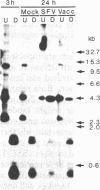
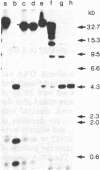
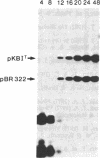
Abstract
A system in which transfected plasmid DNA replicates in the cytoplasm of poxvirus-infected cells is described. A variety of recombinant plasmids was introduced into poxvirus-infected cells by transfection, and replication of input plasmid DNA was monitored by (i) digestion with restriction enzymes that discriminate between input methylated plasmid DNA and unmethylated DNA produced by replication in mammalian cells; (ii) amplification of intracellular plasmid DNA; and (iii) density shift analysis in the presence of BrdUrd. Replication of plasmid DNA was observed in the cytoplasm of cells infected with the tumorigenic leporipoxviruses Shope fibroma virus (SFV) and myxoma, and less extensively with the orthopoxvirus vaccinia, but not in uninfected cells. Unexpectedly, all input plasmids tested, including pBR322, pUC13, polyoma, PM2 phi X174 replicative form (RF), and M13 RF, replicated with equal efficiency in SFV-infected cells, indicating that no specific replication origin sequence is required. The transfected plasmid DNA was replicated concomitantly with the infecting poxviral DNA and by 24 hr post-transfection, it resided predominantly in high molecular weight Dpn I-resistant head-to-tail tandem repeats. The failure to detect unreplicated Dpn I-sensitive plasmid concatemers early in replication together with the absence of significant levels of integrated plasmid sequences in the poxviral genome suggest that replication of the transfected plasmid DNA is not the consequence of nonhomologous recombination of concatemeric plasmid DNA into the poxvirus genome, but rather of an autonomous process that is dependent on trans-acting replication factors produced during virus infection, and that does not require a specific origin sequence on the substrate plasmid DNA.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochran M. A., Mackett M., Moss B. Eukaryotic transient expression system dependent on transcription factors and regulatory DNA sequences of vaccinia virus. Proc Natl Acad Sci U S A. 1985 Jan;82(1):19–23. doi: 10.1073/pnas.82.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dales S., Milovanovitch V., Pogo B. G., Weintraub S. B., Huima T., Wilton S., McFadden G. Biogenesis of vaccinia: isolation of conditional lethal mutants and electron microscopic characterization of their phenotypically expressed defects. Virology. 1978 Feb;84(2):403–428. doi: 10.1016/0042-6822(78)90258-1. [DOI] [PubMed] [Google Scholar]
- Davidson I., Stow N. D. Expression of an immediate early polypeptide and activation of a viral origin of DNA replication in cells containing a fragment of herpes simplex virus DNA. Virology. 1985 Feb;141(1):77–88. doi: 10.1016/0042-6822(85)90184-9. [DOI] [PubMed] [Google Scholar]
- DeLange A. M., Futcher B., Morgan R., McFadden G. Cloning of the vaccinia virus telomere in a yeast plasmid vector. Gene. 1984 Jan;27(1):13–21. doi: 10.1016/0378-1119(84)90234-8. [DOI] [PubMed] [Google Scholar]
- Delange A. M., Macaulay C., Block W., Mueller T., McFadden G. Tumorigenic poxviruses: construction of the composite physical map of the Shope fibroma virus genome. J Virol. 1984 May;50(2):408–416. doi: 10.1128/jvi.50.2.408-416.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban M., Flores L., Holowczak J. A. Topography of vaccinia virus DNA. Virology. 1977 Oct 1;82(1):163–181. doi: 10.1016/0042-6822(77)90040-x. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. I. In vivo DNA synthesis. Virology. 1977 May 1;78(1):57–75. doi: 10.1016/0042-6822(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Esteban M., Holowczak J. A. Replication of vaccinia DNA in mouse L cells. III. Intracellular forms of viral DNA. Virology. 1977 Oct 15;82(2):308–322. doi: 10.1016/0042-6822(77)90006-x. [DOI] [PubMed] [Google Scholar]
- Harland R. M., Laskey R. A. Regulated replication of DNA microinjected into eggs of Xenopus laevis. Cell. 1980 Oct;21(3):761–771. doi: 10.1016/0092-8674(80)90439-0. [DOI] [PubMed] [Google Scholar]
- Kucherlapati R., Skoultchi A. I. Introduction of purified genes into mammalian cells. CRC Crit Rev Biochem. 1984;16(4):349–379. doi: 10.3109/10409238409108719. [DOI] [PubMed] [Google Scholar]
- Loyter A., Scangos G. A., Ruddle F. H. Mechanisms of DNA uptake by mammalian cells: fate of exogenously added DNA monitored by the use of fluorescent dyes. Proc Natl Acad Sci U S A. 1982 Jan;79(2):422–426. doi: 10.1073/pnas.79.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mocarski E. S., Roizman B. Herpesvirus-dependent amplification and inversion of cell-associated viral thymidine kinase gene flanked by viral a sequences and linked to an origin of viral DNA replication. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5626–5630. doi: 10.1073/pnas.79.18.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B., Cooper N. Genetic evidence for vaccinia virus-encoded DNA polymerase: isolation of phosphonoacetate-resistant enzyme from the cytoplasm of cells infected with mutant virus. J Virol. 1982 Aug;43(2):673–678. doi: 10.1128/jvi.43.2.673-678.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Méchali M., Kearsey S. Lack of specific sequence requirement for DNA replication in Xenopus eggs compared with high sequence specificity in yeast. Cell. 1984 Aug;38(1):55–64. doi: 10.1016/0092-8674(84)90526-9. [DOI] [PubMed] [Google Scholar]
- Panicali D., Paoletti E. Construction of poxviruses as cloning vectors: insertion of the thymidine kinase gene from herpes simplex virus into the DNA of infectious vaccinia virus. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4927–4931. doi: 10.1073/pnas.79.16.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peden K. W., Pipas J. M., Pearson-White S., Nathans D. Isolation of mutants of an animal virus in bacteria. Science. 1980 Sep 19;209(4463):1392–1396. doi: 10.1126/science.6251547. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Vaccinia virus replication in enucleate BSC-1 cells: particle production and synthesis of viral DNA and proteins. J Virol. 1974 Feb;13(2):488–493. doi: 10.1128/jvi.13.2.488-493.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G., Berkowitz E. M., Dales S. Investigation of vaccinia virus DNA replication employing a conditional lethal mutant defective in DNA. Virology. 1984 Jan 30;132(2):436–444. doi: 10.1016/0042-6822(84)90048-5. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M. T. The mode of replication of vaccinia virus DNA. Virology. 1978 Jan;84(1):1–8. doi: 10.1016/0042-6822(78)90213-1. [DOI] [PubMed] [Google Scholar]
- Pogo B. G., O'Shea M., Freimuth P. Initiation and termination of vaccinia virus DNA replication. Virology. 1981 Jan 15;108(1):241–248. doi: 10.1016/0042-6822(81)90543-2. [DOI] [PubMed] [Google Scholar]
- Prescott D. M., Kates J., Kirkpatrick J. B. Replication of vaccinia virus DNA in enucleated L-cells. J Mol Biol. 1971 Aug 14;59(3):505–508. doi: 10.1016/0022-2836(71)90313-5. [DOI] [PubMed] [Google Scholar]
- Spaete R. R., Frenkel N. The herpes simplex virus amplicon: a new eucaryotic defective-virus cloning-amplifying vector. Cell. 1982 Aug;30(1):295–304. doi: 10.1016/0092-8674(82)90035-6. [DOI] [PubMed] [Google Scholar]
- Stow N. D. Localization of an origin of DNA replication within the TRS/IRS repeated region of the herpes simplex virus type 1 genome. EMBO J. 1982;1(7):863–867. doi: 10.1002/j.1460-2075.1982.tb01261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarreal E. C., Roseman N. A., Hruby D. E. Isolation of vaccinia virus mutants capable of replicating independently of the host cell nucleus. J Virol. 1984 Aug;51(2):359–366. doi: 10.1128/jvi.51.2.359-366.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldeck W., Rösl F., Zentgraf H. Origin of replication in episomal bovine papilloma virus type 1 DNA isolated from transformed cells. EMBO J. 1984 Sep;3(9):2173–2178. doi: 10.1002/j.1460-2075.1984.tb02109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Bajszár G., Moss B. Mapping of the vaccinia virus thymidine kinase gene by marker rescue and by cell-free translation of selected mRNA. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1210–1214. doi: 10.1073/pnas.79.4.1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills A., Delange A. M., Gregson C., Macaulay C., McFadden G. Physical characterization and molecular cloning of the Shope fibroma virus DNA genome. Virology. 1983 Oct 30;130(2):403–414. doi: 10.1016/0042-6822(83)90095-8. [DOI] [PubMed] [Google Scholar]