This work shows that volatile signals, including methyl jasmonate and methyl salicylate, emitted by UV-irradiated plants can cause an increase in homologous recombination in nearby, nonirradiated bystander plants. Emission of these signals is triggered by the formation of necrotic lesions and depends on NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 function.
Abstract
We have previously shown that local exposure of plants to stress results in a systemic increase in genome instability. Here, we show that UV-C–irradiated plants produce a volatile signal that triggers an increase in genome instability in neighboring nonirradiated Arabidopsis thaliana plants. This volatile signal is interspecific, as UV-C–irradiated Arabidopsis plants transmit genome destabilization to naive tobacco (Nicotiana tabacum) plants and vice versa. We report that plants exposed to the volatile hormones methyl salicylate (MeSA) or methyl jasmonate (MeJA) exhibit a similar level of genome destabilization as UV-C–irradiated plants. We also found that irradiated Arabidopsis plants produce MeSA and MeJA. The analysis of mutants impaired in the synthesis and/or response to salicylic acid (SA) and/or jasmonic acid showed that at least one other volatile compound besides MeSA and MeJA can communicate interplant genome instability. The NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (npr1) mutant, defective in SA signaling, is impaired in both the production and the perception of the volatile signals, demonstrating a key role for NPR1 as a central regulator of genome stability. Finally, various forms of stress resulting in the formation of necrotic lesions also generate a volatile signal that leads to genomic instability.
INTRODUCTION
Plants exposed to environmental stress produce a variety of protective metabolites. Among these, volatile hormones such as methyl salicylate (MeSA) and methyl jasmonate (MeJA) are well-studied signaling molecules involved in the activation of systemic plant defense responses (Baldwin et al., 2006; Heil and Silva Bueno, 2007). It has been proposed that MeSA is also involved in the acquisition of systemic acquired resistance (SAR) in tobacco (Nicotiana tabacum) and Arabidopsis thaliana (Shulaev et al., 1995; Park et al., 2007; Park et al., 2009), although recent work by Attaran et al. (2009) demonstrated that MeSA is not required for systemic signaling and that SAR is established through de novo salicylic acid (SA) synthesis in systemic noninfected leaves that is primed by the production of the mobile metabolite azelaic acid, a nine-carbon dicarboxylic acid (Jung et al., 2009). MeJA has been shown to be produced upon wounding stress (Ryan and Moura, 2002) and is likely to be involved in systemic wound signaling (SWS). Furthermore, both MeSA and MeJA produced by stressed plants have been shown to activate defense responses in neighboring, nonstressed plants (Farmer and Ryan, 1990; Shulaev et al., 1995; Baldwin et al., 2006; Heil and Silva Bueno, 2007).
The concept of plant–plant signaling through volatile organic compounds has been suggested for many species (Farmer and Ryan, 1990; Shulaev et al., 1995; Baldwin et al., 2006; Heil and Silva Bueno, 2007). Plant–plant communication may help bystander plants, which are not directly affected by a given stress, to anticipate the stress and prepare themselves for a more robust response in the event of a direct exposure. The discovery of volatile hormones that mediate stress responses in systemic tissue suggests that the surrounding headspace of a plant may be an important signaling environment, with potential for neighboring plants to “eavesdrop” (Baldwin et al., 2006) on systemic signaling events. Indeed, because the volatile hormones MeSA and MeJA are involved in many aspects of systemic stress signaling, they are top contenders for mediating interplant communication.
Analogously to SAR and SWS, we have shown that exposure of a single leaf to either virus or UV-C irradiation results in systemic changes in the homologous recombination frequency (HRF) in noninfected or nonirradiated somatic tissues (Kovalchuk et al., 2003; Filkowski et al., 2004). Similarly to UV-B, exposure to UV-C primarily results in the formation of pyrimidine dimers that are repaired via photolyase or nucleotide excision repair. Nucleotide excision repair intermediates, in the form of DNA strand breaks, can be repaired by the homologous recombination machinery. UV-C may also lead directly to DNA double-strand breaks, although this type of DNA damage is less frequent than pyrimidine dimer formation.
Changes in HRF can be measured with a luciferase transgene reporter system, in which an intact Luciferase gene is only obtained after the successful recombination of two separate halves. Each recombination event that generates an intact luciferase reporter can be visualized with a sensitive charge-coupled device (CCD) camera as a single luminescent spot, the size of which will depend on how many cell division cycles have occurred since the recombination event. Because homologous recombination is involved in the repair of single and double DNA strand breaks as well as the generation of gene duplications, translocations, and gross chromosomal rearrangements (Puchta, 2005), the level of HRF is an overall indicator of the level of genome rearrangement, which in turn is an indicator of genomic instability. Using an HRF reporter, we previously demonstrated an increase in HRF in response to various environmental stresses, including UV-C (Filkowski et al., 2004; Boyko et al., 2006a, 2006b, 2010a, 2010b; Boyko and Kovalchuk, 2010). We showed that systemic changes in HRF, referred to as the systemic recombination signal, were generated in stressed tissues, transported to naive nonstressed tissues, and inherited in subsequent generations (Kovalchuk et al., 2003; Boyko et al., 2007; Kathiria et al., 2010). However, it has not been determined whether signal dissemination from stressed to systemic tissues is mediated by volatile molecules or transported through the vasculature.
Here, we tested whether UV-C–irradiated plants can emit a volatile signal in their immediate gaseous environment that leads to an increase in HRF in neighboring nonirradiated plants. By monitoring HRF, we observed that when neighboring nonirradiated plants were exposed to volatiles produced by UV-C–irradiated plants, they exhibited a similar increase in genomic instability as the directly irradiated plants. We found that UV-C–irradiated plants emit both MeJA and MeSA and that these two volatile hormones trigger an increase in HRF in nonirradiated plants. Interestingly, analysis of plants deficient in the accumulation, synthesis, or response to SA or jasmonic acid (JA) showed that there is at least one additional volatile signal besides MeJA and MeSA that can trigger in increase in HRF. NONEXPRESSOR OF PATHOGENESIS-RELATED GENES1 (NPR1), a key regulator involved in SA signaling, is necessary for both the generation and reception of the volatile signals.
RESULTS AND DISCUSSION
UV-Irradiated Plants Communicate Genome Instability to Neighboring Naive Plants
HRF was analyzed in transgenic Arabidopsis and tobacco plants carrying a luciferase-based recombination reporter (Ilnytskyy et al., 2004; Boyko et al., 2006c; Kathiria and Kovalchuk, 2010). In these reporter lines, a single recombination event generates a functional luciferase gene, the activity of which can be visualized in vivo with a CCD camera as a luminescent spot (see Supplemental Figure 1 online).
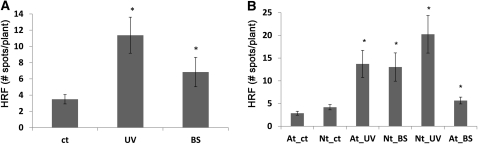
To determine whether UV-C–irradiated plants generate a volatile signal that causes genome instability in neighboring plants, we tested whether naive plants cohabiting with irradiated plants in a gas-impermeable enclosure exhibit changes in their HRF. We irradiated a group of 20 Arabidopsis HRF luciferase reporter plants with UV-C (7.0 J/m2) and immediately placed them in sealed plastic bags (see Methods) alongside 20 nonirradiated bystander reporter plants. After 4 d, the bags were opened, and 3 d later, HRF was determined in both the irradiated and bystander plants by counting the number of luminescent spots in the leaves of each plant, as described in Methods. Each luminescent spot is composed of either a single cell or a cluster of cells derived from a single cell in which a recombination event generated an intact Luciferase reporter. As a control, nonirradiated HRF reporter plants were also placed in a sealed environment side by side with a set of naive plants. Pilot experiments showed that 7 d after irradiation is optimal for detection of new homologous recombination events in irradiated and bystander plants, possibly because cells in which DNA damage has occurred need to replicate their DNA to repair it through homologous recombination or simply because cells in which DNA damage and recombination take place need to accumulate enough luciferase protein for efficient detection. We detected an increase in HRF compared with nonirradiated control plants in both irradiated and bystander plants (Figure 1A). These data suggested that UV-C–irradiated plants produce volatile molecules triggering an increase in HRF in nonirradiated bystander plants.
Figure 1.
UV-C–Irradiated Plants Trigger an Increase in HRF in Bystander Plants.
(A) UV-C causes increased HRF in both irradiated and naive bystander Arabidopsis plants (BS) exposed to volatiles produced by UV-C–irradiated plants (UV). Bars show average HRF ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate a significant difference from control (ct) nonirradiated plants (P < 0.05).
(B) UV-C–irradiated Arabidopsis (At_UV) triggers increased HRF in tobacco naive bystander plants (Nt_BS), whereas UV-C–irradiated tobacco (Nt_UV) triggers increased HRF in naive Arabidopsis plants (At_BS). Bars show average HRF ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate significant difference compared with control nonirradiated Arabidopsis (At_ct) or control tobacco (Nt_ct) plants (P < 0.05).
Interspecies Communication of UV-C–Triggered Genome Instability
Because volatile hormones are highly conserved between species (Santner et al., 2009), we hypothesized that the volatile signals that activate HRF might function interspecifically. Indeed, we found that UV-C–irradiated tobacco or Arabidopsis plants communicated genome instability to nonirradiated bystander Arabidopsis or tobacco plants, respectively (Figure 1B). These data suggest that stressed plants have the ability to transmit signals to both conspecific and heterospecific flora, presumably through the production and perception of conserved volatile compounds. Although it is unknown whether interplant communication mediated by volatile compounds occurs in natural habitats (Heil and Silva Bueno, 2007), interplant communication does occur between cultivated plants (Weber et al., 2007), suggesting that interplant signaling may occur in the wild.
MeSA and MeJA Can Trigger an Increase in HRF
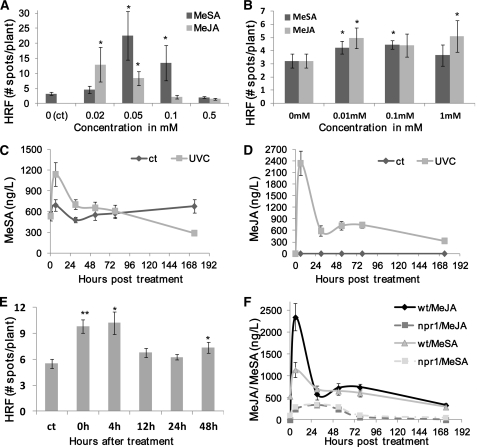
Because MeSA and MeJA are known to function in both local and interplant signaling, we hypothesized that they could be the volatile signals triggering an increase in HRF. First, we tested whether direct exposure to MeSA or MeJA regulates HRF. We found that 0.05 to 0.1 mM MeSA and 0.02 to 0.05 mM MeJA increased the HRF when sprayed directly onto leaves in an open environment, whereas higher concentrations decreased the HRF possibly because high doses of these hormones produce stunted growth that may interfere with cell division, DNA replication, and consequently with recombination (Figure 2A). Next, we tested whether these volatile compounds affected HRF when present in the gaseous headspace. To expose plants to a gaseous environment containing either MeSA or MeJA, we sprayed 5 mL of these volatiles into sealed plastic bags at concentrations ranging from 0.01 to 1 mM and then kept the plants in these enclosed environments for 4 d. We found a modest but statistically significant increase in HRF at 0.01 mM MeSA or MeJA that did not increase at higher concentrations (Figure 2B). These data suggest that either MeSA or MeJA could be the volatile signals contributing to the increased HRF in bystander plants, assuming that these two volatiles are produced and released by plants after UV-C irradiation.
Figure 2.
Role of MeSA and MeJA in Eliciting an Increase in HRF.
(A) HRF in wild-type plants after direct spraying with MeSA or MeJA. Three-week-old Arabidopsis plants were sprayed with MeSA or MeJA at various concentrations, ranging from 0.02 to 0.5 mM. Bars show average HRF ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants . Asterisks indicate significant difference compared with control mock-sprayed Arabidopsis (P < 0.05).
(B) HRF in wild-type Arabidopsis plants after being exposed to a MeSA- or MeJA-enriched environment. Each experimental point consisted of three pots with 12 to 20 plants in each one. Bars represent average HRF ± sd. Asterisks indicate significant difference compared with control (0 mM) unexposed Arabidopsis (P < 0.05).
(C) MeSA levels (ng/L) in the headspace of UV-C–irradiated plants. After irradiation with 7.0 J/m2 UV-C, gas samples were taken and MeSA concentration was determined. The data are the average ± sd of four biological repeats.
(D) MeJA levels (ng/L) in the headspace of UV-C–irradiated plants. Experimental details are the same as in (C).
(E) Time course of volatile production after UV-C irradiation. Bars represent average bystander HRF ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate significant difference compared with control mock-sprayed Arabidopsis (P < 0.05). Asterisks indicate a significant difference from control (*P < 0.05 and **P < 0.01).
(F) MeSA and MeJA levels (in ng/L) in the headspace of UV-C–irradiated wild-type and npr1 plants. Experimental details are the same as in (C).
Exposure to UV-C Triggers an Increase in Volatile MeSA and MeJA
To test whether UV-C irradiation triggers the release of volatile MeSA or MeJA, we used gas chromatography (GC) to measure these compounds in the headspace of UV-irradiated plants. The steady state MeSA level released by nonirradiated Columbia-0 wild-type control plants was ~600 ng/L over the experimental period of 7 d (Figure 2C). UV-C irradiation of wild-type plants resulted in an approximately twofold increase in MeSA 6 h after irradiation. These levels gradually subsided, and by day 4 after irradiation, they were slightly lower than the preirradiation values. The level of MeJA in the headspace of nonirradiated plants was not detectable (<1 ng/L). Similarly to MeSA, UV irradiation of wild-type plants resulted in a substantial increase (>2000 ng/L) in MeJA concentration at 6 h after irradiation, which then decreased to a level of ~400 ng/L at day 7 after irradiation (Figure 2D).
To determine whether the concentrations of MeSA and MeJA observed in the headspace of UV-irradiated plants were comparable to the levels of MeSA and MeJA that are sufficient to induce an increase in the HRF in the experiments described above in Figure 2B, we assessed the actual concentration of these hormones after spraying. After spraying jars with 5 mL of 0.01 mM of MeSA or MeJA, we found that MeSA and MeJA concentrations were ~1500 to 1900 ng/L and ~500 to 700 ng/L, respectively (see Supplemental Figure 2 online). Thus, the amount of MeSA and MeJA in the headspace of UV-irradiated plants observed in Figures 2C and 2D should be sufficient to induce an increase in the HRF. However, it should be noted that the levels of MeSA and MeJA in the bags in which the hormones solutions were sprayed (see Supplemental Figure 2 online) remained high for several days, whereas in the experiment with UV-C–irradiated plants, these volatiles dropped sharply after 24 h of exposure (Figures 2C and 2D).
The Volatile Compounds Increasing HRF Are Produced in Waves after Exposure to UV-C
To test the time frame of production of volatile signals, we placed UV-C–irradiated plants side by side with bystander plants in sealed plastic bags at different times after UV-C irradiation, from 0 to 48 h. Consistent with the data in Figures 2C and 2D, an increase in HRF was observed in bystander plants if the irradiated plants were placed in the bag either immediately after or up to 4 h after irradiation; after this, the HRF in bystander plants dropped to the level of control plants, indicating that the production of most of the volatiles ceased ~4 h after irradiation (Figure 2E). However, it should be noted that a small but significant increase in HRF occurred in bystander plants exposed to UV-C–irradiated plants 48 h after irradiation, suggesting that the production of volatile signaling molecules can continue a relatively long time after irradiation. Previous studies showed that a single short exposure to UV-C or ozone resulted in an increased accumulation of SA lasting over 5 d (Yalpani et al., 1993). Although unlikely, we cannot exclude the possibility that plant volatiles released from UV-C–irradiated plants interact with plastic polymers in the plastic bags, which in turn generates volatiles that enhance the HRF in bystander plants. However, as direct spraying of MeSA and MeJA on plants triggered an increase in HRF even when plants were kept in an open environment (Figure 2A), this latter hypothesis appears unnecessary to explain the activity of MeSA and MeJA.
MeSA and MeJA Are Key Signaling Volatiles but Not the Only Ones Involved in Plant–Plant Communication of Genome Instability
So far, our data show that (1) UV-C–irradiated plants emit volatile signals that trigger an increase in HRF in neighboring naive plants, (2) UV-C–irradiated plants produce MeSA and MeJA, and (3) MeSA and MeJA applied exogenously at concentrations similar to those produced by UV-C–irradiated plants trigger an increase in HRF in nonirradiated plants. These data allow us to hypothesize that MeSA and/or MeJA play a key role in interplant communication of genome instability. Assuming that MeSA and/or MeJA are the sole interplant volatile signals leading to an increase in HRF, mutants impaired in both MeSA and MeJA synthesis should be compromised for emitting an interplant genome instability signal, whereas mutants compromised for MeSA and MeJA signal perception should be “blind” to the signal emitted by UV-C–irradiated plants. To test these hypotheses, we used genetic crosses to introduce the luciferase HR cassette into a variety of mutants impaired in MeSA and/or MeJA perception or synthesis and selected homozygous plants for both the reporter luciferase cassette and each mutation that was tested.
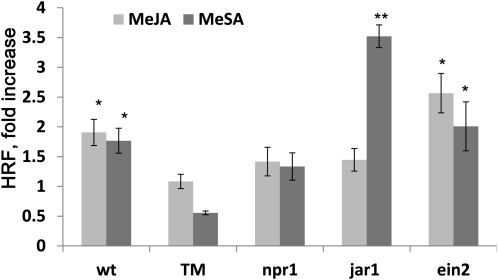
We first tested whether mutants impaired in hormone perception were impaired in the MeSA- or MeJA-elicited increase in HRF. For this analysis, we used mutants impaired in JA (jar1-1), SA (npr1-1), or ethylene (ein2-1) signaling as well as a triple mutant that is deficient in all three signaling pathways (npr1 ein2 jar1 triple mutant; Clarke et al., 2000). After spraying 1 mM MeJA or 1 mM MeSA into sealed plastic bags, we placed 2-week-old mutant plants carrying the HRF reporter cassette into the bags and measured HRF 7 d after exposure (Figure 3). As expected, we found that wild-type and ein2 plants responded to MeSA and MeJA with a significant (P < 0.05 in all cases) increase in HRF, whereas the triple mutant did not (P > 0.05 in all cases) (Figure 3). Also as expected, npr1 and jar1 did not show an increase in HRF after MeSA or MeJA treatments, respectively, whereas jar1 responded with a relatively large increase in HRF in response to MeSA. Consistent with these data, jar1 plants were previously shown to be unable to establish a UV-B–induced protective response against herbivores (Caputo et al., 2006), suggesting a requirement for the jar1-encoded JA–amino acid conjugase in both response to pathogens and to abiotic stress–induced changes in HRF after exposure to UV light.
Figure 3.
Mutants Deficient in NPR1 Are Insensitive to MeJA- or MeSA-Elicited Increase in HRF.
HRF in Arabidopsis wild-type (wt) or mutant plants exposed to a MeSA- and MeJA-enriched environment. Plants were exposed to MeSA or MeJA for 4 d. Bars represent average HRF fold increase (compared with control) ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate significant difference compared mock-sprayed Arabidopsis (P < 0.05). Asterisks indicate a significant difference from control (*P < 0.05 and **P < 0.01). TM, triple mutant.
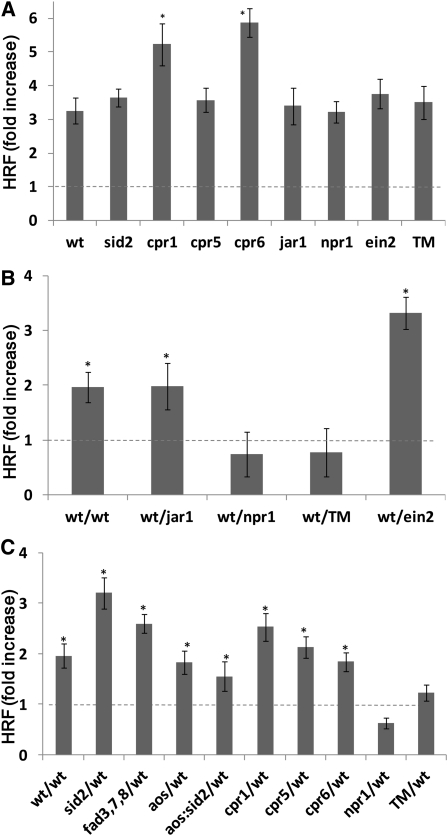
Surprisingly, npr1 did not respond to MeJA. This latter observation was unexpected and led us to hypothesize that NPR1 may be necessary to integrate different signaling pathways leading to increased HRF. To broaden our understanding of the role of MeSA and MeJA as signaling molecules and NPR1 as a regulator of HRF, we tested the above-mentioned hormone signaling mutants and mutants impaired for the synthesis of SA (sid2-2), mutants that overaccumulate SA (cpr1, cpr5, and cpr6), mutants impaired in JA production (aos and fad3 fad7 fad8 triple mutant), and a double mutant impaired in both SA and JA production (aos/sid2) for their capacity to respond to UV-C irradiation by increasing HRF as well as to produce or to perceive volatiles from UV-C–irradiated plants. First, we observed that HRF in all these mutants increased in response to UV-C, showing that none of the genes being tested, including NPR1, EIN2, and JAR1, is required for mediating an increase in HRF after direct irradiation with UV-C (Figure 4A). However, we found that cpr1 and cpr6 plants displayed a higher fold increase in the level of recombination after UV-C exposure compared with wild-type plants and the other mutants tested (P < 0.05) (Figure 4A), suggesting that SA accumulation may have a priming effect on recombination. Interestingly, the cpr5 mutant, which also overaccumulates SA, showed an increase in HRF similar to that of wild-type plants, suggesting that there may be other factors besides SA overaccumulation contributing to the postulated priming of the HRF. In contrast with cpr1 and cpr6, cpr5 plants exhibit a spontaneous programmed cell death phenotype (Kirik et al., 2001). Hence, it is possible that the spontaneous programmed cell death phenotype observed in cpr5 suppresses the priming effect of SA, which in turn may prevent this mutant from displaying the high levels of HRF observed in cpr1 and cpr6. Combined with the observation that SA levels have been shown to increase after UV-C exposure, these latter data suggest that SA may play a role in potentiating HRF (Wang et al., 2010).
Figure 4.
Changes in HRF in Hormone Signaling and Hormone Synthesis Mutants.
Three-week-old Arabidopsis wild-type (wt) plants (line #15d8) and various mutants (npr1 jar1 ein2 triple mutant [TM]) were either UV-C irradiated to test volatile emission or used as bystander. For (B) and (C), irradiated plants were immediately placed into plastic bags together with nonirradiated plants. Control plants (the wild type or mutants) were placed into sealed bags without irradiation.
(A) HRF in UV-C–irradiated wild-type and mutant plants is shown as average (±sd) fold increase over HRF in unexposed plants calculated from four independent experiments, with each experiment consisting of ~100 plants. Asterisks indicate a significant difference from control plants (P < 0.05). Dashed line refers to control, assigned a value of 1.
(B) HRF in bystander hormone signaling mutants exposed to volatiles from wild-type irradiated plants. Bars represent average HRF fold increase (compared with control) ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate a significant difference from control plants (P < 0.05). Dashed line refers to control, assigned a value of 1.
(C) HRF in bystander wild-type plants exposed to volatiles from irradiated hormone signaling or hormone synthesis/accumulation mutants. Bars represent average HRF fold increase (compared with control) ± sd of three biological repeats, each one consisting of three pots containing 12 to 20 plants. Asterisks indicate a significant difference from control plants (P < 0.05). Dashed line refers to control, assigned a value of 1.
Next, we tested various hormone mutants (jar1, ein2, npr1, and the triple mutant npr1 ein2 jar1) for their capacity to perceive the UV-C–induced volatile signal leading to genome instability. In this experiment, wild-type plants were irradiated and then placed in a sealed plastic bag with the hormone mutants harboring the HRF reporter. As bystanders, the hormone signaling mutants jar1 and ein2 showed an increase in HRF, whereas npr1 and the npr1 ein2 jar1 triple mutant showed no increase in HRF compared with nonirradiated cognate plants (Figure 4B). This experiment showed that the JA and ethylene pathways are not involved in perception of the volatile signal, whereas functional NPR1 is necessary to perceive the volatile signal. We were not able to test HRF in either directly exposed or bystander fad3 fad7 fad8, aos, or aos sid2 plants since these mutants have not been crossed with the recombination reporter line.
We also tested the various hormone mutants (sid2, cpr1, cpr5, cpr6, aos, fad3 fad7 fad8, and aos sid2) for their ability to generate the volatile signal that causes an increase in HRF in naive wild-type bystander plants. As shown in Figure 4C, all mutants tested were able to produce the HRF signal and communicate genome instability to bystander wild-type plants, except npr1 and the npr1 ein2 jar1 triple mutant. Although npr1 is not known to be involved in SA or JA biosynthesis, the data in Figure 4C suggest that the npr1 mutant may be impaired for MeSA and MeJA biosynthesis following UV-C irradiation. Indeed, we found that npr1 was compromised for the production of both hormones after UV-C irradiation (Figure 2F). As expected, analysis of the levels of MeSA and MeJA released by UV-C–irradiated mutants showed that mutants compromised for SA synthesis (sid2 and aos sid2) released significantly lower levels of MeSA compared with wild-type plants (P < 0.05 in all cases) (see Supplemental Figure 3A online). Similarly, mutants impaired in JA biosynthesis (fad3 fad7 fad8, aos, and aos sid2) showed lower levels of MeJA (P < 0.05) (see Supplemental Figure 3B online). Despite not showing an increase in either MeSA or MeJA after UV-C irradiation, the aos sid2 double mutant was still able to communicate genome instability to bystander naive wild-type plants (Figure 4C), which suggests that there is at least one volatile signaling molecule in addition to MeSA and MeJA involved in activating HRF.
To summarize the key results in this section, the npr1 mutant, which is compromised for SA-mediated responses, appears to be necessary for both perception of the volatile HRF-eliciting signal from UV-C–irradiated plants as well as production of the volatile signal after UV-C irradiation (Figure 4). In addition, fad3 fad7 fad8, aos, and aos sid2 mutants are significantly impaired in the production of MeJA or MeSA or both but are not impaired in either sending or receiving the volatile signal. Based on these data, we conclude that (1) NPR1 plays a key role in HRF, (2) MeSA and MeJA are not the only volatile signals that are able to trigger genomic instability, and (3) the lack of signal production in the npr1 mutant is likely not a consequence of the lack of production of MeSA or MeJA upon UV-C irradiation, which suggests that npr1 is compromised in the production of other active HRF-eliciting volatiles besides MeSA and MeJA.
Basal HRF Is Compromised in npr1 Mutants
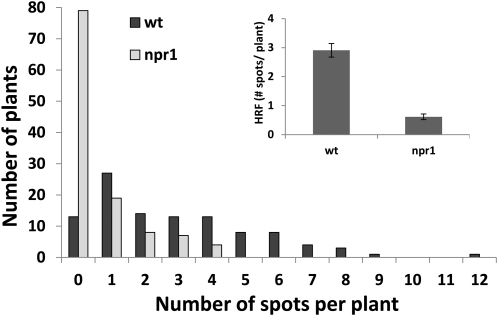
What are the possible roles of SA and NPR1 in the recombination process? Durrant et al. (2007) proposed that NPR1 may function as a negative regulator of SNI1, which is an important negative regulator of pathogenesis gene (PR) gene expression and recombination in the absence of induction by pathogen stress (Durrant et al., 2007). Durrant et al. suggest that the activation of SAR through SA releases SNI1-mediated suppression in an NPR1-dependent manner and thus results in the induction of PR gene expression and activation of homologous recombination (Durrant et al., 2007). In an npr1 mutant, however, the negative regulatory activity of SNI1 on recombination cannot be released. This model suggests that npr1 mutants should have low basal frequencies of homologous recombination. Indeed, although npr1 is still capable of increasing HRF after UV-C irradiation (Figure 4A), our analysis shows that the basal HRF is nearly fivefold lower in npr1 compared with wild-type plants (Figure 5).
Figure 5.
The npr1 Mutant Has a Low Basal Level of Homologous Recombination.
Distribution of plants (y axis) by number of recombination spots (zero, one, two, three, or more) per plants (x axis). The inset shows the HRF in wild-type (wt) and npr1 plants as an average HRF ± sd of four biological repeats, with each experiment consisting of ~100 plants.
Another connection between NPR1 and the homologous recombination machinery is suggested by the work of Wang et al. (2010), who showed that downstream of NPR1, BRCA2A is a major regulator of transcription of genes associated with the defense response. Using chromatin immunoprecipitation analysis, the authors demonstrated that a key protein involved in homologous recombination, RAD51, is specifically recruited to the promoters of defense genes during SAR through a mechanism involving SA production and the function of the DNA binding and recombination protein BRCA2 (Wang et al., 2010).
Dying and Necrotized Tissue Produces the Plant–Plant Genome Instability Signal
UV-C irradiation of plants has been shown to induce necrotic lesions (Danon et al., 2004). To test whether necrotic lesions per se emit volatiles that trigger an increase in HRF in bystander plants and whether or not the lesions are caused by UV-C irradiation, we wounded leaves from 6-week-old tobacco plants by making several 2- to 3-cm-long incisions, detached them, and placed them in a closed environment with Arabidopsis plants. Indeed, we found an increase of >50% in HRF in bystander Arabidopsis plants (see Supplemental Figure 4 online).
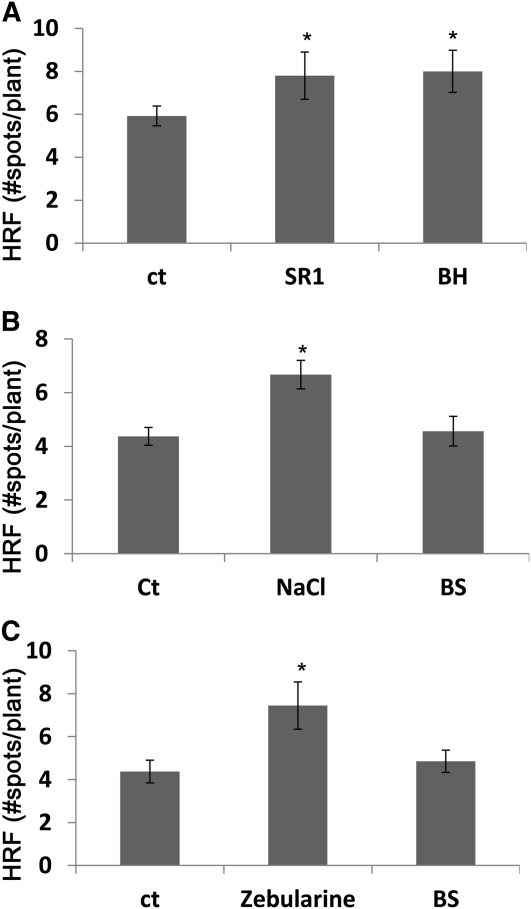
Tissue damage and necrotic lesions are also produced after inoculation of plants with either compatible or incompatible pathogens. To test whether volatile signals that upregulate the HRF emanate from pathogen-infected plants, we infected Big Havana or SR1 tobacco plants with tobacco mosaic virus (TMV) and placed them in closed bags with HRF reporter plants. Control tobacco plants were kept untreated and placed together with naive tobacco plants as a negative control. Big Havana is resistant to TMV because it expresses the N resistance gene, whereas SR1 is susceptible to TMV. Resistant plants form necrotic lesions at the site of TMV infection, and although infection of susceptible plants with TMV does not result in large amounts of tissue necrosis, there apparently is a substantial production of free radicals and activation of SA-dependent signaling pathways (Love et al., 2005; Grant and Lamb, 2006). Both Big Havana and SR1 that were infected with TMV produced a volatile signal that significantly increased the HRF in the bystander reporter plants (Figure 6A).
Figure 6.
Changes in HRF in Bystander Plants Exposed to Volatiles from Plants Treated with Necrotizing or Non-Necrotizing Types of Stress.
(A) HRF in bystander tobacco plants exposed to volatiles from TMV-infected tobacco. Two leaves of 4-week-old tobacco plants (cultivars SR1, susceptible to TMV, or Big Havana [BH], resistant to TMV) were infected with 200 ng of TMV. Infected SR1 or Big Havana plants were placed in a sealed bag side by side with naive tobacco plants of the same cultivar. Each experimental point consisted of three pots with 8 to 12 plants per pot. Bars represent the average HRF ± sd calculated from three biological repeats. Asterisks indicate a significant difference compared with control (ct) (P < 0.05).
(B) HRF in Arabidopsis plants exposed to NaCl or to volatiles from NaCl-treated plants (BS). Two-week-old Arabidopsis plants were grown on soil and watered with 250 mM NaCl for 2 d, after which they were placed side by side with naive Arabidopsis plants in sealed plastic bags for 4 d. At day 4 after exposure, bags were opened and HRF was measured 3 d later (7 d after treatment). Each experimental point consisted of three pots with 12 to 20 plants per pot. Bars represent the average HRF ± sd calculated from three biological repeats. Asterisks indicate a significant difference compared with control (P < 0.05).
(C) HRF in Arabidopsis plants exposed to zebularine or to volatiles from zebularine-treated plants (BS). Two-week-old Arabidopsis plants grown on soil were sprayed with zebularine and immediately placed side by side with naive Arabidopsis plants in sealed plastic bags for 4 d. At day 4 after exposure, bags were opened and HRF was measured 3 d later (7 d after treatment). Experimental details are the same as in (B). Asterisks indicate a significant difference compared with control (P < 0.05).
In contrast with stress leading to necrosis, exposure to NaCl or zebularine (demethylation agent), two types of stresses that do not directly lead to tissue necrosis, despite triggering an increase in the HRF in treated plants, did not lead to an increase in the HRF in bystander plants (Figures 6B and 6C), which suggests that stress-triggered necrosis is sufficient for the production of volatiles but not for the activation of recombination in directly stress plants.
The data in Figure 6 suggest that stress that results in tissue damage, such as TMV infection of tobacco leaves, results in the production of volatile signals. It is possible that UV-C stress causes the formation of necrotic lesions similarly to pathogen-associated stresses, leading to the activation of SA signaling pathways and the activation of both PR gene expression, an increase in the HRF, and the generation of volatile signaling molecules. Indeed, Yalpani et al. (1993) showed that exposure to UV-C or ozone stimulated production of SA and an increase in PR1 expression within 24 h of exposure (Yalpani et al., 1993). Distant, shielded, unexposed secondary leaves exhibited a similar level of SA increase, PR1 expression, and protection against TMV as the treated primary leaves. The observed effect of UV-C and ozone on SA levels and disease resistance appears to mimic the effect of necrotizing pathogens, suggesting that biotic and abiotic inducers of SA accumulation and disease resistance may share a common signal transduction pathway. In support of this model, UV light, ozone, and necrotizing pathogens elicit a burst of reactive oxygen species in plant tissues (Apostol et al., 1989). Moreover, UV-triggered changes in PR gene expression and activation of SA have been documented for several plant species (Green and Fluhr, 1995; Jenkins, 2009), and UV-induced transition to flowering has been found to be SA dependent (Martínez et al., 2004). Finally, UV exposure can activate defense mechanisms against insect herbivory in a JA-dependent manner (Stratmann et al., 2000; Agrawal et al., 2003; Caputo et al., 2006; Foggo et al., 2007; Clarke et al., 2009; Demkura et al., 2010). All in all, these data suggest that UV-induced systemic recombination signal and SWS may share common signaling molecules.
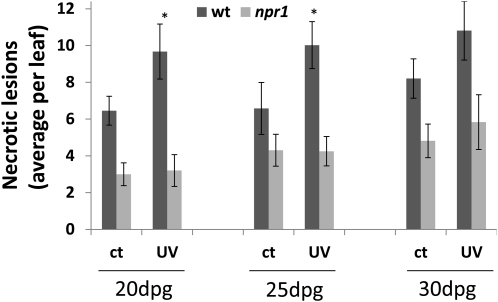
The npr1 Mutant Does Not Develop Necrotic Lesion after UV-C Irradiation
Because the defect in npr1 mutant plants in communicating UV-C–triggered genome instability to bystander plants is not simply due to the lack of production of MeSA and MeJA, we hypothesized that npr1 plants might also be compromised for other UV-C–triggered responses besides the production of these two volatile hormones. Based on our results showing that NPR1 plays a key role in both the generation and reception of the volatile signal following UV-C irradiation and that necrotic lesions per se can trigger an increase in HRF, we hypothesized that NPR1 might be compromised for the formation of necrotic lesions following UV-C irradiation, which in turn is required for the induction of volatiles followed by an increase in HRF. To test whether UV-C induces an increase in necrotic lesion formation, we analyzed the formation of necrotic lesions in UV-C–treated wild-type and npr1 plants. Using trypan blue staining, we found that whereas spontaneous levels of necrotic lesions were similar in wild-type and npr1 plants, a statistically significant increase in the formation of necrotic lesions upon UV-C irradiation was only observed for wild-type (P < 0.05) but not for npr1 (P > 0.05) plants (Figure 7). As the differences in the formation of necrotic lesions after UV-C irradiation of wild-type plants compared with control plants are rather small, it is possible that necrotic lesions are not the only source of volatiles triggering HRF increase in bystander plants.
Figure 7.
UV-C–Induced Necrosis.
Plants were irradiated at 20, 25, and 30 d postgermination (dpg). Necrotic lesions were scored 24 h after irradiation and trypan blue staining. Bars show the average number of necrotic lesions ± sd per leaf. Data were obtained from three biological repeats, each one consisting of 20 to 30 leaves. Asterisks indicate a significant difference (P < 0.05) between irradiated (UV) and control (ct) plants. wt, wild type.
Conclusions
To summarize, in this study, we demonstrate that UV-C–irradiated and pathogen-infected plants are able to generate an airborne signal leading to an increase in the HRF in neighboring naive plants. Extrapolating from current paradigms of interplant stress signaling, MeSA and MeJA were hypothesized to be the potential volatile signals. Indeed, when applied exogenously, both MeSA and MeJA elicit an increase in the HRF. Moreover, UV-C–irradiated plants produce levels of gaseous MeSA and MeJA that are sufficiently high to induce an increase in HRF in neighboring plants. However, the use of Arabidopsis mutants impaired in the synthesis of both SA and JA demonstrate that at least one additional volatile signal that increases the HRF is still generated in these mutants after UV-C irradiation. It is not clear what these other volatile signals may be, but the fact that the fad3 fad7 fad8 triple mutant still produces the signal makes it unlikely that trienoic fatty acids are involved. It is possible that the volatile signal is actually a bouquet of molecules.
Importantly, our data indicate that NPR1 plays a key role in both the generation and the perception of the volatile signal. On the one hand, the lack of perception of the signal and the low HRF observed in npr1 might be due to the suppressive activity of SNI1 on homologous recombination. On the other hand, the lack of production of the signal in npr1 may be due to the partial loss of ability of this mutant to develop necrotic lesions in response to UV-C irradiation.
It is unclear what role plant-to-plant communication of genome instability may play in adaptation to UV-C irradiation or other stresses. Possibilities include higher DNA repair capacity and increased genome reshuffling. The increased HRF observed in bystander plants could be a consequence of local chromatin modifications, such as hypomethylation, acquisition of permissive chromatin marks, and nucleosome repositioning, which may be part of the adaptation response to UV-C irradiation. The increase in HRF and stress tolerance observed in the progeny of plants exposed to stress is associated with changes in DNA methylation (Boyko et al., 2010a). UV-mediated DNA damage is modulated by DNA methylation and chromatin modifications, which affect chromatin accessibility and DNA repair (Rochette et al., 2009). The links between the increase in HRF in bystander plants, the role of chromatin modification in this process, and the potential adaptive advantage for these plants in coping with UV-C irradiation and/or other stresses remain to be established.
METHODS
Plant Material
The HRF was analyzed in transgenic Arabidopsis thaliana and tobacco (Nicotiana tabacum) plants carrying a luciferase-based recombination reporter (Ilnytskyy et al., 2004; Boyko et al., 2006c; Kathiria and Kovalchuk, 2010). These Arabidopsis and tobacco lines carry in their genomes two nonfunctional overlapping luciferase half genes, which are the substrate for homologous recombination. A single recombination event in the recombination cassette that restores luciferase activity can be visualized in vivo with a CCD camera as a luminescent spot (see Supplemental Figure 1 online).
For the analysis of recombination rates in mutants, we crossed individual mutant plants with a transgenic Arabidopsis line named #15d8 (Columbia-0 background), which carries the recombination reporter construct conferring BASTA resistance. We crossed #15d8 with mutants deficient in SA synthesis (sid2-2; Wildermuth et al., 2001), SA-mediated responses (npr1-1; Cao et al., 1997), SA overaccumulation (cpr1-1, Bowling et al., 1994; cpr5-1, Bowling et al., 1997; and cpr6-1, Clarke et al., 1998), JA signaling (jar1-1; Staswick et al., 2002), ethylene-mediated responses (ein2-1; Alonso et al., 1999), and mutants deficient in SA-JA-ethylene–dependent pathways (npr1 ein2 jar1 triple mutant; Clarke et al., 2000). Homozygous plants for both the luciferase recombination reporter substrate and the corresponding mutation/s were obtained by analyzing BASTA segregation and the corresponding hormone-related phenotype (jar1-1 and ein2-1), respectively. In the case of sid2-2 and npr1-1, mutations were confirmed by PCR genotyping. For volatile emission experiments, we used the aforementioned mutants in addition to mutants compromised for JA synthesis (fad3/7/8, Rao et al., 2000; aos, Salk_017756 from ABRC stock center, Alonso et al., 2003) and a double mutant compromised for SA and JA synthesis (sid2 aos).
Experimental Setup
Unless indicated otherwise, plants were grown in soil in standard 5 × 5-cm plastic pots at 22°C under a 12-h photoperiod at 100 μM m−2 s−1 light irradiance. For UV-C irradiation, 3-week old Arabidopsis or tobacco plants were irradiated with 7.0 J/m2 (70,000,000 ergs, with an intensity of ~100 ergs/cm2/s for 70 s) of UV-C using a laminar flow UV-C germicidal lamp. This relatively low level of irradiation was chosen because it approximates the rate of UV-B irradiation that plants receive in the wild over a comparable amount of time (Ries et al., 2000) and because this level of irradiation does not have any obvious observable deleterious effects on the growth of the plants. After irradiation, the plants were immediately placed into plastic zip-lock bags (~3-liter volume) containing nonirradiated plants. Precautions were taken that nonirradiated bystander plants were not touching the irradiated plants. Control plants were placed in the bags without being UV-C treated.
For the experiments with Arabidopsis mutants, a reciprocal approach was used: Irradiated mutant plants were placed with the bystander wild-type (line #15d8) plants. Alternatively, irradiated wild-type plants were placed with bystander mutant plants. For the experiments with MeJA and MeSA, plants were either directly sprayed with 0, 0.02, 0.05, 0.1, and 0.5 mM solutions of the hormones and grown in a open environments until HRF was determined or placed in the bags that were previously sprayed with 0, 0.01, 0.1, and 1.0 mM MeJA or MeSA (5 mL per bag). To determine the actual concentration of MeSA and MeJA in the form of gas to which the plants were exposed inside the bags, we sprayed 5 mL of MeSA or MeJA (at the concentrations indicated above) into wide-mouth glass jars, and the concentration of volatiles was determined by GC as described below.
To determine whether UV-C–irradiated plants emit a volatile signal, several groups of Arabidopsis plants were irradiated with 7.0 J/m2 UV-C and placed into plastic bags containing bystander naive plants at different times after irradiation, namely, at 0, 4, 12, 24, and 48 h. For NaCl stress, plants were watered with 100 mM NaCl for 2 d and transferred to sealed bags side by side with naive bystander plants. For zebularine treatment, plants were sprayed with 100 μM zebularine and placed in plastic bags with bystander plants. Control plants were placed in plastic bags without treatment. To test the production of volatiles after wounding, we made several 2- to 3-cm-long incisions with a scalpel across the leaves of 6-week-old tobacco plants. Wounded leaves were detached, and three leaves were placed into a sealed bag side by side with naive Arabidopsis plants.
In all cases, a single experiment contained three individual bags, with 12 to 20 exposed and bystander plants per bag. Each experiment was repeated three to five times. The bags were opened after 4 d, and HRF was analyzed 3 d after the bags were opened (7 d after exposure).
Viral Infection of Tobacco
Tobacco cultivars SR1 and Big Havana, each carrying the HRF reporter (Kovalchuk et al., 2003), were germinated and grown on soil. Four-week-old SR1 or Big Havana plants were rub-inoculated with 200 ng (100 ng per each of two leaves) of TMV in phosphate buffer with carborundum used as an abrasive or rub-inoculated with phosphate buffer only or not treated at all. Infected SR1 or Big Havana plants were placed in the same sealed bag with naive bystander tobacco of the same cultivar immediately after TMV infection. Control plants were placed in the bags with mock-treated plants. Plastic bags were kept sealed for 4 d, and HRF was checked 3 d later.
Analysis of HRF
The HRF in Arabidopsis and tobacco SR1 and BH plants carrying the luciferase reporter was analyzed by scoring luminescent spots on a dark background with a CCD camera after spraying with luciferin (Boyko et al., 2006c). Regardless of the luciferase spot size, each spot is the product of a single recombination event. HRF was calculated by relating the number of recombination events in the entire population of plants tested to the total number of plants scored. The average HRF was calculated from three to five independent experiments, with each experiment consisting of three different pots, each one containing 12 to 20 plants. The ratio between treatments over control was calculated for each of the three to five independent experiments and then the average ratio ± sd was calculated.
Determination of MeSA and MeJA Using Headspace Solid-Phase Microextraction and GC
Isolation of selected active esters was performed using solid-phase microextraction (SPME). A manual SPME holder and a fiber with polydimethylsiloxane with a film thickness of 100 μm (SUPELCO) were used in the experiment. The SPME fiber was conditioned at 250°C for 30 min before use. For headspace-SPME sampling, plants were grown in open wide-mouth glass jars (10 × 10 × 10 cm). Three-week-old plants were either exposed to 7.0 J/m2 UV-C or mock treated. The jars were immediately sealed. The SPME fiber was inserted into the jars through a septum placed on a jar cap and volatiles isolated for 1 h at room temperature. Volatiles from treated and control plants were taken at the same time of day 6, 30, 54, 78, and 174 h after irradiation. The amounts of MeSA and MeJA in the headspace were calculated using external calibration with standards.
For GC analysis, volatiles from the fiber were immediately desorbed for 5 min directly into a GC injection port held at 250°C and set into splitless mode. Isolated volatiles were separated on a gas chromatograph Trace GC Ultra (Thermo Electron) equipped with a DB-35MS capillary column (25 m × 0.20 mm × 0.33 μm; JandW Scientific) and a flame ionization detector. Column temperature was programmed starting at 40°C for 5 min, then increased to 190°C at rate of 15°C min−1, held for 1 min, further programmed to 260°C at rate of 5°C min−1, and the final temperature held for 10 min. Injector and detector temperatures were set at 250 and 300°C, respectively. Hydrogen was used as the carrier gas at a flow of 1.5 mL/min. Identity of esters was verified by GC–mass spectrometry analysis, comparing mass spectra of standards with isolated analytes. A Varian CP-3800 GC coupled to Varian Saturn 4000 ion trap mass spectrometer was used with the same column and parameters as described above. The transfer line was held at 230°C and the ion trap at 180°C; mass range from 40 to 350 m/z at one spectra per second was scanned.
Analysis of Necrotic Lesions in Wild-Type and npr1 Plants
Wild-type and npr1 plants were grown on soil at 22°C under 12-h-day/12-h-night conditions and illumination at 100 μM m−2 s−1. Plants were irradiated with 7.0 J/m2 UV-C at 20, 25, and 30 d after germination and used for the analysis 24 h after irradiation. Necrotic lesions were analyzed using trypan blue staining. In brief, individual leaves were submerged in trypan blue (in 15-mL Falcon tubes) and placed into a boiling water bath for 2 min. Then, tubes were moved to room temperature for 20 min and then destained multiple times with 70% ethanol. Areas that physically connected with each other were considered as single lesions. Twenty to thirty leaves were used per each experiment, and three repetitions were performed.
Statistical Treatment of Data
In all cases, the average and sd in three independent experiments were calculated. The statistical significance of experiments was confirmed by performing either a Student’s t test (two-tailed paired or nonpaired) or single-factor or two-factor analysis of variance tests. Statistical analyses were performed using the MS Excel software and Microcal Origin 6.0.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Detection of Recombination Events in Transgenic Line #15d8.
Supplemental Figure 2. Measurement of MeSA and MeJA Levels.
Supplemental Figure 3. MeSA and MeJA Levels in the Headspace of UV-C–Exposed Plants.
Supplemental Figure 4. HRF Increase in Arabidopsis Bystander Plants Exposed to Volatiles Produced by Wounded Tobacco Leaves.
Acknowledgments
Financial support was provided by The Natural Sciences and Engineering Research Council of Canada, Alberta Agriculture Research Institute, and Human Frontiers Scientific Program grants to I.K. and by National Institutes of Health Grant R37 GM48707 and National Science Foundation Grant MCB-0519898 awarded to F.M.A.
AUTHOR CONTRIBUTIONS
Y.Y. and R.P. performed research and analyzed data, C.H.D. performed research, analyzed data, and wrote the article. F.J.Z. performed research and wrote the article. V.T. and O.N.C. performed research. F.M.A. analyzed data and wrote the article. I.K. designed research, analyzed data, and wrote the article.
References
- Agrawal G.K., Jwa N.S., Shibato J., Han O., Iwahashi H., Rakwal R. (2003). Diverse environmental cues transiently regulate OsOPR1 of the “octadecanoid pathway” revealing its importance in rice defense/stress and development. Biochem. Biophys. Res. Commun. 310: 1073–1082 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., Hirayama T., Roman G., Nourizadeh S., Ecker J.R. (1999). EIN2, a bifunctional transducer of ethylene and stress responses in Arabidopsis. Science 284: 2148–2152 [DOI] [PubMed] [Google Scholar]
- Alonso J.M., et al. (2003). Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science 301: 653–657 [DOI] [PubMed] [Google Scholar]
- Apostol I., Heinstein P.F., Low P.S. (1989). Rapid stimulation of an oxidative burst during elicitation of cultured plant cells: Role in defense and signal transduction. Plant Physiol. 90: 109–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attaran E., Zeier T.E., Griebel T., Zeier J. (2009). Methyl salicylate production and jasmonate signaling are not essential for systemic acquired resistance in Arabidopsis. Plant Cell 21: 954–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin I.T., Halitschke R., Paschold A., von Dahl C.C., Preston C.A. (2006). Volatile signaling in plant-plant interactions: “Talking trees” in the genomics era. Science 311: 812–815 [DOI] [PubMed] [Google Scholar]
- Bowling S.A., Clarke J.D., Liu Y., Klessig D.F., Dong X. (1997). The cpr5 mutant of Arabidopsis expresses both NPR1-dependent and NPR1-independent resistance. Plant Cell 9: 1573–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowling S.A., Guo A., Cao H., Gordon A.S., Klessig D.F., Dong X. (1994). A mutation in Arabidopsis that leads to constitutive expression of systemic acquired resistance. Plant Cell 6: 1845–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A., Blevins T., Yao Y., Golubov A., Bilichak A., Ilnytskyy Y., Hollunder J., Meins F., Jr, Kovalchuk I. (2010a). Transgenerational adaptation of Arabidopsis to stress requires DNA methylation and the function of Dicer-like proteins. PLoS ONE 5: e9514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A., Golubov A., Bilichak A., Kovalchuk I. (2010b). Chlorine ions but not sodium ions alter genome stability of Arabidopsis thaliana. Plant Cell Physiol. 51: 1066–1078 [DOI] [PubMed] [Google Scholar]
- Boyko A., Greer M., Kovalchuk I. (2006a). Acute exposure to UVB has a more profound effect on plant genome stability than chronic exposure. Mutat. Res. 602: 100–109 [DOI] [PubMed] [Google Scholar]
- Boyko A., Hudson D., Bhomkar P., Kathiria P., Kovalchuk I. (2006b). Increase of homologous recombination frequency in vascular tissue of Arabidopsis plants exposed to salt stress. Plant Cell Physiol. 47: 736–742 [DOI] [PubMed] [Google Scholar]
- Boyko A., Kathiria P., Zemp F.J., Yao Y., Pogribny I., Kovalchuk I. (2007). Transgenerational changes in the genome stability and methylation in pathogen-infected plants: (virus-induced plant genome instability). Nucleic Acids Res. 35: 1714–1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A., Kovalchuk I. (2010). Transgenerational response to stress in Arabidopsis thaliana. Plant Signal. Behav. 5: 995–998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyko A., Zemp F., Filkowski J., Kovalchuk I. (2006c). Double-strand break repair in plants is developmentally regulated. Plant Physiol. 141: 488–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H., Glazebrook J., Clarke J.D., Volko S., Dong X. (1997). The Arabidopsis NPR1 gene that controls systemic acquired resistance encodes a novel protein containing ankyrin repeats. Cell 88: 57–63 [DOI] [PubMed] [Google Scholar]
- Caputo C., Rutitzky M., Ballaré C.L. (2006). Solar ultraviolet-B radiation alters the attractiveness of Arabidopsis plants to diamondback moths (Plutella xylostella L.): Impacts on oviposition and involvement of the jasmonic acid pathway. Oecologia 149: 81–90 [DOI] [PubMed] [Google Scholar]
- Clarke J.D., Liu Y., Klessig D.F., Dong X. (1998). Uncoupling PR gene expression from NPR1 and bacterial resistance: Characterization of the dominant Arabidopsis cpr6-1 mutant. Plant Cell 10: 557–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke J.D., Volko S.M., Ledford H., Ausubel F.M., Dong X. (2000). Roles of salicylic acid, jasmonic acid, and ethylene in cpr-induced resistance in Arabidopsis. Plant Cell 12: 2175–2190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke S.M., Cristescu S.M., Miersch O., Harren F.J., Wasternack C., Mur L.A. (2009). Jasmonates act with salicylic acid to confer basal thermotolerance in Arabidopsis thaliana. New Phytol. 182: 175–187 [DOI] [PubMed] [Google Scholar]
- Danon A., Rotari V.I., Gordon A., Mailhac N., Gallois P. (2004). Ultraviolet-C overexposure induces programmed cell death in Arabidopsis, which is mediated by caspase-like activities and which can be suppressed by caspase inhibitors, p35 and Defender against Apoptotic Death. J. Biol. Chem. 279: 779–787 [DOI] [PubMed] [Google Scholar]
- Demkura P.V., Abdala G., Baldwin I.T., Ballaré C.L. (2010). Jasmonate-dependent and -independent pathways mediate specific effects of solar ultraviolet B radiation on leaf phenolics and antiherbivore defense. Plant Physiol. 152: 1084–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrant W.E., Wang S., Dong X. (2007). Arabidopsis SNI1 and RAD51D regulate both gene transcription and DNA recombination during the defense response. Proc. Natl. Acad. Sci. USA 104: 4223–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer E.E., Ryan C.A. (1990). Interplant communication: Airborne methyl jasmonate induces synthesis of proteinase inhibitors in plant leaves. Proc. Natl. Acad. Sci. USA 87: 7713–7716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filkowski J., Kovalchuk O., Kovalchuk I. (2004). Genome stability of vtc1, tt4, and tt5 Arabidopsis thaliana mutants impaired in protection against oxidative stress. Plant J. 38: 60–69 [DOI] [PubMed] [Google Scholar]
- Foggo A., Higgins S., Wargent J.J., Coleman R.A. (2007). Tri-trophic consequences of UV-B exposure: Plants, herbivores and parasitoids. Oecologia 154: 505–512 [DOI] [PubMed] [Google Scholar]
- Grant M., Lamb C. (2006). Systemic immunity. Curr. Opin. Plant Biol. 9: 414–420 [DOI] [PubMed] [Google Scholar]
- Green R., Fluhr R. (1995). UV-B-induced PR-1 accumulation is mediated by active oxygen species. Plant Cell 7: 203–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heil M., Silva Bueno J.C. (2007). Within-plant signaling by volatiles leads to induction and priming of an indirect plant defense in nature. Proc. Natl. Acad. Sci. USA 104: 5467–5472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilnytskyy Y., Boyko A., Kovalchuk I. (2004). Luciferase-based transgenic recombination assay is more sensitive than beta-glucoronidase-based. Mutat. Res. 559: 189–197 [DOI] [PubMed] [Google Scholar]
- Jenkins G.I. (2009). Signal transduction in responses to UV-B radiation. Annu. Rev. Plant Biol. 60: 407–431 [DOI] [PubMed] [Google Scholar]
- Jung H.W., Tschaplinski T.J., Wang L., Glazebrook J., Greenberg J.T. (2009). Priming in systemic plant immunity. Science 324: 89–91 [DOI] [PubMed] [Google Scholar]
- Kathiria P., Kovalchuk I. (2010). Reporter gene-based recombination lines for studies of genome stability. Methods Mol. Biol. 631: 243–252 [DOI] [PubMed] [Google Scholar]
- Kathiria P., Sidler C., Golubov A., Kalischuk M., Kawchuk L.M., Kovalchuk I. (2010). Tobacco mosaic virus infection results in an increase in recombination frequency and resistance to viral, bacterial, and fungal pathogens in the progeny of infected tobacco plants. Plant Physiol. 153: 1859–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirik V., Bouyer D., Schöbinger U., Bechtold N., Herzog M., Bonneville J.M., Hülskamp M. (2001). CPR5 is involved in cell proliferation and cell death control and encodes a novel transmembrane protein. Curr. Biol. 11: 1891–1895 [DOI] [PubMed] [Google Scholar]
- Kovalchuk I., Kovalchuk O., Kalck V., Boyko V., Filkowski J., Heinlein M., Hohn B. (2003). Pathogen-induced systemic plant signal triggers DNA rearrangements. Nature 423: 760–762 [DOI] [PubMed] [Google Scholar]
- Love A.J., Yun B.W., Laval V., Loake G.J., Milner J.J. (2005). Cauliflower mosaic virus, a compatible pathogen of Arabidopsis, engages three distinct defense-signaling pathways and activates rapid systemic generation of reactive oxygen species. Plant Physiol. 139: 935–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez C., Pons E., Prats G., León J. (2004). Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J. 37: 209–217 [DOI] [PubMed] [Google Scholar]
- Park S.W., Kaimoyo E., Kumar D., Mosher S., Klessig D.F. (2007). Methyl salicylate is a critical mobile signal for plant systemic acquired resistance. Science 318: 113–116 [DOI] [PubMed] [Google Scholar]
- Park S.W., Liu P.P., Forouhar F., Vlot A.C., Tong L., Tietjen K., Klessig D.F. (2009). Use of a synthetic salicylic acid analog to investigate the roles of methyl salicylate and its esterases in plant disease resistance. J. Biol. Chem. 284: 7307–7317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchta H. (2005). The repair of double-strand breaks in plants: Mechanisms and consequences for genome evolution. J. Exp. Bot. 56: 1–14 [DOI] [PubMed] [Google Scholar]
- Rao M.V., Lee H., Creelman R.A., Mullet J.E., Davis K.R. (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12: 1633–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries G., Heller W., Puchta H., Sandermann H., Seidlitz H.K., Hohn B. (2000). Elevated UV-B radiation reduces genome stability in plants. Nature 406: 98–101 [DOI] [PubMed] [Google Scholar]
- Rochette P.J., Lacoste S., Therrien J.P., Bastien N., Brash D.E., Drouin R. (2009). Influence of cytosine methylation on ultraviolet-induced cyclobutane pyrimidine dimer formation in genomic DNA. Mutat. Res. 665: 7–13 [DOI] [PubMed] [Google Scholar]
- Ryan C.A., Moura D.S. (2002). Systemic wound signaling in plants: A new perception. Proc. Natl. Acad. Sci. USA 99: 6519–6520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santner A., Calderon-Villalobos L.I., Estelle M. (2009). Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 5: 301–307 [DOI] [PubMed] [Google Scholar]
- Shulaev V., Leon J., Raskin I. (1995). Is salicylic acid a translocated signal of systemic acquired resistance in tobacco? Plant Cell 7: 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick P.E., Tiryaki I., Rowe M.L. (2002). Jasmonate response locus JAR1 and several related Arabidopsis genes encode enzymes of the firefly luciferase superfamily that show activity on jasmonic, salicylic, and indole-3-acetic acids in an assay for adenylation. Plant Cell 14: 1405–1415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratmann J.W., Stelmach B.A., Weiler E.W., Ryan C.A. (2000). UVB/UVA radiation activates a 48 kDa myelin basic protein kinase and potentiates wound signaling in tomato leaves. Photochem. Photobiol. 71: 116–123 [DOI] [PubMed] [Google Scholar]
- Wang S., Durrant W.E., Song J., Spivey N.W., Dong X. (2010). Arabidopsis BRCA2 and RAD51 proteins are specifically involved in defense gene transcription during plant immune responses. Proc. Natl. Acad. Sci. USA 107: 22716–22721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber W., Daoud-El Baba M., Fussenegger M. (2007). Synthetic ecosystems based on airborne inter- and intrakingdom communication. Proc. Natl. Acad. Sci. USA 104: 10435–10440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildermuth M.C., Dewdney J., Wu G., Ausubel F.M. (2001). Isochorismate synthase is required to synthesize salicylic acid for plant defence. Nature 414: 562–565 [DOI] [PubMed] [Google Scholar]
- Yalpani N., Leon J., Lawton M.A., Raskin I. (1993). Pathway of salicylic acid biosynthesis in healthy and virus-inoculated tobacco. Plant Physiol. 103: 315–321 [DOI] [PMC free article] [PubMed] [Google Scholar]