The first 30 years. J. Proteomics 72: 452–474 [DOI] [PubMed] [Google Scholar]
- Yoo K.S., Ok S.H., Jeong B.-C., Jung K.W., Cui M.H., Hyoung S., Lee M.-R., Song H.K., Shin J.S. (2011). Single cystathionine β-synthase domain–containing proteins modulate development by regulating the thioredoxin system in Arabidopsis. Plant Cell 23: 3577–3594 [DOI] [PMC free article] [PubMed] [Google Scholar]