Abstract
Periodontitis, a chronic inflammatory periodontal disease that develops from gingivitis, is caused by periodontal pathogenic bacteria such as Porphyromonas gingivalis. Recent studies have focused on the antioxidant, anti–human immunodeficiency virus, anticarcinogenic, and anti-inflammatory properties of gomisins. However, the anti-inflammatory activities of gomisin plants through heme oxygenase-1 (HO-1) signals remain poorly defined. We found that gomisins' anti-inflammatory activity occurs via the induction of HO-1 expression. Gomisins G and J inhibit the production of the pro-inflammatory cytokines tumor necrosis factor-α, interleukin-1β, and interleukin-6 and also block nuclear factor-κB activation in Raw264.7 cells stimulated with P. gingivalis lipopolysaccharide. Furthermore, pro-inflammatory cytokine production is inhibited through the induction of HO-1 expression. HO-1 expression is induced by all gomisins, but their anti-inflammatory activity via HO-1 signaling is observed with gomisins G and J, and not A. We found that gomisins G and J extracted from Schisandria chinensis can inhibit the P. gingivalis lipopolysaccharide induced-inflammatory responses in Raw264.7 cells.
Key Words: gomisins A, G, and J; heme oxygenase-1; nuclear factor E2-related factor 2; Porphyromonas gingivalis; lipopolysaccharide; pro-inflammatory cytokines
Introduction
Inflammation occurs in response to various harmful stimuli. Symptoms of inflammation include increased blood flow, heat, redness, and swelling. Acute inflammation involves the initiation of cell protection and healing at the injury site, whereas chronic inflammation, which affects tissues, can cause serious damage such as cancer.1
Periodontitis is a chronic inflammatory periodontal disease. It is not life-threatening, but once it develops, it affects the tissue supporting the teeth, leading to tooth loss. Periodontal chronic inflammatory disease is a risk factor for systemic problems such as cardiovascular disease, diabetes mellitus, and osteoporosis because the pathogen moves through blood vessels.2,3 Thus, periodontitis should be prevented and treated in the initial stages of infection. The primary cause of periodontitis is infection with rod-shaped, Gram-negative, anaerobic bacteria.4,5 Porphyromonas gingivalis infection is considered a major cause of periodontitis.
Heme oxygenase-1 (HO-1), a microsomal enzyme induced by several stimuli, has a cytoprotective capacity, enabling resistance to oxidative stress. HO-1 degrades free heme and produces carbon monoxide, ferrous iron (Fe2+), and biliverdin.6 Numerous studies have demonstrated the protective role played by HO-1 through the anti-inflammatory activity of carbon monoxide and cell death regulation with Fe2+ as well as the antioxidant activity of bilirubin converted from biliverdin by biliverdin reductase.7,8 HO-1 expression is induced by modulating ho-1 gene transcription. Under standard cell conditions, several transcription factors associated with HO-1 expression, such as nuclear factor E2-related factor 2 (Nrf-2), exist in the cytoplasm, bound with inhibitory proteins such as Keap-1. However, once activated by various stimuli, they separate from the complex and are translocated into the nucleus where they bind to DNA sequences, including antioxidant-responsive elements (AREs) in the HO-1 promoter region.9,10 The protective function of HO-1 is connected with the down-regulation of nuclear factor-κB (NF-κB) activation and decreased production of the pro-inflammatory cytokines interleukin (IL)-1β, IL-6, and tumor necrosis factor-α (TNF-α).11 HO-1 and its by-products are therapeutic targets in inflammatory diseases. Wiesel et al.12 found that HO-1 knockout mice show increased end-organ damage and death. Pae et al.13 also reported that HO-1 has protective properties in allergic inflammation, and other researchers have shown that HO-1 is a major regulator of the inflammatory autoimmune process.14,15
Gomisins are pharmacologically available from natural plants. These bioactive compounds known as phytoestrogens are dibenzocyclooctadiene-type lignans and possess antioxidant, anticarcinogenic, and anti-inflammatory properties. There are several reports on the advantages of gomisins A, G, and J. Gomisin A protects the liver by blocking the metabolism of harmful chemicals by inhibiting cytochrome P450-3A4;16 it also protects against neurotoxicity.17 Gomisin G's anti–human immunodeficiency virus effects occur via the inhibition of human immunodeficiency virus type 1 reverse transcriptase.18 Gomisin J has antioxidative effects in myoblast cells19 and suppresses inflammatory responses.20 The antiproliferative activity of gomisins A and J in cancer cells has also been studied.21 However, thus far, no studies have reported on the anti-inflammatory mechanism by which gomisins regulate HO-1 expression.
In this study, we investigated the anti-inflammatory effects of gomisins through HO-1 signaling, using mouse macrophage Raw264.7 cells, and compared the efficacy of gomisins A, G, and J. Our results demonstrated that gomisins G and J are excellent inhibitors of pro-inflammatory mediators, inducing HO-1 expression in P. gingivalis lipopolysaccharide (LPS)-stimulated Raw264.7 cells.
Materials and Methods
Materials
Fruits of Schisandra chinensis (Turcz.) Baill were collected in September 2005 from Moonkyong, Korea. A voucher specimen (accession number SC-PDRL-1) was deposited in the herbarium of Pusan National University (Miryang, Korea). The plant was identified by one of the authors (Y.-W.C.). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and other reagents were purchased from Sigma (St. Louis, MO, USA). Tin-protoporphyrin IX (SnPP) and antibodies for HO-1, Nrf-2, NF-κB, and TATA box binding protein (TBP) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). LPS (phenol extract of P. gingivalis) was purchased from Invitrogen (San Diego, CA, USA).
Isolation of gomisins A, G, and J
The dried fruits of S. chinensis (2.5 kg) were ground and then successively extracted at room temperature with n-hexane, CHCl3, and methanol. The hexane extract (308 g) was evaporated in vacuo and chromatographed on a silica gel (particle size, 40 μm; Baker, Phillipsburg, NJ, USA) column (100×10 cm) with a step gradient (0%, 5%, and 20%) of ethyl acetate in hexane and 5% methanol in CHCl3 to obtain 38 fractions as described before.22 Fraction 11 (KH11, 3,476 mg) was separated on a silica gel column (100×3.0 cm) with hexane–chloroform–methanol (75:25:1 by volume) to obtain four fractions. Fraction 3 (KH11IC, 1,116 mg) was separated on a Sephadex column (100×3.0 cm) with methanol (yield, 453 mg) (KH11ICIC). Finally, KH11ICIC was separated on a silica gel column (115×3.0 cm) with 5% acetone in chloroform to yield gomisin A (KH11ICICPA) (336.8 mg). Fraction 26 (KH26, 744 mg) was separated on a silica gel column (105×3.0 cm) with a step gradient of 7.5% acetone and 10% methanol in CH2Cl2 in order to obtain 20 fractions. Next, fraction 10 (KH26IJ, 244 mg) was rechromatographed on a silica gel column (105×3.0 cm) with 10% acetone in CHCl3 to yield gomisin J (KH26IJPG, 115.1 mg). Fraction 28 (KH28, 504 mg) was separated on a silica gel column (105×3.0 cm) with 5% acetone in CHCl3 to yield gomisin G (KH28PA, 7.5 mg). Pure gomisins A, G, and J were identified by high-performance liquid chromatograpy on a Phenomenex (Torrance, CA, USA) Luna C18 column (150 mm×4.6 mm i.d.; particle size, 5 μm) with a methanol–acetonitrile gradient at a flow rate of 1.0 mL/minute. Gomisins A, G and J, isolated from S. chinensis fruits, were identified on the basis of the 1H, 13C, and distortionless enhancement of polarization transfer nuclear magnetic resonance spectra in CDCl3 after comparison with previously reported spectral data.23–25
Cell culture
The murine macrophage cell line RAW 264.7 was obtained from American Type Culture Collection (Rockville, MD, USA). The cells were grown in Dulbecco's modified Eagle's medium (GIBCO, Grand Island, NY, USA), supplemented with 10% fetal bovine serum, and incubated at 37°C in a humidified atmosphere of 5% CO2 and 95% air.
Immunofluorescence confocal microscopy
RAW264.7 cells were cultured directly on glass coverslips in a 35-mm-diameter dish and fixed with 3.5% paraformaldehyde in phosphate-buffered saline for 10 minutes at room temperature. Next, they were permeabilized with 100% methanol for 10 minutes. To investigate the cellular localization of NF-κB, the cells were treated for 2 hours with a polyclonal antibody (diluted 1:100) against NF-κB. After extensive washing with phosphate-buffered saline, the cells were further incubated with a secondary fluorescein isothiocyanate–conjugated donkey anti-rabbit immunoglobulin G antibody diluted at 1:1,000 in phosphate-buffered saline for 1 hour at room temperature. Nuclei were stained with 1 μg/mL 4′,6-diamidino-2-phenylindole and then analyzed by confocal microscopy, using a Zeiss (Carl Zeiss Company Ltd., Shinjuku-ku, Tokyo, Japan) LSM 510 Meta microscope.
Transient transfection and dual-luciferase assay
RAW 264.7 cells were transfected with a κB-luc reporter plasmid (consisted of three κB concatamers from the immunoglobulin γ chain) and ARE reporter plasmid (Stratagene, Grand Island) using FuGENE®HD reagent (Roche Diagnostics Co., Roche Applied Science, Indianapolis, IN, USA) according to the manufacturer's instructions. The Renilla luciferase control plasmid pRL-CMV (Promega, Madison, WI, USA) was cotransfected as the internal control to determine transfection efficiency. Twenty-four hours after transfection, the cells were incubated with the indicated reagents for 1 hour and then treated with LPS (1 μg/mL) for 24 hours. Luciferase activity was assayed with a dual-luciferase assay kit (Promega), according to the manufacturer's instructions. Luminescence was measured with a microplate luminometer (Wallac 1420, Perkin Elmer, Norwalk, CT, USA).
Western blot analysis
Cells were harvested in ice-cold lysis buffer comprising 1% Triton X-100, 1% deoxycholate, and 0.1% sodium dodecyl sulfate. The protein content of the cell lysates was then determined using Bradford's reagent (Bio-Rad, Hercules, CA, USA). The proteins in each sample were resolved by 7.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to a polyvinylidine difluoride membrane, and exposed to the appropriate antibodies. The proteins were visualized using an enhanced chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ, USA), using horseradish peroxidase–conjugated anti-rabbit or anti-mouse secondary antibodies. Images were acquired using an ImageQuant™ 350 analyzer (Amersham Biosciences).
Reverse transcription real-time polymerase chain reaction
Total cellular RNA was isolated using RNA spin mini RNA isolation kits (GE Healthcare, Waukesha, WI, USA) according to the manufacturer's instructions. Total RNA (1 μg) was reverse-transcribed using Maxime reverse transcription PreMix (iNtRON Biotechnology, DCC-BIONET, Kyungki-Do, Korea) and anchored oligo(dT15) primers. Real-time polymerase chain reaction was performed using a Chromo4™ instrument (Bio-Rad) with SYBR® Green Master Mix (Applied Biosystems, Foster City, CA, USA). The real-time polymerase chain reaction cycling conditions were as follows: 95°C for 5 minutes, followed by 40 cycles of 30 seconds at 95°C, 20 seconds at 55°C, and 30 seconds at 72°C, ending with fluorescence measurement. The primer sequences were as follows: HO-1 sense, 5′-acaggttgacagaagaggctaa-3′; HO-1 antisense, 5′-aacaggaagctgagagtgagg-3′; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) sense, 5′-aggtggtctcctctgacttc-3′; and GAPDH antisense, 5′-taccaggaaatgagcttgac-3′.
Measurement of TNF-α, IL-1β, and IL-6 concentrations
Cells were incubated first with various concentrations of gomisins A, G, and J for 1 hour and then with P. gingivalis LPS (1 μg/mL) for 24 hours. Following the 24-hour incubation, TNF-α, IL-1β, and IL-6 levels were quantified in the culture medium using an enzyme-linked immunosorbent assay kit (R&D Systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Statistical analysis
Data are expressed as mean±SE values. Each experiment was repeated at least three times. Statistical analysis was performed with SPSS version 16.0 software (SPSS Inc., Chicago, IL, USA) to determine significant differences. We used either one- or two-way analysis of variance, followed by Dunn's post hoc tests for analyses. P<.05 was considered statistically significant.
Results
Isolation of gomisin A, G, and J from S. chinensis
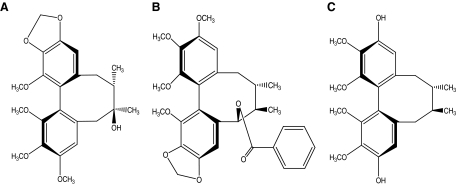
Gomisin A, G, and J were extracted from S. chinensis in large quantities compared with the other lignans, and their structures were identified by nuclear magnetic resonance analysis23 (Fig. 1). Gomisins A, G, and J were validated as composing more than 95% of the extract by chromatographic verification and were used in these experiments to determine their anti-inflammatory effects.
FIG. 1.
Chemical structures of (A) gomisin A, (B) gomisin G, and (C) gomisin J isolated from S. chinensis.
Effects of gomisins on production of pro-inflammatory cytokines secreted from Raw264.7 cells stimulated with P. gingivalis LPS
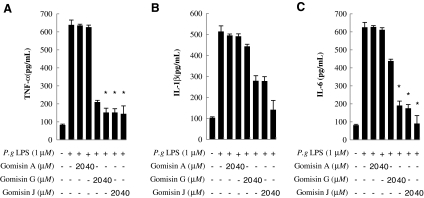
TNF-α, IL-1β, and IL-6 are pro-inflammatory cytokines that mediate the development of inflammation.15 To determine the anti-inflammatory activity of gomisins, cells were treated with 20 and 40 μM gomisins A, G, and J, and levels of TNF-α, IL-1β, and IL-6 produced in the medium after stimulation with P. gingivalis LPS were measured. The results were compared with those of cells not treated with gomisins and P. gingivalis LPS (Fig. 2). Treatment with gomisins G and J but not gomisin A decreased the production of all three pro-inflammatory cytokines in a dose-dependent manner. The amounts of all cytokines produced following treatment with gomisin A were similar to those produced by cells untreated with gomisins. The cell viability assay showed that gomisins A, G, and J are not cytotoxic for RAW 264.7 cells up to 40 μM. Treatment with gomisins A, G, and J did not cause cell necrosis and detachment from the culture plates, as determined by light microscopy (data not shown). These results suggest that the anti-inflammatory effect induced by gomisins G and J is caused by inhibition of P. gingivalis LPS-induced pro-inflammatory cytokines and not by the destruction of RAW 264.7 cells.
FIG. 2.
Effects of gomisins on P. gingivalis (P.g) lipopolysaccharide (LPS)-induced production of pro-inflammatory cytokines in Raw264.7 cells. Cells were incubated with various concentrations of gomisins for 1 hour prior to a 16-hour exposure to LPS (1 μg/mL). We measured (A) tumor necrosis factor-α (TNF-α), (B) interleukin (IL)-1β, and (C) IL-6 in the culture supernatant by enzyme-linked immunosorbent assay. Data are mean±SE values from three independent experiments in each group. *P<.05 versus the LPS-treated group.
Effects of gomisins on NF-κB nuclear translocation in Raw264.7 cells stimulated by P. gingivalis LPS
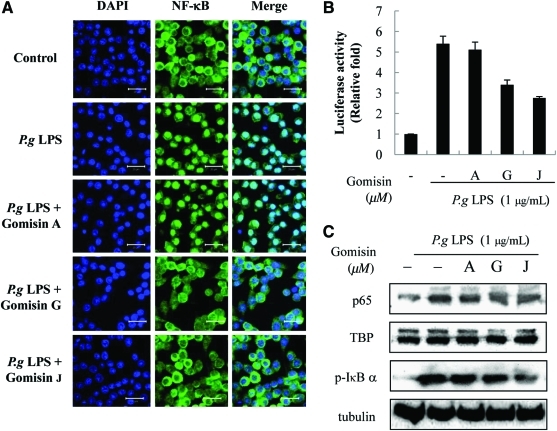
We investigated the effect of gomisins on NF-κB activation. Confocal microscopy revealed that gomisins G and J inhibit the nuclear translocation of NF-κB. However, gomisin A has no such effect (Fig. 3A). To confirm the effect of the gomisins at the transcription level, we conducted a luciferase assay to assess the promoter activity. Gomisins G and J but not gomisin A obstructed promoter activation and blocked the luminance of luciferase (Fig. 3B). The phosphorylation degrees of inhibitor of nuclear factor of κ light polypeptide gene enhancer in B-cells, α (IκBα) also indicate the same tendency (Fig. 3C). Western blotting revealed that gomisins G and J, but not gomisin A, significantly inhibit phosphorylation of IκBα. Because our results were consistent with those of others,26,27 our results support the proposal that gomisins G and J block the P. gingivalis LPS-induced nuclear translocation of NF-κB.
FIG. 3.
Effects of gomisins on P.g LPS-induced nuclear factor-κB (NF-κB) nuclear translocation in Raw264.7 cells. (A) Nuclear translocation of NF-κB was assessed using confocal microscopy. Raw 264.7 cells were pretreated with gomisins for 1 hour and stimulated with LPS (1 μg/mL) for 1 hour. Fixed cells were stained with 4′,6-diamidino-2-phenylindole (DAPI) and anti-NF-κB p65 antibody and fluorescein isothiocyanate–conjugated anti-rabbit immunoglobulin G antibody. (B) Cells were cotransfected with κB-luc reporter and control Renilla luciferase plasmid pRL-CMV. Then, 24 hours later, the cells were incubated with indicated concentrations of gomisins for 1 hour and stimulated with LPS (1 μg/mL) for 24 hours. Equal amounts of cell extracts were assayed for dual-luciferase activity. κB-Luciferase activity was normalized to control Renilla luciferase expression. (C) Nuclear translocation of NF-κB was confirmed by western blotting. Nuclear extracts were prepared and analyzed by western blotting. Cytosolic extracts were analyzed by western blotting with phosphorylated inibitor of nuclear factor of κ light polypeptide gene enhancer in B-cells, α (p-IκB α) antibody. TBP, TATA box binding protein. Color images available online at www.liebertonline.com/jmf
Effects of HO-1 expression induced by the gomisin families on production of pro-inflammatory cytokines secreted from Raw264.7 cells stimulated with P. gingivalis LPS
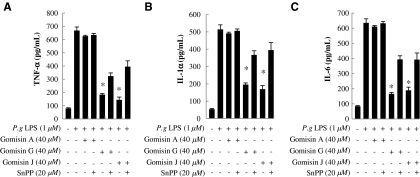
To verify the hypothesis that gomisins exert anti-inflammatory activity by inducing HO-1 expression in mouse macrophage cells, we investigated the effects of HO-1 expression on the production of the typical pro-inflammatory cytokines TNF-α, IL-1β, and IL-6, using the HO-1 inhibitor SnPP.26 The cells were preincubated with 40 μM gomisin and 20 μM SnPP and then stimulated with P. gingivalis LPS. In cells treated with gomisins G and J but not SnPP, the production of TNF-α, IL-1β, and IL-6 decreased, as reported earlier (Fig. 2). Besides, HO-1 activity was blocked by SnPP, and the production of TNF-α, IL-1β, and IL-6 was revived (Fig. 4). Gomisin A showed no anti-inflammatory activity, as reported previously.14,26 Thus, as expected, HO-1 plays a major role in suppressing TNF-α, IL-1β, and IL-6 production.
FIG. 4.
Effects of heme oxygenase-1 on production of P.g LPS-induced pro-inflammatory cytokines secreted by Raw264.7 cells. Cells were pretreated with 40 μM gomisins in the presence of tin-protoporphyrin IX (SnPP) for 1 hour and then stimulated with LPS (1 μg/mL) for 16 hours. We measured the amounts of (A) TNF-α, (B) IL-1β, and (C) IL-6 in the culture supernatant by enzyme-linked immunosorbent assay.
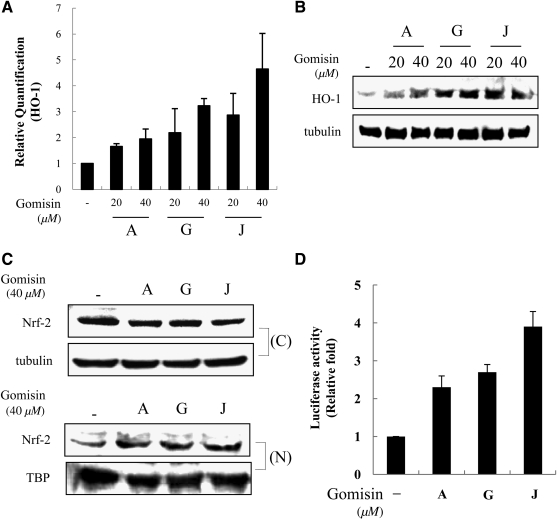
HO-1 expression was induced by gomisins in Raw264.7 cells
HO-1 and its by-products have been studied for their cell-protective and anti-inflammatory activities.7,14 We assessed the effects of gomisins on HO-1 expression in Raw264.7 cells and found that gomisins induced HO-1 expression (Fig. 5A and B). The effects of 20 and 40 μM gomisins A, G, and J were examined. All gomisins induced HO-1 mRNA and protein expressions in a dose-dependent manner (Fig. 5A and B). To understand the regulation of HO-1 expression at the transcriptional level, we investigated Nrf-2 nuclear translocation in Raw 264.7 cells treated with gomisins (Fig. 5C). Nrf-2 is a redox-sensitive transcription factor that binds to AREs in order to regulate HO-1 expression.8,9 In cell total extracts, gomisins increased Nrf-2 expression. In the same context, the Nrf-2 level increased in the nucleus but decreased in cytoplasm. To elucidate the effect of gomisins on Nrf-2 transactivity, we examined the activity of the luciferase reporter gene driven by Nrf-2 bound to AREs. As shown in Figure 5D, treatment of RAW 264.7 cells with gomisins increased ARE promoter activity. These results indicate that Nrf-2 is markedly regulated by gomisins in HO-1 expression.
FIG. 5.
Induction effects of gomisins on heme oxygenase-1 (HO-1) expression in Raw264.7 cells. Cells were cultured with increasing concentrations of gomisins for 8 hours (A) prior to analysis of HO-1 mRNA using reverse transcription real-time polymerase chain reaction. Relative HO-1 mRNA expression (by the 2-ΔCt method) was determined by real-time polymerase chain reaction and calculated by subtracting the Ct value for glyceraldehyde 3-phosphate dehydrogenase from the Ct value for HO-1 as determined by reverse transcription real-time polymerase chain reaction relative to glyceraldehyde 3-phosphate dehydrogenase mRNA (ΔCt=Ct of HO-1 – Ct of glyceraldehyde 3-phosphate dehydrogenase). Relative content of each mRNA was indicated as fold change from control. (B) Cells were incubated for 16 hours with the indicated concentrations of gomisins. Total cellular extracts were prepared and examined by western blotting. Western blot detection of α-tubulin was used as a protein loading control for each lane. (C) Cells were incubated with 40 μM gomisins for 2 hours. The cytosolic extracts (C) and nuclear extracts (N) were prepared and examined by western blotting. (D) Cells were transfected with the antioxidant response elements–luciferase construct and then treated with indicated concentrations of gomisins. Equal amounts of cell extract were assayed for dual-luciferase activity. Expression from the Renilla luciferase control was used to normalize antioxidant response elements–luciferase activity. Data are mean±SE values from three independent experiments in each group. Nrf-2, nuclear factor E2-related factor 2.
Discussion
Periodontitis is a chronic inflammatory disease of the oral cavity and one of the periodontal diseases that develop from gingivitis in the gingival soft tissue. Periodontitis can develop into various systemic diseases, leading to stroke and adverse pregnancy outcomes.1–3 Local inflammation is a beneficial response when triggered by infection; however, excessive or dysregulated inflammatory response can lead to disease. P. gingivalis is a Gram-negative bacterium that forms communities and is frequently found in the human oral cavity. P. gingivalis LPS is considered an important virulence factor because LPS is closely associated with the inflammatory response. P. gingivalis LPS is functionally and chemically quite different from enterobacterial LPS.3–5 The inflammatory response to P. gingivalis LPS has been well documented in many different types of human cells. P. gingivalis LPS activates the host to produce pro-inflammatory cytokines such as TNF-α, IL-6, and IL-1β in macrophages. P. gingivalis LPS initiates macrophage pro-inflammatory cytokine production, and these cytokines damage the periodontal tissue.2,27 Many recent studies have reported that natural compounds extracted from fruits, vegetables, or herbs may prevent or cure inflammatory periodontal diseases.26,27 Because P. gingivalis LPS-induced infections have been linked with periodontitis and various chronic inflammatory diseases, it is possible that the modulation of the inflammatory response is one of the major actions of S. chinensis extract.
When the fruits of S. chinensis are brewed in hot water and drunk as a traditionally prepared tea in Korea or China, the brew prevents diseases of the oral cavity. Therefore, we attempted to determine the relationship between S. chinensis extract and periodontal inflammation in vitro. First, we screened for the inhibitory effects of gomisins on nitrite production of P. gingivalis LPS-stimulated Raw264.7 cells. Gomisins G and J but not gomisin A inhibited nitrite production (data not shown). Next, we investigated the production of pro-inflammatory cytokines in Raw264.7 cells stimulated with P. gingivalis LPS and treated with gomisins. Production of the representative pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 was significantly inhibited with 20 μM gomisins G and J. To confirm this result, we examined the results of NF-κB activation using confocal microscopy, a luciferase assay, and western blotting. NF-κB regulates the production of pro-inflammatory mediators as a transcriptional factor.26 We found that gomisins G and J obstruct NF-κB nuclear translocation successfully. The nuclear translocation level of p65, a major active subunit of NF-κB, and the phosphorylation level of IκBα decreased in cells co-cultured with P. gingivalis LPS and gomisins G and J compared with P. gingivalis LPS-stimulated control cells. Promoter activity showed the same propensity. Previous studies have reported that extracts from S. chinensis inhibit the phosphorylation of the mitogen-activated protein kinase family, including extracellular signal-regulated kinase, p38, and c-Jun N-terminal kinase18,19 as well as nuclear translocation of NF-κB inhibitory capacity. However, there are few studies investigating the HO-1 signaling pathway.
HO-1, which plays a major role in resisting oxidative stress, was recently revealed to be involved in the inflammatory response. In this study, we investigated whether HO-1 mediates the anti-inflammatory activity of gomisins. All the gomisins examined in this study induced the expression of HO-1 mRNA and protein (Fig. 5). Our results revealed that gomisins enhance Nrf-2 nuclear translocation, induce HO-1 expression, and function as an anti-inflammatory mediator. Nrf-2 is a key factor in HO-1 expression. A recent report found that p65 represses Nrf-2 transcription activity.28 Therefore, we can infer from the results that the gomisins' anti-inflammatory effects via induction of HO-1 are possibly due to inhibition of p65 activation. The data in Figure 4 support the proposal that the anti-inflammatory effect of gomisins G and J is through HO-1 signaling. When cells were treated with the HO-1 inhibitor SnPP, the levels of TNF-α, IL-1β, and IL-6 returned to those following treatment with gomisin G and J.
In Asia, food and herbs are traditionally used to prevent and treat several disorders.29 Phytoestrogens, including isoflavones, flavonoids, and lignans,29 are found in various plants, and their therapeutic effects have been confirmed by modern science. As already known, the induction of HO-1 has many advantages in the immune system, oxidative stress, and cancer. Thus, to investigate various protective roles of HO-1 induction by phytoestrogens elucidates the beneficial effects of many natural compounds.
Thus, our study showed that gomisins G and J, the lignan type of phytoestrogen, have anti-inflammatory properties that are exerted through the induction of HO-1 expression in P. gingivalis LPS-stimulated Raw264.7 cells. Hence, gomisins G and J, extracted from S. chinensis, can be used as therapeutic agents for periodontal diseases. However, gomisin A did not reveal any anti-inflammatory response despite inducing HO-1 expression. We speculate that the HO-1 expression induced by gomisin A is mediated by some other signaling pathway. In conclusion, this is the first report on the relationship between gomisins and oral inflammation through HO-1 expression.
Acknowledgment
This study was supported by grant 106048031SB010 from the Technology Development Program for Agriculture and Forestry, Ministry for Food, Agriculture, Forestry and Fisheries, Korea.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Ferrero-Miliani L. Nielsen OH. Anderson PS. Girardin SE. Chronic inflammation: importance of NOD2 and NALP3 in interleukin-1beta generation. Clin Exp Immunol. 2007;147:227–235. doi: 10.1111/j.1365-2249.2006.03261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim J. Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology. 2006;94:10–21. doi: 10.1007/s10266-006-0060-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour GJ. Ford PJ. Cullinan MP. Leishman S. Tamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13(Suppl 4):3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 4.Tanner A. Kent R. Maiden MF. Taubman MA. Clinical, microbiological and immunological profile of healthy, gingivitis and putative active periodontal subjects. J Periodontal Rev. 1996;31:195–204. doi: 10.1111/j.1600-0765.1996.tb00484.x. [DOI] [PubMed] [Google Scholar]
- 5.Slots J. Listgarten MA. Bacteroides gingivalis, Bacteroides intermedius and Actinobacillus actinomycetemcomitans in human periodontal diseases. J Clin Periodontol. 1998;15:85–93. doi: 10.1111/j.1600-051x.1988.tb00999.x. [DOI] [PubMed] [Google Scholar]
- 6.Tenhunen R. Marver HS. Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61:748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Otterbein LE. Choi AMK. Heme oxygenase: colors of defense against cellular stress. Am J Physiol Lung Cell Mol Physiol. 2000;279:L1029–L1037. doi: 10.1152/ajplung.2000.279.6.L1029. [DOI] [PubMed] [Google Scholar]
- 8.Paine A. Eiz-Vesper B. Blasczyk R. Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 9.Itoh K. Wakabayashi N. Katoh Y. Ishii T. Igarashi K. Engel JD. Yamamoto M. Keap1 represses nuclear activation of antioxidant response elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi M. Yamamoto M. Molecular mechanisms activating the Nrf2-keap1 path way of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 11.Morse D. Pischke SE. Zhou Z. Davis RJ. Flavell RA. Loop T. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK path way and AP-1. J Biol Chem. 2003;278:36993–36998. doi: 10.1074/jbc.M302942200. [DOI] [PubMed] [Google Scholar]
- 12.Wiesel P. Patel AP. Difonzo N. Marria PB. Sim CU. Pellacani A. Maemura K. LeBlanc BW. Marino K. Doerschuk CM. Yet SF. Lee ME. Perrella MA. Endotoxin-induced mortality is related to increased oxidative stress and end-organ dysfunction, not refractory hypotension, in heme oxygenase-1 deficient mice. Circulation. 2000;102:3015–3022. doi: 10.1161/01.cir.102.24.3015. [DOI] [PubMed] [Google Scholar]
- 13.Pae HO. Lee YC. Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov. 2008;2:159–165. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 14.Chora AA. Fontoura P. Cunha A. Pais TF. Cardoso S. Ho PP. Lee LY. Sobel RA. Steinman L. Soares MP. Heme oxygenase-1 and carbon monoxide suppress autoimmune neuroinflammation. J Clin Invest. 2007;117:438–447. doi: 10.1172/JCI28844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobayashi H. Takeno M. Saito T. Takeda Y. Kirino Y. Noyori K. Hayashi T. Ueda A. Ishigatsubo Y. Regulatory role of heme oxygenase 1 in inflammation of rheumatoid arthritis. Arthritis Rheum. 2006;54:1132–1142. doi: 10.1002/art.21754. [DOI] [PubMed] [Google Scholar]
- 16.Wan CK. Tse AK. Yu ZL. Zhu GY. Wang H. Fong DWF. Inhibition of cytochrome P450 3A4 activity by schisandrol A and gomisin A isolated from Fructus Schisandrae shinensis. Phytomedicine. 2010;17:702–705. doi: 10.1016/j.phymed.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Kim SR. Lee MK. Koo KA. Kim SH. Sung SH. Lee NG. Markelonis GJ. Oh TH. Yang JH. Kim YC. Dibenzocyclooctadiene lignans from Schisandra chinensis protect primary cultures of rat cortical cells from glutamate-induced toxicity. J Neurosci Res. 2004;76:397–405. doi: 10.1002/jnr.20089. [DOI] [PubMed] [Google Scholar]
- 18.Chen DF. Zhang SX. Xie L. Xie JX. Chen K. Kashiwada Y. Zhou BN. Wang P. Cosentino LM. Lee KH. Anti-AIDS agents—XXVI. Structure-activity correlations of gomisin-G-related anti-HIV lignans from Kadsura interior and of related synthetic analogues. Bioorg Med Chem. 1997;5:1715–1723. doi: 10.1016/s0968-0896(97)00118-1. [DOI] [PubMed] [Google Scholar]
- 19.Yoshikawa A. Saito Y. Maruyama K. Lignan compounds and 4,4′-dihydroxybiphenyl protect C2C12 cells against damage from oxidative stress. Biochem Biophys Res Commun. 2006;344:394–399. doi: 10.1016/j.bbrc.2006.03.107. [DOI] [PubMed] [Google Scholar]
- 20.Oh SY. Kim YH. Bae DS. Um BH. Pan CH. Kim CH. Lee HJ. Lee JK. Anti-inflammatory effects of gomisin N, gomisin J, and schisandrin C isolated from the fruit of Schisandra chinensis. Biosci Biotechnol Biochem. 2010;74:285–291. doi: 10.1271/bbb.90597. [DOI] [PubMed] [Google Scholar]
- 21.Slaninova I. Brezinova L. Koubikova L. Slanina J. Dibenzocyclooctadiene lignans overcome drug resistance in lung cancer cells—study of structure-activity relationship. Toxicol In Vitro. 2009;23:1047–1054. doi: 10.1016/j.tiv.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 22.Choi YW. Kim HJ. Park SS. Chung HW. Lee SO. Oh BS. Kim JB. Kim HY. Chung BP. Yu CD. Kim SY. Inhibition of endothelial cell adhension by the new anti-inflammatory agent alpha-iso-cubebene. Vascul Pharmacol. 2009;51:215–224. doi: 10.1016/j.vph.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 23.Choi YW. Takamatsu S. Khan SI. Srinivas PV. Ferreira D. Zhao J. Khan IA. Schisandrene, a dibenzocyclooctadiene lignan from Schisandra chinensis; structure-antioxidant activity relationships of dibenzocyclooctadiene lignans. J Nat Prod. 2006;69:356–359. doi: 10.1021/np0503707. [DOI] [PubMed] [Google Scholar]
- 24.Ikeya Y. Taguchi H. Yosioka I. Kobayashi H. The constituents of Schizandra chinensis Baill. I. Isolation and structure determination of five new lignans, gomisin A, B, C, F and G, and the absolute structure of schizandrin. Chem Pharm Bull (Tokyo) 1979;27:1383–1394. doi: 10.1248/cpb.27.1383. [DOI] [PubMed] [Google Scholar]
- 25.Ikeya Y. Taguchi H. Sasaki K. NaKajima K. Yosioka I. The constituents of Schizandra chinensis Baill. VI. 13C nuclear magnetic resonance spectroscopy of dibenzocyclooctadiene lignans. Chem Pharm Bull (Tokyo) 1980;28:2414–2421. [Google Scholar]
- 26.Park SY. Kim YH. Kim EK. Ryu EY. Lee SJ. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with Porphyromonas gingivalis lipopolysaccharide. Chem Biol Interact. 2010;188:437–445. doi: 10.1016/j.cbi.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 27.Chen D. Nie M. Fan MW. Bian Z. Anti-inflammatory activity of curcumin in macrophages stimulated by lipopolysaccharides from Porphyromonas gingivalis. Pharmacology. 2008;82:264–269. doi: 10.1159/000161127. [DOI] [PubMed] [Google Scholar]
- 28.Yu M. Li H. Liu Q. Liu F. Tang L. Li C. Yuan Y. Zhan Y. Xu W. Li W. Chen H. Ge C. Wang J. Yang X. Nuclear factor p65 interacts with Keap1 to repress the Nrf2-ARE pathway. Cell Signal. 2011;23:883–892. doi: 10.1016/j.cellsig.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 29.Hwang YP. Jeong HG. Mechanism of phytoestrogen puerarin-mediated cytoprotection following oxidative injury: estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol Appl Pharmacol. 2008;233:371–381. doi: 10.1016/j.taap.2008.09.006. [DOI] [PubMed] [Google Scholar]