This work shows that a blue light photoreceptor, phototropin 2, promotes cylindrical palisade cell development in response to the light stimulus in a tissue-autonomous manner in the leaf. Even a constitutively active fragment of phototropin 2 induces cell elongation along the predetermined axis without the directional cue provided by light.
Abstract
Light is an important environmental information source that plants use to modify their growth and development. Palisade parenchyma cells in leaves develop cylindrical shapes in response to blue light; however, the photosensory mechanism for this response has not been elucidated. In this study, we analyzed the palisade cell response in phototropin-deficient mutants. First, we found that two different light-sensing mechanisms contributed to the response in different proportions depending on the light intensity. One response observed under lower intensities of blue light was mediated exclusively by a blue light photoreceptor, phototropin 2 (PHOT2). Another response was elicited under higher intensities of light in a phototropin-independent manner. To determine the tissue in which PHOT2 perceives the light stimulus to regulate the response, green fluorescent protein (GFP)–tagged PHOT2 (P2G) was expressed under the control of tissue-specific promoters in the phot1 phot2 mutant background. The results revealed that the expression of P2G in the mesophyll, but not in the epidermis, promoted palisade cell development. Furthermore, a constitutively active C-terminal kinase fragment of PHOT2 fused to GFP (P2CG) promoted the development of cylindrical palisade cells in the proper direction without the directional cue provided by light. Hence, in response to blue light, PHOT2 promotes the development of cylindrical palisade cells along a predetermined axis in a tissue-autonomous manner.
INTRODUCTION
Light is an important information source for plants and helps them to optimize their growth and development. The red and blue regions of the light spectrum are most effective in eliciting various physiological and developmental responses. To perceive blue light signals for diverse responses, plants have evolved multiple classes of blue light photoreceptors, including cryptochrome, phototropin, ZEITLUPE, FLAVIN BINDING/KELCH REPEAT/F-BOX1, and LOV KELCH PROTEIN2 proteins (Briggs and Christie, 2002; Demarsy and Fankhauser, 2009). Using these photoreceptors, plants regulate various physiological and developmental processes, such as deetiolation, floral induction, and gene expression (Briggs and Huala, 1999). Among these responses, phototropins mediate responses that include phototropism, chloroplast movement, stomatal opening, and leaf flattening (Huala et al., 1997; Kagawa et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001; Sakamoto and Briggs, 2002), all of which influence photosynthetic efficiency (Takemiya et al., 2005).
Because they are the main photosynthetic organs, leaves effectively change their overall shapes and their interior structures according to the surrounding light conditions. The interior space in leaves is filled with tissue called the mesophyll, which is further divided into adaxial (upper) palisade and abaxial (lower) spongy layers in many dicotyledonous plants (Haberlandt, 1914). When plants are grown under high light conditions, leaves develop palisade tissue, in which cylindrical cells are aligned perpendicular to the epidermis to maximize the efficiency of photosynthesis (Esau, 1977; Terashima and Saeki, 1983; Terashima et al., 2006). Interestingly, the development of cylindrical palisade cells is promoted by blue light in multiple plant species, such as Capsicum annuum (Schuerger et al., 1997), Arabidopsis thaliana (López-Juez et al., 2007), Pelargonium zonale (Fukuda et al., 2008), and Alternanthera brasiliana (Macedo et al., 2011).
The spectral dependence of the palisade cell response suggests that it is controlled by photoreceptors such as phototropins (Schuerger et al., 1997; López-Juez et al., 2007; Fukuda et al., 2008; Macedo et al., 2011). Although López-Juez et al. (2007) reported that cylindrical palisade cells developed even in a phototropin-deficient mutant under 400 μmol m−2 s−1 white light, it remains possible that phototropins mediate the palisade cell response at lower intensities of blue light. We therefore reexamined the palisade cell response using irradiation with mono- and dichromatic blue and red light. As described below, we found that blue light at 30 μmol m−2 s−1 promoted the development of cylindrical palisade cells in the presence of background red light in wild-type Arabidopsis but not in the phototropin-deficient mutant. Hence, phototropins were shown to promote the development of cylindrical palisade cells under lower intensities of blue light.
Phototropin is a light-activated kinase belonging to the cAMP-dependent kinase, cGMP-dependent kinase, and protein kinase C (AGC) VIII Ser/Thr kinase family (Galván-Ampudia and Offringa, 2007). The LOV (light, oxygen, or voltage) domains residing in the N-terminal moiety bind a flavin mononucleotide chromophore to act as a blue light–sensing module (Christie et al., 1999). Upon absorption of light, the inhibitory LOV domain is released from the kinase domain to trigger physiological responses (Matsuoka and Tokutomi, 2005). Isolated C-terminal kinase fragments of phototropin, such as P2CG, exhibit constitutive kinase activity both in vitro and in vivo (Matsuoka and Tokutomi, 2005; Kong et al., 2007).
In Arabidopsis, there are two phototropins (PHOT1 and PHOT2) (Huala et al., 1997; Kagawa et al., 2001), of which PHOT1 is more sensitive to light (Sakai et al., 2001). Both PHOT1 and PHOT2 are localized mainly in the plasma membrane region (Sakamoto and Briggs, 2002; Kong et al., 2006; Wan et al., 2008). In addition, parts of PHOT1 and PHOT2 translocate from the plasma membrane to the cytoplasm (Sakamoto and Briggs, 2002; Han et al., 2008) and Golgi apparatus (Kong et al., 2006), respectively, in response to blue light stimuli.
PHOT1 and PHOT2 are expressed in almost all plant tissues (Sakamoto and Briggs, 2002; Kong et al., 2006; Wan et al., 2008). However, the identities of the tissues in which phototropins regulate growth responses, such as phototropism and leaf flattening, have not previously been determined. For example, phytochrome B in the mesophyll regulates flowering through suppression of Flowering Locus T expression in veins (Endo et al., 2005). It is possible that phototropin expressed outside of the palisade tissue mediates the growth response in palisade cells. Hence, it is important to determine the photoperceptive site at the tissue level. Recently, tissue-specific promoters of CHLOROPHYLL A/B BINDING PROTEIN3 (CAB3; mesophyll-specific) and 3-KETOACYL-COA SYNTHASE6 (CER6; epidermis-specific) genes have been used to express cryptochrome 2 in target tissues (Endo et al., 2007). This approach should be useful to address the problem of phototropin signaling localization.
Because the cylindrical palisade cells are oriented perpendicular to the leaf surface, the incident light within the leaf might guide the directional growth of the palisade cells. If this is the case, the mechanism by which phototropins determine the growth axis according to the direction of incident light is of great interest. Alternatively, phototropin might trigger the onset of cell growth along a predetermined axis.
In this study, we genetically identified phototropins to be the photoreceptor that regulates palisade cell development under lower-intensity blue light. Spectral and genetic analyses further revealed that the response was mediated mainly by PHOT2. We then established transgenic lines that expressed green fluorescent protein (GFP)–tagged PHOT2 (P2G), in a tissue-specific manner. PHOT2 was thereby found to promote the palisade cell development in a tissue-autonomous manner. In addition, the C-terminal kinase fragment of PHOT2 fused to GFP (P2CG) could induce the development of cylindrical palisade cells in the proper direction without any light stimulus, indicating that the incident light did not provide a directional cue at the cellular level.
RESULTS
A Blue Light Photoreceptor, Phototropin, Promotes Development of Cylindrical Palisade Cells
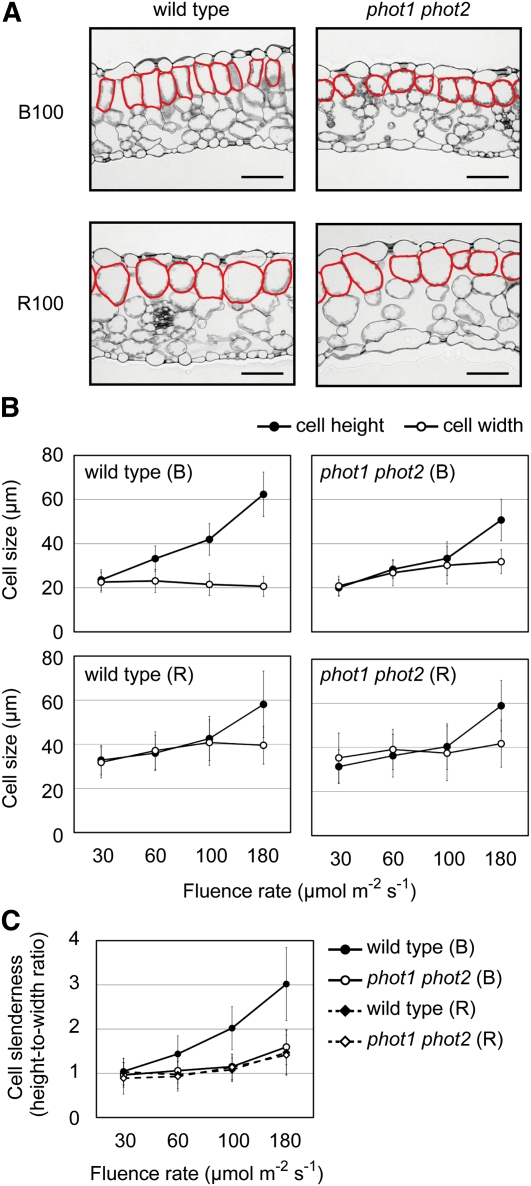
It has previously been reported that cylindrical palisade cells develop in response to blue, but not red, light (Schuerger et al., 1997; López-Juez et al., 2007; Fukuda et al., 2008; Macedo et al., 2011). First, we confirmed those reports under monochromatic lights with different intensities from 30 to 180 μmol m−2 s−1. Transverse sections were prepared from the fourth leaves of wild-type Arabidopsis seedlings grown under 100 μmol m−2 s−1 blue light for 10 d or under red light for 20 d (Figure 1A). As expected, cylindrical cells were observed in the subepidermal palisade layer in leaves grown under blue light but not in leaves grown under red light (Figure 1A).
Figure 1.
Palisade Cell Development under Monochromatic Blue and Red Light.
(A) Transverse sections of fourth leaves collected from wild-type and phot1 phot2 seedlings grown under 100 μmol m−2 s−1 blue (B100) or 100 μmol m−2 s−1 red (R100) light. Subepidermal palisade cells are outlined in red. Bars = 50 μm.
(B) Heights and widths of the subepidermal palisade cells in the fourth leaves under different fluence rates of monochromatic blue (B) and red (R) lights. Data are means ± sd (n ≥ 80 from at least 11 leaves).
(C) Slenderness of the subepidermal palisade cells was determined by the ratios of heights to widths from (B). Data are means ± sd (n ≥ 80 from at least 11 leaves).
We then determined cell heights in the anticlinal direction and cell widths in the periclinal direction on the sections. In red light, statistically significant increases in both cell height and width were observed with increasing light intensity up to 100 μmol m−2 s−1 (P < 0.05, Student’s t test; Figure 1B). By contrast, blue light increased cell height but not cell width up to 180 μmol m−2 s−1. To compare the cell slenderness under different light conditions, the height-to-width ratios were calculated (Figure 1C). Cell slenderness increased with increasing the blue light intensity, whereas cells remained almost spherical in red light up to 100 μmol m−2 s−1 (Figure 1C). Similarly, the cell slenderness was increased under strong white light (see Supplemental Figures 1A and 1C online). Although the effect was not clear in very young leaves, the cells became more slender as they developed (see Supplemental Figure 1B online).
Two phototropins, PHOT1 and PHOT2, function redundantly in Arabidopsis (Huala et al., 1997; Kagawa et al., 2001, 2009; Kinoshita et al., 2001; Sakai et al., 2001; Sakamoto and Briggs, 2002). We therefore examined the response in the phot1 phot2 double mutant under the same light conditions. The phot1 phot2 palisade cells remained spherical even under 100 μmol m−2 s−1 blue (Figures 1A to 1C) or 350 μmol m−2 s−1 white light (see Supplemental Figures 1A to 1C online), indicating that phototropins almost exclusively mediated the response under these conditions. Furthermore, cell slenderness was increased even in phot1 phot2 under blue light ranging from 100 to 180 μmol m−2 s−1 (Figure 1C).
PHOT2 Is Primarily Responsible for Regulating the Development of Cylindrical Palisade Cells
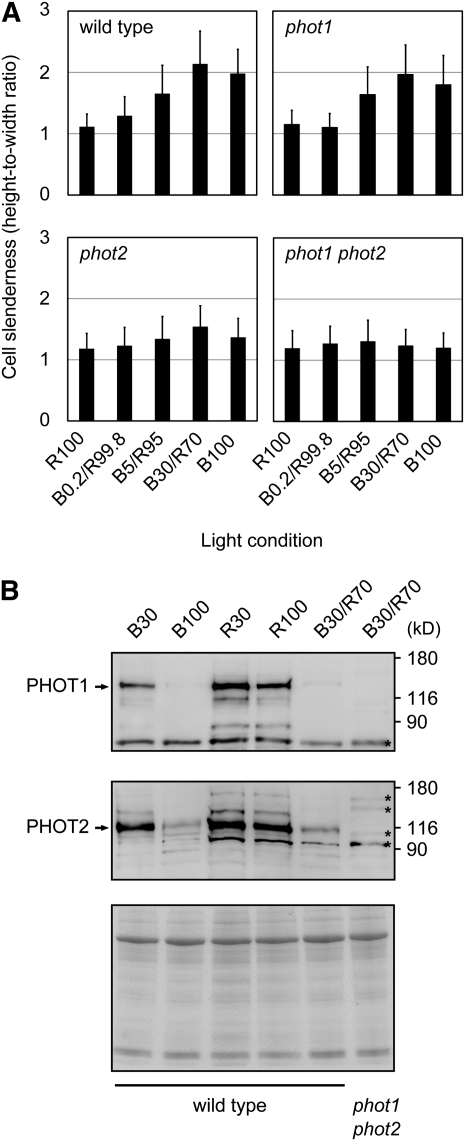
No palisade cell response was observed under monochromatic blue light at 30 μmol m−2 s−1 (Figure 1C). Hence, the observed response appeared to be less sensitive than other phototropin-mediated responses, such as phototropism, chloroplast avoidance movement, and stomatal opening (Huala et al., 1997; Kagawa et al., 2001; Kinoshita et al., 2001; Sakai et al., 2001). To further investigate this phenomenon, we compared the effects of lower intensities of blue light combined with background red light (Figure 2A). The response under 30 μmol m−2 s−1 blue light in the presence of 70 μmol m−2 s−1 red light (B30/R70) was indistinguishable from that under monochromatic 100 μmol m−2 s−1 blue light (Figure 2A). Even 5.0 μmol m−2 s−1 blue light in combination with 95 μmol m−2 s−1 red light (B5/R95) was partially effective in eliciting the response (Figure 2A). Hence, the development of slender cells may require not only phototropin activation but also a certain amount of light to sustain cell growth.
Figure 2.
Palisade Cell Development in phot1 and phot2 Single Mutants and in the phot1 phot2 Double Mutant.
(A) Slenderness of the subepidermal palisade cells was determined as for Figure 1C. Blue (B) and red (R) light intensities are given in μmol m−2 s−1. The total fluence rate was adjusted to 100 μmol m−2 s−1 in all the combinations. Data are means ± sd (n ≥ 90 from at least 11 leaves).
(B) Immunoblot analysis of PHOT1 and PHOT2 in young third and fourth leaves. Crude protein extracts were prepared from third and fourth leaves grown for 10 d under different light conditions as indicated on the top of the blot. Blue (B) and red (R) light intensities are given in μmol m−2 s−1. The blots were probed with anti-PHOT1 (top) or anti-PHOT2 (middle) polyclonal antibodies. Arrows indicate the positions of PHOT1 and PHOT2. Nonspecific bands are marked with asterisks. Aliquots of the samples were stained with Coomassie blue (bottom). The molecular mass marker sizes in kilodaltons are shown on the right of the blots.
PHOT1 is usually more sensitive to light than PHOT2 (Sakai et al., 2001). We therefore examined the palisade cell response in the phot1 and phot2 single mutants. The response was substantially reduced in the phot2 mutant, whereas the phot1 mutant showed an almost normal response (Figure 2A). These results indicated that the blue light–dependent development of cylindrical palisade cells was mediated principally by PHOT2. Some responses mediated by PHOT2, such as the chloroplast avoidance response, are clearly less sensitive than the present response (Harada and Shimazaki, 2007). However, the chloroplast accumulation response mediated by PHOT2 is elicited by only 2 μmol m−2 s−1 blue light (Kagawa et al., 2001; Sakai et al., 2001). Hence, the blue light sensitivity of the palisade cell response was relatively high but within the range of known PHOT2-mediated responses.
The levels of PHOT1 and PHOT2 can be affected by light conditions (Sakamoto and Briggs, 2002; Kong et al., 2006). Hence, we examined the levels of both proteins by immunoblotting (Figure 2B). The PHOT1 level in the fourth leaves was substantially reduced under 100 μmol m−2 s−1 blue light, whereas the level remained relatively high under 30 μmol m−2 s−1 blue light (Figure 2B). As expected, PHOT1 was more stable under monochromatic red light at either 30 or 100 μmol m−2 s−1. Interestingly, the level was substantially reduced under B30/R70 light (Figure 2B), indicating that 30 μmol m−2 s−1 blue and 70 μmol m−2 s−1 red lights synergistically reduced the level of PHOT1. In contrast with PHOT1, the PHOT2 level has been reported not to be affected by blue light (Sakamoto and Briggs, 2002; Kong et al., 2006). However, it was reduced to some extent under both B100 and B30/R70 conditions compared with R100 (Figure 2B).
PHOT2 Regulates the Development of Cylindrical Palisade Cells in a Tissue-Autonomous Manner
The PHOT2 promoter is active in most leaf tissues, including the mesophyll and epidermis (Kong et al., 2006). To elucidate the photoperceptive site within the leaf for the palisade cell response, we developed the transgenic plants that expressed P2G under the control of the CAB3 and CER6 promoters, which have been used for mesophyll-specific (CAB3) and epidermis-specific (CER6) expression of exogenous proteins (Endo et al., 2007). As a control, the P2G gene was expressed under the control of the native PHOT2 promoter (Kong et al., 2006).
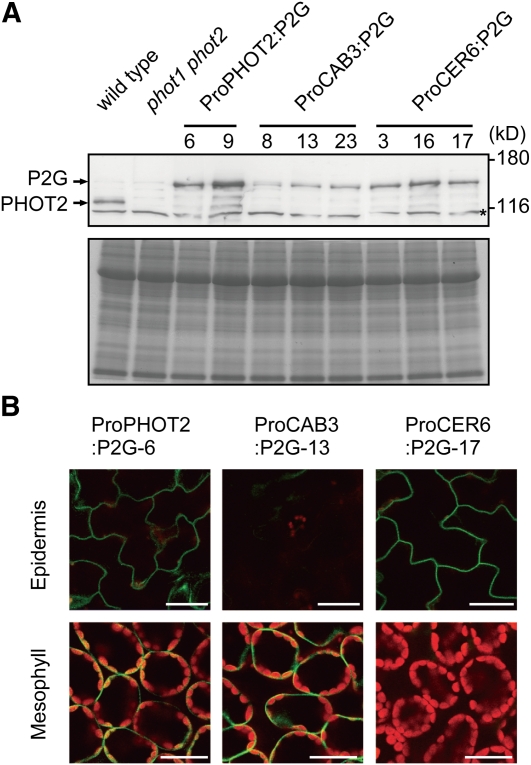
Each of these three P2G fusion constructs was transformed into the phot1 phot2 double mutant to establish lines named ProPHOT2:P2G, ProCAB3:P2G, and ProCER6:P2G, respectively. Immunoblot analysis with anti-PHOT2 polyclonal antibody revealed that the P2G protein was expressed at the predicted size (~130 kD) in all the lines (Figure 3A). We then examined the tissue-specific expression of P2G under a confocal laser scanning microscope. As expected, GFP fluorescence was detected only in the mesophyll and epidermis in ProCAB3:P2G and ProCER6:P2G plants, respectively (Figure 3B; see Supplemental Figure 2 online). By contrast, the fluorescence was observed in both tissues in the ProPHOT2:P2G plants (Figure 3B; see Supplemental Figure 2 online).
Figure 3.
Analysis of P2G Expression in ProCAB3:P2G and ProCER6:P2G.
(A) The P2G protein was detected by immunoblotting with an anti-PHOT2 polyclonal antibody. Crude protein extracts were prepared from leaves grown under continuous 70 μmol m−2 s−1 white light for 3 weeks. Arrows indicate the positions of P2G and the endogenous PHOT2 (top). A nonspecific band is marked with an asterisk. Aliquots of the samples were stained with Coomassie blue (bottom). The molecular mass marker sizes in kilodaltons are shown on the right of the blot.
(B) Intracellular distribution of the P2G protein. GFP and chlorophyll fluorescence were observed in the fourth leaves under a confocal laser scanning microscope. Plants were grown under continuous 70 μmol m−2 s−1 white light for 21 d for the observation of GFP-tagged proteins. Paradermal views in the epidermal (top) and subepidermal (bottom) layers are shown. The images are false-colored to indicate GFP (green) and chlorophyll (red) fluorescence. Bars = 20 μm.
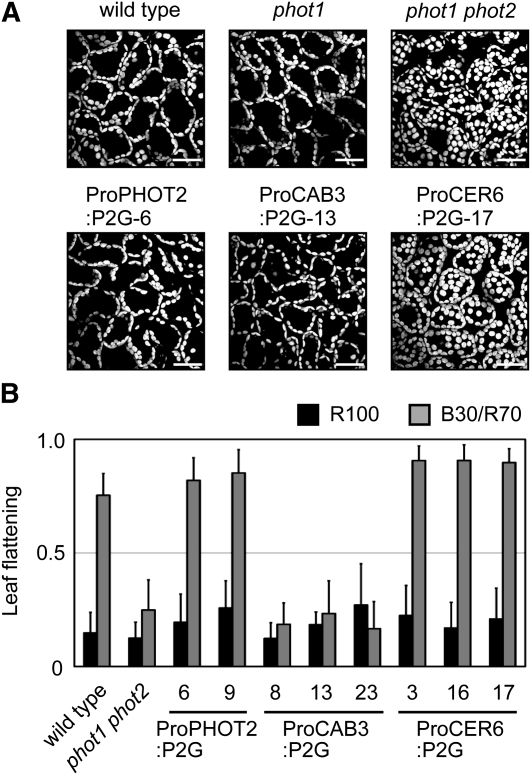
To validate the tissue-specific expression of the above lines functionally, we examined the chloroplast avoidance response, which is known to be regulated by PHOT2 in a cell-autonomous manner (Kagawa et al., 2001). Under 50 μmol m−2 s−1 blue light, chloroplasts avoided the light and accumulated at the anticlinal sides of the cells in wild-type, phot1, and ProPHOT2:P2G plants (Figure 4A), as previously reported (Kagawa et al., 2001; Kong et al., 2007). As expected, the same response was observed in ProCAB3:P2G plants, whereas chloroplasts stayed at the tops of the cells in phot1 phot2 and ProCER6:P2G plants (Figure 4A).
Figure 4.
Functional Analysis of P2G Expression in ProCAB3:P2G and ProCER6:P2G.
(A) Chloroplast avoidance responses under 50 μmol m−2 s−1 blue light in the transgenic plants. Chloroplast autofluorescence was observed in the fourth leaves under a confocal laser scanning microscope. Bars = 50 μm.
(B) Leaf flattening response in the transgenic lines expressing P2G. Plants were grown under 100 μmol m−2 s−1 red light (R100) or a combination of 30 μmol m−2 s−1 blue and 70 μmol m−2 s−1 red lights (B30/R70). Leaf transverse sections were prepared and subjected to flattening analysis (as described in Methods). Data are means ± sd (n ≥ 12).
PHOT1 and PHOT2 redundantly regulate the leaf-flattening response (Sakamoto and Briggs, 2002; Inoue et al., 2008; de Carbonnel et al., 2010). Accordingly, curled leaves were observed in phot1 phot2 (Figure 4B; see Supplemental Figure 3A online). In contrast with the chloroplast avoidance response, ProCER6:P2G, but not ProCAB3:P2G, complemented this phenotype (Figure 4B; see Supplemental Figure 3A online). Namely, the ProCAB3:P2G plants had curled leaves even under blue light (Figure 4B; see Supplemental Figure 3A online). P2G in the epidermis, but not in the mesophyll, therefore restored the leaf flattening response. Taken together, this evidence showed that P2G was expressed in the target tissues.
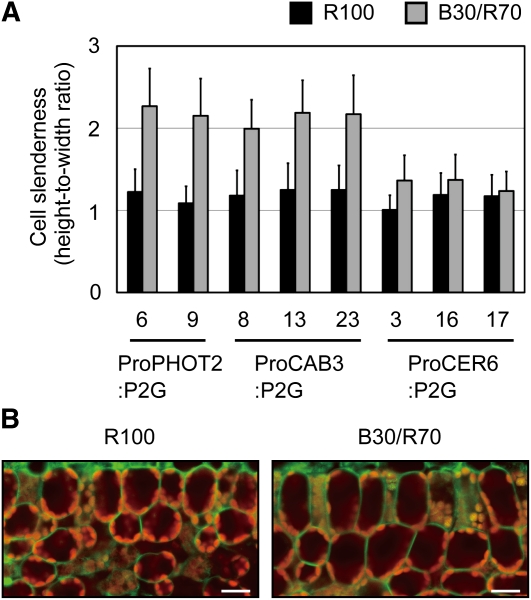
We then examined the palisade cell development in the above lines under different light conditions. As expected, no response was observed under 100 μmol m−2 s−1 red light in all the lines (Figure 5A). Importantly, cylindrical palisade cells were observed under B30/R70 light in the ProCAB3:P2G plants, but not in the ProCER6:P2G plants. Hence, the mesophyll was demonstrated to be the photoperceptive site for this response.
Figure 5.
Regulation of Palisade Cell Development by PHOT2 in a Tissue-Autonomous Manner.
Plants were grown under 100 μmol m−2 s−1 red light (R100) or a combination of 30 μmol m−2 s−1 blue and 70 μmol m−2 s−1 red lights (B30/R70).
(A) Slenderness of the subepidermal palisade cells in transgenic plants was determined as for Figure 1C. Data are means ± sd (n ≥ 75 from at least 10 leaves).
(B) GFP fluorescence distribution in palisade cells of ProPHOT2:P2G-6. Transverse sections of young leaves were observed under a confocal laser scanning microscope. The images are false-colored to indicate GFP (green) and chlorophyll (red) fluorescence. Bars = 20 μm.
The Direction of the Development of Cylindrical Palisade Cells Is Determined Independently of the Direction of the Incident Light
To examine whether PHOT2 was distributed along the developmental axis in palisade cells, we observed the P2G fluorescence in ProPHOT2:P2G plants. In the transverse sections of leaves, the P2G fluorescence was uniformly detected in the plasma membrane region regardless of light conditions (Figure 5B). Hence, no correlation was observed between P2G intracellular distribution and the directional development of the cells.
We further examined the possibility that incident light acted as a directional cue for the development of cylindrical cells with the aid of the Pro35S:P2CG transgenic plants (previously described as 35-P2CG/p1p2-14 in Kong et al., 2007). In this line, PHOT2-mediated physiological responses, such as stomatal opening and chloroplast avoidance, are induced without light stimulus due to constitutive expression of the P2CG fragment (Kong et al., 2007).
The Pro35S:P2CG plants were grown under 100 μmol m−2 s−1 red light or B30/R70 light and subjected to the anatomical analyses. Cylindrical palisade cells were found to have developed even in red light (Figure 6A). Furthermore, those cylindrical cells were oriented perpendicular to the leaf surface (Figure 6A). The slenderness values were the same in the two light conditions (Figure 6B; see Supplemental Figure 4A online). Since the P2CG fragment lacks the photosensory N-terminal LOV domains (Kong et al., 2007), we conclude that the direction of the development of cylindrical cells is determined independent of the direction of incident light.
Figure 6.
Promotion of Palisade Cell Development in Pro35S:P2CG.
The Pro35S:P2CG plants were grown under the light conditions described in Figure 5.
(A) Transverse sections of the fourth leaves. The subepidermal palisade cells are outlined in red. Bars = 50 μm.
(B) Slenderness of the subepidermal palisade cells in Pro35S:P2CG was determined as for Figure 1C. Data are means ± sd (n ≥ 100 from at least 15 leaves).
We also examined the intracellular distribution of P2CG in the Pro35S:P2CG plants. The localization of P2CG was uniformly detected to be in the plasma membrane region in palisade cells (see Supplemental Figure 4B online), as is the case with P2G (Figure 5B), though the expression level was much lower in the Pro35S:P2CG plants. Hence, we confirmed that the phototropin localization in the cells was not directly related to the determination of the developmental axis.
The Development of Cylindrical Palisade Cells Is Independent of the Chloroplast Position
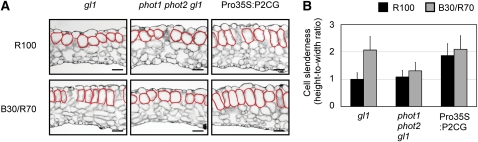
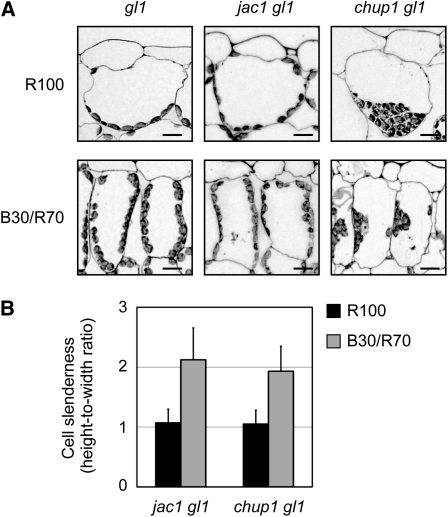
PHOT2 promotes chloroplast accumulation at the anticlinal side of palisade cells under relatively strong blue light (Kagawa et al., 2001). This avoidance response is constitutively induced in the Pro35S:P2CG cells (Kong et al., 2007). Therefore, we thought that the development of cylindrical cells might be a direct consequence of the position adopted by chloroplasts to avoid light. To test this possibility, we examined the palisade cell development in the chloroplast-positioning mutants j-domain protein required for chloroplast accumulation response1 (jac1) glabra1 (gl1) (Suetsugu et al., 2005) and chloroplast unusual positioning1 (chup1) gl1 (Kasahara et al., 2002; Oikawa et al., 2003).
We first confirmed that the chloroplast light avoidance response was induced under B30/R70. For this experiment, wild-type plants carrying the gl1 mutation were used because the other mutants examined here were all in the gl1 mutant background. Consequently, gl1 plants but not phot1 phot2 gl1 plants exhibited the response (see Supplemental Figure 5A online). As reported previously (Suetsugu et al., 2005), avoidance-like distribution of chloroplasts was observed in jac1 gl1 under both 100 μmol m−2 s−1 red light and B30/R70 light (Figure 7A; see Supplemental Figure 5A online). Nevertheless, cylindrical palisade cells were observed only under B30/R70 light (Figure 7B; see Supplemental Figure 5B online). Hence, accumulation of chloroplasts at the anticlinal side of the palisade cells did not necessarily induce the development of cylindrical cells.
Figure 7.
Palisade Cell Development Is Independent of Chloroplast Position.
The jac1 gl1 and chup1 gl1 mutants were grown under different light conditions as for Figure 5.
(A) Chloroplast localization patterns in jac1 gl1 and chup1 gl1. Chloroplasts were observed in the transverse sections of subepidermal palisade cells. Bars = 50 μm.
(B) Slenderness of the subepidermal palisade cells in jac1 gl1 and chup1 gl1 was determined as for Figure 1C. Data are means ± sd (n ≥ 100 from at least 15 leaves).
In chup1 gl1 plants, chloroplasts aggregated at the bottoms or anticlinal sides of the cells depending on the light conditions (Figure 7A; see Supplemental Figure 5A online) (Kasahara et al., 2002). Despite this abnormal chloroplast positioning, cylindrical palisade cells developed normally in B30/R70 (Figure 7B; see Supplemental Figure 5B online). Taken together, this evidence shows that the development of cylindrical cells was independent of the position of the chloroplasts.
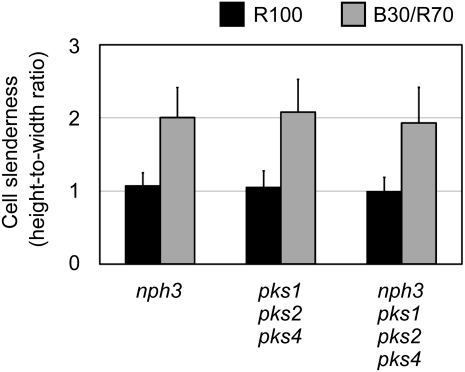
NPH3 and PKS Are Not Involved in the Palisade Cell Response
It has previously been reported that NON-PHOTOTROPIC HYPOCOTYL3 (NPH3) and PHYTOCHROME KINASE SUBSTRATE (PKS) family proteins function in the phototropic and leaf flattening responses downstream of phototropins (Motchoulski and Liscum, 1999; Lariguet et al., 2006; Inoue et al., 2008; de Carbonnel et al., 2010). To address whether those factors are involved in the palisade cell response, the palisade cells of the nph3 single, pks1 pks2 pks4 triple, and nph3 pks1 pks2 pks4 quadruple mutants were observed. The measurement of palisade cell sizes revealed that these mutants normally developed cylindrical palisade cells under B30/R70 light (Figure 8; see Supplemental Figure 6 online). The above-mentioned factors were therefore not involved in the palisade cell response.
Figure 8.
Palisade Cell Development in nph3 and pks1 pks2 pks4.
The nph3 single, pks1 pks2 pks4 triple, and nph3 pks1 pks2 pks4 quadruple mutants were grown as for Figure 5. Slenderness of the subepidermal palisade cells was determined as for Figure 1C. Data are means ± sd (n ≥ 150 from at least 20 leaves).
The blue light photoreceptor PHOT2 was found to promote the development of cylindrical palisade cells in a tissue-autonomous manner. However, the molecular mechanism for this response remains unclear. Phototropism is caused by the differential cell growth between the lit and shaded sides of a cell or tissue. Nevertheless, factors such as NPH3 and PKS proteins, which were involved in phototropism, were not required for the present response. Hence, PHOT2 might regulate the palisade cell growth by a different mechanism. In this respect, it will be worth testing whether the plant hormone auxin, which is known to be involved in phototropism, participates in this response.
DISCUSSION
Palisade Cell Development Is Regulated Differently by Red and Blue Light
In this study, we examined palisade cell development in Arabidopsis leaves grown under mono- and dichromatic red and blue light conditions. As reported previously (Schuerger et al., 1997; López-Juez et al., 2007; Fukuda et al., 2008; Macedo et al., 2011), blue light was more effective than red light in promoting the development of cylindrical palisade cells (Figure 1C). It should be noted here that not only blue but also red light at 180 μmol m−2 s−1 increased cell slenderness to some extent (Figure 1C). This response was shown to be phototropin independent, as phot1 phot2 exhibited similar responses to blue and red light at 180 μmol m−2 s−1 (Figure 1C). Under bright sunlight, intensities of red and blue light would reach this value (Smith, 1982). Hence, palisade cell development might be regulated by multiple mechanisms under natural conditions, each of which could contribute to a different extent depending on the light intensity and spectrum. In earlier studies, intense white light rather than monochromatic light was used to study palisade cell development (Esau, 1977; López-Juez et al., 2007). Hence, these two responses might have been inadvertently confused in those studies.
Under light intensities below 100 μmol m−2 s−1, only blue light increased cell slenderness (Figure 1C). Furthermore, this promotion was not significant in phot1 phot2 (Figure 1C). Hence, phototropins almost exclusively mediated the palisade cell response to lower intensities of blue light. Phototropins have been found to function in a range of the intensities from 0.01 to 100 μmol m−2 s−1 of blue light to mediate the phototropic response (Sakai et al., 2001). Consistent with this observation, the present response was almost saturated with 30 μmol m−2 s−1 blue light when combined with background red light (Figure 2A).
In contrast with the blue light responses mediated by phototropins, the mechanism by which higher intensities of light promoted palisade cell development remains less clear. It is well established that irradiation with intense light increases the levels of reactive oxygen species (ROS) (Li et al., 2009). Interestingly, ROS are involved in cell expansion during organ morphogenesis (Carol and Dolan, 2006). Furthermore, ROS promote root hair growth through the regulation of calcium ion influx (Foreman et al., 2003; Takeda et al., 2008). Hence, it is possible that ROS are involved in palisade cell development under high-intensity light.
López-Juez et al. (2007) reported that cylindrical palisade cells developed even in phot1 phot2 plants grown under 400 μmol m−2 s−1 white light. As shown in Figure 1C, wavelength-independent cylindrical cell development was observed under intense monochromatic lights at 180 μmol m−2 s−1. In addition, we confirmed that cell slenderness increased to some extent in phot1 phot2 and ProCER6:P2G plants under 350 μmol m−2 s−1 white light (see Supplemental Figures 1A and 1C online). However, the extent of the response was much weaker than the one reported by López-Juez et al. (2007).
Possible explanations for the apparent discrepancy are as follows. First, the white light source used in this study (see Supplemental Figure 1D online) was spectrally different from the one used before (López-Juez et al., 2007). For instance, the former light is more enriched in red light than the present one (see Supplemental Figure 1D online), which might explain why the palisade cell development was observed even in phot1 phot2 in the previous work. Another explanation is that the phot1-101 phot2-5 double mutant used in the previous study was established in genetically heterogeneous background between Landsberg erecta and Wassilewskija (López-Juez et al., 2007), whereas all the mutant lines used in this study were in pure Columbia background.
The cylindrical shape of palisade cells has been proposed to increase the photosynthetic efficiency (Terashima and Saeki, 1983; Terashima et al., 2006). Hence, we compared the photosynthetic activities in wild-type and phot1 phot2 leaves by measuring the CO2 assimilation rate (see Supplemental Figure 7 online). Interestingly, the activity was higher in the leaves grown under B30/R70 than the ones grown under R100 regardless of the genotype. However, no difference was observed between the wild type and phot1 phot2 under both light conditions. Hence, it remains unclear to what extent the palisade cell shape contributes to the photosynthesis. The difference might be too small to be detected in the method we employed.
PHOT2 but Not PHOT1 Mediates the Palisade Cell Response
The palisade cell response was almost abolished in the phot2 mutant, whereas the phot1 mutant exhibited a normal response under B30/R70 (Figure 2A). Hence, we conclude that this response is mediated principally by PHOT2. Although PHOT1 and PHOT2 have different photosensitivities, which are determined by the N-terminal moiety (Aihara et al., 2008), they function redundantly in most responses, including phototropism, chloroplast accumulation, stomatal opening, and leaf flattening (Briggs and Christie, 2002; Sakamoto and Briggs, 2002; Inoue et al., 2008; Kagawa et al., 2009). The chloroplast avoidance response, however, was regulated only by PHOT2 (Kagawa et al., 2001; Sakai et al., 2001).
PHOT1 is less stable than PHOT2 under blue light (Sakamoto and Briggs, 2002; Kong et al., 2006). Indeed, the PHOT1 level was strongly reduced in blue light, but not in red light (Figure 2B). Furthermore, PHOT2 was more stable than PHOT1 under B30/R70 light (Figure 2B). Hence, the degradation of PHOT1 might be promoted under the conditions in which the palisade cell development was observed. However, it remains unclear why PHOT1 fails to respond to the B0.2/R99.8 light regardless of the high sensitivity of PHOT1 observed for other responses. It will be interesting to test whether PHOT1 can induce the response when it is expressed in mesophyll under stronger promoters, such as the 35S and CAB3 promoters.
Cell-Autonomous Regulation of Palisade Cell Development by PHOT2
To understand how PHOT2 regulates the palisade cell development, it is essential to know in which tissue the blue light stimulus is perceived. The CAB3 and CER6 promoters functioned successfully to express PHOT2 in the mesophyll and epidermis, respectively (Figures 3A and 3B). The epidermis has been found to restrict cell expansion in the inner tissue layer, although the intertissue signal remains unknown (Savaldi-Goldstein et al., 2007). However, PHOT2 in the mesophyll was found to promote the palisade cell development (Figure 5A). Hence, the mechanism to coordinately regulate the cell growth in an organ may vary depending on the organ type.
Although we could not exclude the possibility that PHOT2 in the spongy mesophyll tissue layer promoted palisade cell development, it is simpler to assume that the response is regulated in a cell-autonomous manner. First, P2G expressed in the epidermis, but not that in the mesophyll, restored the leaf flattening response probably through cell-autonomous regulation of cell growth (Figure 4B). Stomatal opening and chloroplast movement are regulated by PHOT2 in cell-autonomous manners (Kagawa et al., 2001; Kinoshita et al., 2001). Hence, it might be a characteristic of phototropin-mediated responses that the cell directly and promptly responds to the light stimulus.
The mesophyll is the main site of photosynthesis. It therefore seems reasonable that mesophyll cells monitor their surrounding light environment by themselves. It is intriguing that PHOT2 appears to regulate both the development of cylindrical cells and chloroplast movement in the same palisade cells (Kagawa et al., 2001). Indeed, chloroplasts can avoid damage by strong light more efficiently in cylindrical cells, where there is more room to escape from the light. Nevertheless, these processes were regulated independently by PHOT2 because the chloroplast-positioning mutants, such as jac1 gl1 and chup1 gl1, still exhibited the cylindrical palisade cell development (Figure 7).
In addition to PHOT2, phytochrome B and cryptochrome 2 have been shown to regulate developmental processes such as floral initiation in mesophyll and vascular cells, respectively (Endo et al., 2005, 2007). Hence, a picture emerges that plants use photoreceptors at different sites in the leaf to coordinately regulate various developmental and physiological processes.
Signal Transduction Mechanisms for PHOT2 in Mesophyll Cells
This study provides insights into the mechanism by which PHOT2 promotes the development of cylindrical palisade cells. It was attractive to hypothesize that the incident light directly provided a directional cue for palisade cell development. However, the constitutively active P2CG fragment promoted the development of cylindrical cells regardless of the fact that it lacked the N-terminal photosensory domain (Figures 6A and 6B). In addition, the intracellular distribution of P2G was found to be in the plasma membrane region regardless of the light conditions (Figure 5B). Thus, palisade cells do not appear to use the light as a directional cue to determine their developmental axes.
It is also possible that PHOT2 regulates the direction of palisade cell development through the regulation of chloroplast positioning. Indeed, the development of cylindrical cells and the avoidance position of chloroplasts correlated in wild-type, phot1 phot2, and Pro35S:P2CG plants (Kagawa et al., 2001; Kong et al., 2007). However, cylindrical cell development was partially induced in response to B5/R95 (Figure 2A), in which chloroplasts were accumulated in the upper surface of the cell (Sakai et al., 2001). Furthermore, the jac1 gl1 and chup1 gl1 mutants, in which chloroplasts are abnormally distributed (Figure 7A; Kasahara et al., 2002; Suetsugu et al., 2005), exhibited normal palisade cell responses (Figure 7B). Hence, these two processes have been demonstrated to be independently regulated by PHOT2.
Several phototropin-interacting proteins have been identified. The NPH3/RPT2 and the PKS family proteins, which physically interact with phototropins, are involved in phototropic and leaf flattening responses (Motchoulski and Liscum, 1999; Lariguet et al., 2006; Inoue et al., 2008; de Carbonnel et al., 2010). However, we found that the nph3 and pks multiple mutants exhibited normal palisade cell responses (Figure 8). Hence, the PHOT2 signaling pathway for palisade cell regulation was independent of those factors.
The phototropic response has been proposed to be regulated by asymmetric auxin distribution caused by polar PIN-FORMED3 (PIN3) localization (Friml et al., 2002; Blakeslee et al., 2004). Furthermore, the auxin response is globally elevated in P2CG seedlings (Kong et al., 2007). Hence, the auxin responses elicited by blue light in the mesophyll might lead to the observed palisade cell development. To test this possibility, effects of exogenous auxin on palisade cell development should be examined in future studies. Likewise, auxin transport inhibitors, such as 1-N-naphthylphthalamic acid, and the auxin resistant mutants, such as axr1 and axr2, in which auxin signals are strongly suppressed (Lincoln et al., 1990; Wilson et al., 1990), would provide alternative tools to address this question.
It is noteworthy that phototropins belong to the AGC VIII kinase family (Bögre et al., 2003). Another member of this family, PINOID, phosphorylates the auxin-efflux carriers, such as PIN proteins, and also regulates their polar localization through intracellular trafficking (Geldner et al., 2001; Kleine-Vehn et al., 2009; Huang et al., 2010). Furthermore, ADP ribosylation factor, which is involved in vesicle trafficking (Takeuchi et al., 2002), has been shown to interact with PHOT1 in vitro (Sullivan et al., 2009). It has been reported that a part of PHOT2 is translocated in response to blue light from plasma membrane to the Golgi apparatus (Kong et al., 2006), which plays a crucial role in vesicle trafficking (Hawes, 2005). Hence, PHOT2 might promote the palisade cell development by altering the auxin distribution through its interaction with ADP ribosylation factor proteins.
METHODS
Plant Materials
Arabidopsis thaliana mutants and transgenic plants were used in this study. The Columbia accession was used as the wild type. The phot1-5 gl1-1 (Huala et al., 1997), phot2-1 gl1-1 (Kagawa et al., 2001), and phot1-5 phot2-1 gl1-1 (Kinoshita et al., 2001) mutant plants were crossed with the wild type to obtain respective mutants in the wild-type background. The gl1-1 (Oppenheimer et al., 1991), phot1-5 phot2-2 gl1-1 (Onodera et al., 2005), jac1-1 gl1-1 (Suetsugu et al., 2005), chup1-2 gl1-1 (Kasahara et al., 2002), nph3-6 (Motchoulski and Liscum, 1999), pks1-1 pks2-1 pks4-1 (Lariguet et al., 2006), and nph3-6 pks1-1 pks2-1 pks4-1 (de Carbonnel et al., 2010) mutants are in the Columbia background. The Pro35S:P2CG plants are in the phot1-5 phot2-2 gl1-1 background (Kong et al., 2007).
Growth Conditions and Light Sources
The seeds were sown on rockwool moistened with 0.5% (v/v) Hyponex solution (Hyponex) and kept at 4°C in darkness for 3 d (Kozuka et al., 2010). The seedlings were then placed under continuous white light from fluorescent tubes (70 μmol m−2 s−1) at 22°C and grown for 7 d before the light treatments (Kozuka et al., 2010). Plants were then treated with monochromatic red (peak at 660 nm) and/or blue (peak at 470 nm) light from light-emitting diodes (IS-Series; CCS) to observe light responses.
Anatomical Analysis
Leaves were numbered from the first rosette leaf that emerged after the cotyledons. After the light treatment, the blades of the fourth rosette leaves were detached from the plants and fixed overnight in formaldehyde acetic acid solution at 4°C (Tsukaya et al., 1993). To determine the cell sizes and chloroplast positions, leaf samples were dehydrated in an ethanol series on ice. The ethanol was then replaced with propylene oxide in an ethanol/propylene oxide series at room temperature. Finally, the samples were embedded in Spurr’s resin (Polysciences) (Yano and Terashima, 2004), from which 1-μm-thick sections were sliced with a glass knife on an ultramicrotome (UCT-GA-D/E-1/00; Leica). The sections were stained with 0.1% toluidine blue solution (Tsukaya et al., 1993) before observation under a microscope (BX51; Olympus). Seven to 10 subepidermal palisade cells inside a small central region of either the right or left half of the leaf blade were observed. The cell height in the anticlinal direction and the cell width in the periclinal direction were determined in the sections with the aid of ImageJ image analysis software (http://rsbweb.nih.gov/ij/; NIH).
For conventional observation of the leaves (Figures 1A and 6A and Supplemental Figure 1A online), fixed leaves were dehydrated with ethanol on ice and immediately embedded in Technovit 7100 resin (Kulzer) (Tsukaya et al., 1993). Ten-micrometer-thick sections were slicedwith a disposable blade (TC-65; Leica) on a microtome (RM2135; Leica) and then stained with 0.1% toluidine blue solution (Tsukaya et al., 1993) before observation under a microscope (BX51; Olympus). Because the rates of growth and the final sizes of leaves varied substantially depending on the light conditions, the fourth leaves were collected after different durations of the monochromatic light treatment: after 10 d in blue light, 20 d in red light, and between 15 and 18 d in combined blue and red lights.
Plasmid Construction and Plant Transformation
Full-length PHOT2-GFP (P2G) cDNA was cut from the 35S:PHOT2-GFP/pUC vector (Kong et al., 2006) with XbaI and SmaI and ligated into the XbaI and SmaI sites in the pPZP211 transformation vector (P2G/pPZP211). CAB3 and CER6 promoter fragments cut from the pCAB-C2G/pPZP211/NP and pCER-C2G/pPZP211/NP vectors (Endo et al., 2007) with SalI and PstI and inserted into the SalI and PstI sites in the pGEM-3Z vector (Promega) (ProCAB3/pGEM-3Z and ProCER6/pGEM-3Z, respectively). The CAB3 promoter fragment cut from ProCAB3/pGEM-3Z with XbaI and SpeI was inserted into the XbaI and SpeI sites of the P2G/pPZP211 vector to give the ProCAB3:P2G transformation vector. A DNA fragment containing the CER6 promoter cut from ProCER6/pGEM-3Z with XbaI was inserted into the XbaI sites of the P2G/pPZP211 vector to give the ProCER6:P2G transformation vector. The phot1-5 phot2-1 mutant was transformed with ProPHOT2:P2G (Kong et al., 2006), ProCAB3:P2G, or ProCER6:P2G by the floral dip method (Clough and Bent, 1998). Kanamycin-resistant (50 mg L−1) plants were selected on Murashige and Skoog agar and the T3 homogenous lines were used for all experiments.
Microscopy Observation of GFP and Chlorophyll Fluorescence
Fluorescence from GFP-tagged proteins and chlorophyll was observed under a confocal laser scanning microscope (LSM510; Zeiss). The fourth leaves were collected from the plants and vacuum-infiltrated in water. For transverse observation, leaves were sliced with vibratome sectioning (Microslicer DTK-1000; Dosaka EM) as described previously (Endo et al., 2005). Green fluorescence from GFP was observed with a 500- to 530-nm filter set with 488-nm excitation, and red fluorescence from chlorophyll was observed with a >560-nm cutoff filter with 543-nm excitation. The images were false-colored to reflect actual colors of GFP (green) and chlorophyll (red) fluorescence.They were then overlaid electronically.
Immunoblot Analysis
Immunoblot analysis was performed essentially as described previously (Kong et al., 2006). Fifty micrograms of total crude proteins were prepared from the leaf blades of plants grown under B30/R70 or 70 μmol m−2 s−1 white light for indicated periods and then subjected to 7.5% SDS-PAGE. The blots were probed with anti-PHOT1 (Aihara et al., 2008) and anti-PHOT2 (Kong et al., 2006) polyclonal antibodies.
Analysis of Chloroplast Avoidance Response
To examine the avoidance response, plants grown under a 16-h-day/8-h-night cycle for 3 weeks were adapted in darkness at 23°C for 12 h before being treated with 50 μmol m−2 s−1 blue light for 2 h. Chloroplast distribution was observed under a laser scanning microscope (FV300; Olympus) in subepidermal palisade cells of the fourth leaves, essentially as described previously (Kong et al., 2007).
Analysis of Leaf Flattening Response
The leaf flattening response was observed in the fourth leaves collected from plants that were treated with B30/R70 light for 15 d or with monochromatic red light at 100 μmol m−2 s−1 for 21 d. The leaves were embedded in 5% low-melting-point agarose to prepare transverse sections with a razor blade across the approximate center of the leaf blade. Photographs of the sections were taken with a stereomicroscope (MZFL; Leica) and measured with ImageJ (http://rsbweb.nih.gov/ij/; NIH). The ratio of the straight-line distance between the two edges of the leaf to the actual leaf width along the curved surface was then determined (see Supplemental Figure 3B online).
Measurement of Photosynthetic Rate
Photosynthetic CO2 fixation was determined essentially as described (Takemiya et al., 2005). Gas exchange was measured in the fifth leaves under dark and 600 μmol m−2 s−1 white light conditions. To estimate the photosynthetic CO2 assimilation rate, differences in gas exchange characteristics between the dark and light conditions were determined and then expressed on the basis of the leaf blade area.
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative or GenBank/EMBL database under the following accession numbers: At1g29910 (CAB3), At1g68530 (CER6), At3g25690 (CHUP1), At3g27920 (GL1), At1g75100 (JAC1), At5g64330 (NPH3), At2g02950 (PKS1), At1g14280 (PKS2), At5g04190 (PKS4), At3g45780 (PHOT1), and At5g58140 (PHOT2).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Palisade Cell Development under Weak and Strong White Light.
Supplemental Figure 2. Analysis of P2G Expression in Transverse Sections.
Supplemental Figure 3. The Leaf Flattening Response.
Supplemental Figure 4. Analysis of the Pro35S:P2CG Transgenic Plants.
Supplemental Figure 5. Promotion of the Development of Cylindrical Palisade Cells in jac1 gl1 and chup1 gl1.
Supplemental Figure 6. Heights and Widths of Palisade Cells in nph3, pks1 pks2 pks4, and nph3 pks1 pks2 pks4.
Supplemental Figure 7. Net CO2 Assimilation Rate in Leaves of the Wild Type and phot1 phot2.
Acknowledgments
We thank M. Endo (Kyoto University, Japan) and T. Nishimura (University of Geneva, Switzerland) for providing the plasmid vectors, N. Mochizuki (Kyoto University), T. Suzuki (Kyoto University), and S. Yano (National Institute for Basic Biology, Japan) for helpful discussion. We thank M. Wada (Kyushu University, Japan) for the gifts of the jac1 gl1 and chup1 gl1 mutant seeds, C. Fankhauser (University of Geneva, Switzerland) for the gifts of the nph3 and pks multiple mutant seeds, and I. Hara-Nishimura (Kyoto University), T. Shimada (Kyoto University), and K. Tamura (Kyoto University) for technical support. This work was supported by a Grant-in-Aid for Scientific Research on Priority Areas (17084002 to A.N.), a Grant-in-Aid for Scientific Research (B) (21370020 to A.N.), a Grant-in-Aid for Scientific Research on Innovative Areas (22120002 to A.N.), a Grant-in-Aid for the Global COE Program ‘Formation of a strategic base for biodiversity and evolutionary research: from genome to ecosystem’ (to A.N.).
AUTHOR CONTRIBUTIONS
T.K. designed the research, performed the research, analyzed the data, and wrote the article. S.-G.K. prepared the plant materials. M.D. and K.S. contributed to the analysis of photosynthetic activity. A.N. designed the research and wrote the article.
References
- Aihara Y., Tabata R., Suzuki T., Shimazaki K., Nagatani A. (2008). Molecular basis of the functional specificities of phototropin 1 and 2. Plant J. 56: 364–375 [DOI] [PubMed] [Google Scholar]
- Blakeslee J.J., Bandyopadhyay A., Peer W.A., Makam S.N., Murphy A.S. (2004). Relocalization of the PIN1 auxin efflux facilitator plays a role in phototropic responses. Plant Physiol. 134: 28–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bögre L., Okrész L., Henriques R., Anthony R.G. (2003). Growth signalling pathways in Arabidopsis and the AGC protein kinases. Trends Plant Sci. 8: 424–431 [DOI] [PubMed] [Google Scholar]
- Briggs W.R., Christie J.M. (2002). Phototropins 1 and 2: versatile plant blue-light receptors. Trends Plant Sci. 7: 204–210 [DOI] [PubMed] [Google Scholar]
- Briggs W.R., Huala E. (1999). Blue-light photoreceptors in higher plants. Annu. Rev. Cell Dev. Biol. 15: 33–62 [DOI] [PubMed] [Google Scholar]
- Carol R.J., Dolan L. (2006). The role of reactive oxygen species in cell growth: Lessons from root hairs. J. Exp. Bot. 57: 1829–1834 [DOI] [PubMed] [Google Scholar]
- Christie J.M., Salomon M., Nozue K., Wada M., Briggs W.R. (1999). LOV (light, oxygen, or voltage) domains of the blue-light photoreceptor phototropin (nph1): binding sites for the chromophore flavin mononucleotide. Proc. Natl. Acad. Sci. USA 96: 8779–8783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- de Carbonnel M., Davis P., Roelfsema M.R., Inoue S., Schepens I., Lariguet P., Geisler M., Shimazaki K., Hangarter R., Fankhauser C. (2010). The Arabidopsis PHYTOCHROME KINASE SUBSTRATE2 protein is a phototropin signaling element that regulates leaf flattening and leaf positioning. Plant Physiol. 152: 1391–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demarsy E., Fankhauser C. (2009). Higher plants use LOV to perceive blue light. Curr. Opin. Plant Biol. 12: 69–74 [DOI] [PubMed] [Google Scholar]
- Endo M., Mochizuki N., Suzuki T., Nagatani A. (2007). CRYPTOCHROME2 in vascular bundles regulates flowering in Arabidopsis. Plant Cell 19: 84–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M., Nakamura S., Araki T., Mochizuki N., Nagatani A. (2005). Phytochrome B in the mesophyll delays flowering by suppressing FLOWERING LOCUS T expression in Arabidopsis vascular bundles. Plant Cell 17: 1941–1952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau K. (1977). Anatomy of Seed Plants, 2nd ed (New York: John Wiley & Sons; ). [Google Scholar]
- Foreman J., Demidchik V., Bothwell J.H., Mylona P., Miedema H., Torres M.A., Linstead P., Costa S., Brownlee C., Jones J.D., Davies J.M., Dolan L. (2003). Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422: 442–446 [DOI] [PubMed] [Google Scholar]
- Friml J., Wiśniewska J., Benková E., Mendgen K., Palme K. (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Fukuda N., Fujita M., Ohta Y., Sase S., Nishimura S., Ezura H. (2008). Directional blue light irradiation triggers epidermal cell elongation of abaxial side resulting in inhibition of leaf epinasty in geranium under red light condition. Sci. Hortic. (Amsterdam) 115: 176–182 [Google Scholar]
- Galván-Ampudia C.S., Offringa R. (2007). Plant evolution: AGC kinases tell the auxin tale. Trends Plant Sci. 12: 541–547 [DOI] [PubMed] [Google Scholar]
- Geldner N., Friml J., Stierhof Y.D., Jürgens G., Palme K. (2001). Auxin transport inhibitors block PIN1 cycling and vesicle trafficking. Nature 413: 425–428 [DOI] [PubMed] [Google Scholar]
- Haberlandt G. (1914). Physiological Plant Anatomy (London: Macmillan; ). [Google Scholar]
- Han I.S., Tseng T.S., Eisinger W., Briggs W.R. (2008). Phytochrome A regulates the intracellular distribution of phototropin 1-green fluorescent protein in Arabidopsis thaliana. Plant Cell 20: 2835–2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada A., Shimazaki K. (2007). Phototropins and blue light-dependent calcium signaling in higher plants. Photochem. Photobiol. 83: 102–111 [DOI] [PubMed] [Google Scholar]
- Hawes C. (2005). Cell biology of the plant Golgi apparatus. New Phytol. 165: 29–44 [DOI] [PubMed] [Google Scholar]
- Huala E., Oeller P.W., Liscum E., Han I.S., Larsen E., Briggs W.R. (1997). Arabidopsis NPH1: a protein kinase with a putative redox-sensing domain. Science 278: 2120–2123 [DOI] [PubMed] [Google Scholar]
- Huang F., Zago M.K., Abas L., van Marion A., Galván-Ampudia C.S., Offringa R. (2010). Phosphorylation of conserved PIN motifs directs Arabidopsis PIN1 polarity and auxin transport. Plant Cell 22: 1129–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue S., Kinoshita T., Takemiya A., Doi M., Shimazaki K. (2008). Leaf positioning of Arabidopsis in response to blue light. Mol. Plant 1: 15–26 [DOI] [PubMed] [Google Scholar]
- Kagawa T., Kimura M., Wada M. (2009). Blue light-induced phototropism of inflorescence stems and petioles is mediated by phototropin family members phot1 and phot2. Plant Cell Physiol. 50: 1774–1785 [DOI] [PubMed] [Google Scholar]
- Kagawa T., Sakai T., Suetsugu N., Oikawa K., Ishiguro S., Kato T., Tabata S., Okada K., Wada M. (2001). Arabidopsis NPL1: A phototropin homolog controlling the chloroplast high-light avoidance response. Science 291: 2138–2141 [DOI] [PubMed] [Google Scholar]
- Kasahara M., Kagawa T., Oikawa K., Suetsugu N., Miyao M., Wada M. (2002). Chloroplast avoidance movement reduces photodamage in plants. Nature 420: 829–832 [DOI] [PubMed] [Google Scholar]
- Kinoshita T., Doi M., Suetsugu N., Kagawa T., Wada M., Shimazaki K. (2001). Phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kleine-Vehn J., Huang F., Naramoto S., Zhang J., Michniewicz M., Offringa R., Friml J. (2009). PIN auxin efflux carrier polarity is regulated by PINOID kinase-mediated recruitment into GNOM-independent trafficking in Arabidopsis. Plant Cell 21: 3839–3849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong S.G., Kinoshita T., Shimazaki K., Mochizuki N., Suzuki T., Nagatani A. (2007). The C-terminal kinase fragment of Arabidopsis phototropin 2 triggers constitutive phototropin responses. Plant J. 51: 862–873 [DOI] [PubMed] [Google Scholar]
- Kong S.G., Suzuki T., Tamura K., Mochizuki N., Hara-Nishimura I., Nagatani A. (2006). Blue light-induced association of phototropin 2 with the Golgi apparatus. Plant J. 45: 994–1005 [DOI] [PubMed] [Google Scholar]
- Kozuka T., Kobayashi J., Horiguchi G., Demura T., Sakakibara H., Tsukaya H., Nagatani A. (2010). Involvement of auxin and brassinosteroid in the regulation of petiole elongation under the shade. Plant Physiol. 153: 1608–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lariguet P., Schepens I., Hodgson D., Pedmale U.V., Trevisan M., Kami C., de Carbonnel M., Alonso J.M., Ecker J.R., Liscum E., Fankhauser C. (2006). PHYTOCHROME KINASE SUBSTRATE 1 is a phototropin 1 binding protein required for phototropism. Proc. Natl. Acad. Sci. USA 103: 10134–10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Wakao S., Fischer B.B., Niyogi K.K. (2009). Sensing and responding to excess light. Annu. Rev. Plant Biol. 60: 239–260 [DOI] [PubMed] [Google Scholar]
- Lincoln C., Britton J.H., Estelle M. (1990). Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2: 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Juez E., Bowyer J.R., Sakai T. (2007). Distinct leaf developmental and gene expression responses to light quantity depend on blue-photoreceptor or plastid-derived signals, and can occur in the absence of phototropins. Planta 227: 113–123 [DOI] [PubMed] [Google Scholar]
- Macedo A.F., Leal-Costa M.V., Tavares E.S., Lage C.L.S., Esquibel M.A. (2011). The effect of light quality on leaf production and development of in vitro-cultured plants of Alternanthera brasiliana Kuntze. Environ. Exp. Bot. 70: 43–50 [Google Scholar]
- Matsuoka D., Tokutomi S. (2005). Blue light-regulated molecular switch of Ser/Thr kinase in phototropin. Proc. Natl. Acad. Sci. USA 102: 13337–13342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motchoulski A., Liscum E. (1999). Arabidopsis NPH3: A NPH1 photoreceptor-interacting protein essential for phototropism. Science 286: 961–964 [DOI] [PubMed] [Google Scholar]
- Oikawa K., Kasahara M., Kiyosue T., Kagawa T., Suetsugu N., Takahashi F., Kanegae T., Niwa Y., Kadota A., Wada M. (2003). Chloroplast unusual positioning1 is essential for proper chloroplast positioning. Plant Cell 15: 2805–2815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onodera A., Kong S.G., Doi M., Shimazaki K., Christie J., Mochizuki N., Nagatani A. (2005). Phototropin from Chlamydomonas reinhardtii is functional in Arabidopsis thaliana. Plant Cell Physiol. 46: 367–374 [DOI] [PubMed] [Google Scholar]
- Oppenheimer D.G., Herman P.L., Sivakumaran S., Esch J., Marks M.D. (1991). A myb gene required for leaf trichome differentiation in Arabidopsis is expressed in stipules. Cell 67: 483–493 [DOI] [PubMed] [Google Scholar]
- Sakai T., Kagawa T., Kasahara M., Swartz T.E., Christie J.M., Briggs W.R., Wada M., Okada K. (2001). Arabidopsis nph1 and npl1: Blue light receptors that mediate both phototropism and chloroplast relocation. Proc. Natl. Acad. Sci. USA 98: 6969–6974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto K., Briggs W.R. (2002). Cellular and subcellular localization of phototropin 1. Plant Cell 14: 1723–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savaldi-Goldstein S., Peto C., Chory J. (2007). The epidermis both drives and restricts plant shoot growth. Nature 446: 199–202 [DOI] [PubMed] [Google Scholar]
- Schuerger A.C., Brown C.S., Stryjewski E.C. (1997). Anatomical features of pepper plants (Capsicum annuum L.) grown under red light-emitting diodes supplemented with blue or far-red light. Ann. Bot. (Lond.) 79: 273–282 [DOI] [PubMed] [Google Scholar]
- Smith H. (1982). Light quality, photoperception, and plant strategy. Annu. Rev. Plant Physiol. 33: 481–518 [Google Scholar]
- Suetsugu N., Kagawa T., Wada M. (2005). An auxilin-like J-domain protein, JAC1, regulates phototropin-mediated chloroplast movement in Arabidopsis. Plant Physiol. 139: 151–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan S., Thomson C.E., Kaiserli E., Christie J.M. (2009). Interaction specificity of Arabidopsis 14-3-3 proteins with phototropin receptor kinases. FEBS Lett. 583: 2187–2193 [DOI] [PubMed] [Google Scholar]
- Takeda S., Gapper C., Kaya H., Bell E., Kuchitsu K., Dolan L. (2008). Local positive feedback regulation determines cell shape in root hair cells. Science 319: 1241–1244 [DOI] [PubMed] [Google Scholar]
- Takemiya A., Inoue S., Doi M., Kinoshita T., Shimazaki K. (2005). Phototropins promote plant growth in response to blue light in low light environments. Plant Cell 17: 1120–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi M., Ueda T., Yahara N., Nakano A. (2002). Arf1 GTPase plays roles in the protein traffic between the endoplasmic reticulum and the Golgi apparatus in tobacco and Arabidopsis cultured cells. Plant J. 31: 499–515 [DOI] [PubMed] [Google Scholar]
- Terashima I., Hanba Y.T., Tazoe Y., Vyas P., Yano S. (2006). Irradiance and phenotype: Comparative eco-development of sun and shade leaves in relation to photosynthetic CO2 diffusion. J. Exp. Bot. 57: 343–354 [DOI] [PubMed] [Google Scholar]
- Terashima I., Saeki T. (1983). Light environment within a leaf 1. Optical properties of paradermal sections of Camellia leaves with special reference to differences in the optical-properties of palisade and spongy tissues. Plant Cell Physiol. 24: 1493–1501 [Google Scholar]
- Tsukaya H., Naito S., Redei G.P., Komeda Y. (1993). A new class of mutations in Arabidopsis thaliana, acaulis1, affecting the development of both inflorescences and leaves. Development 118: 751–764 [Google Scholar]
- Wan Y.L., Eisinger W., Ehrhardt D., Kubitscheck U., Baluska F., Briggs W. (2008). The subcellular localization and blue-light-induced movement of phototropin 1-GFP in etiolated seedlings of Arabidopsis thaliana. Mol. Plant 1: 103–117 [DOI] [PubMed] [Google Scholar]
- Wilson A.K., Pickett F.B., Turner J.C., Estelle M. (1990). A dominant mutation in Arabidopsis confers resistance to auxin, ethylene and abscisic acid. Mol. Gen. Genet. 222: 377–383 [DOI] [PubMed] [Google Scholar]
- Yano S., Terashima I. (2004). Developmental process of sun and shade leaves in Chenopodium album L. Plant Cell Environ. 27: 781–793 [Google Scholar]