Abstract
Background
We have demonstrated that soy isoflavones radiosensitize cancer cells. Prostate cancer patients receiving radiotherapy (RT) and soy tablets had reduced radiation toxicity to surrounding organs. We have now investigated the combination of soy with RT in lung cancer (NSCLC), for which RT is limited by radiation-induced pneumonitis.
Methods
Human A549 NSCLC cells were injected i. v. in nude mice to generate lung tumor nodules. Lung tumor-bearing mice were treated with left lung RT at 12 Gy and with oral soy treatments at 1mg/day for 30 days. Lung tissues were processed for histology.
Results
Compared to lung tumor nodules treated with soy isoflavones or radiation, lung tissues from mice treated with both modalities showed that soy isoflavones augmented radiation-induced destruction of A549 lung tumor nodules leading to small residual tumor nodules containing degenerating tumor cells with large vacuoles. Soy isoflavones decreased the hemorrhages, inflammation and fibrosis caused by radiation in lung tissue, suggesting protection of normal lung tissue.
Conclusions
Soy isoflavones augment destruction of A549 lung tumor nodules by radiation, and also mitigate vascular damage, inflammation and fibrosis caused by radiation injury to normal lung tissue. Soy could be used as a non-toxic complementary approach to improve RT in NSCLC.
Keywords: Lung cancer, soy isoflavones, radiation
INTRODUCTION
Lung cancer is the second most common malignancy in both men and women in the USA and the leading cause of death. It is estimated that over 215,000 people per year will be diagnosed with lung cancer [1]. Approximately, 85% of lung cancers are classified as non-small cell lung cancer (NSCLC), which includes squamous cell carcinoma, adenocarcinoma and large cell carcinoma. A third of patients with newly diagnosed NSCLC present with unresectable stage III locally advanced disease with an overall 5-year survival rate of 16%. Locally advanced disease is currently treated by chemo-radiotherapy [2]. Several trials showed that concurrent cisplatin chemotherapy with radiotherapy (RT) is superior to sequential chemotherapy followed by RT or to RT alone; however the median survival is only about 17 months [2]. The treatment success has been severely constrained by poor local tumor control and radiation-induced injury to normal lung tissue. The early and late effects of radiation on normal lung tissue cause pneumonitis which is due to a severe inflammatory response to tissue damage [3, 4]. This condition leads to fibrosis and the formation of scar tissue in the lungs that affect patient breathing and their quality of life [3, 4].
We have explored the use of soy isoflavones, which are plant estrogens extracted from soy beans, as a complementary strategy to simultaneously increase radiation effectiveness on lung tumors while reducing pneumonitis and fibrosis in the normal lung tissue. The rationale for selecting soy isoflavones to combine with radiotherapy for NSCLC is based on our work demonstrating that these compounds not only cause tumor cell apoptosis but also sensitize cancer cells to radiation in human cancer cell lines including NSCLC, prostate and kidney [5–9]. We have shown that the mechanisms of potentiation of radiotherapy by soy isoflavones involve the inhibition of radiation-induced activation of survival signaling pathways [5–9]. We have identified three molecular targets APE1/Ref-1, NF-κB and HIF-1α, which are activated by tumor cells in their survival response to radiation but are inhibited by soy isoflavones which leads to increased cell killing and tumor growth inhibition [5–9]. APE1/Ref-1 is a protein involved in DNA repair and redox activation of transcription factors including NF-κB and HIF-1α. Recent findings suggest that inhibition of APE1/Ref-1 DNA repair activity by soy is involved in the mechanism by which soy inhibits DNA repair and leads to greater radiation-induced cell killing in NSCLC cells [7]. Radiosensitization of tumors by soy isoflavones was also demonstrated in vivo in pre-clinical orthotopic models of prostate cancer and renal cell carcinoma [6, 10-3].
Our goals for the current study are to evaluate the effect of soy isoflavones to modulate the radiation response of both tumors and normal tissues in a mouse orthotopic model of NSCLC. The attraction to investigate the role of natural soy isoflavones as radiosensitizers and radioprotectors in NSCLC is based on their safety for human use, as demonstrated in controlled clinical trials [14]. Furthermore, soy isoflavones can also act as anti-oxidants in normal tissues thus protecting them from radiation-induced toxicity. This protective effect was observed in our clinical trial for prostate cancer patients showing that soy isoflavone pills, taken in conjunction with radiotherapy, reduced radiation toxicity resulting in improved urinary, gastrointestinal and sexual functions [15]. We present now recent data from pre-clinical animal studies demonstrating enhanced tumor eradication and normal lung protection by soy isoflavones.
MATERIALS AND METHODS
Establishment of NSCLC lung tumor model
The human non-small cell lung carcinoma (NSCLC) A549 (purchased from ATCC) was cultured in F-12K culture medium containing 7% heat-inactivated fetal bovine serum with supplements. A549 cells, at 2×106 in 200 μ1 HBSS, were injected i. v. in the tail vein of 5–6 week old female Hsd Athymic Nude-Foxn1nu nu/nu nude mice (Harlan, Indianapolis, IN). Mice were housed and handled under sterile conditions using sterilized shoebox cages and micro-filter tops and supplied autoclaved food and water ad libitum. Our assigned animal rooms have been declared pathogen free for the past several years. Any manipulations are carried out using a BSL safety cabinet. Animal facilities are accredited by the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). The animal protocol was approved by Wayne State University Institutional Animal Care and Use Committee (IACUC).
Soy Isoflavones
The soy isoflavones mixture G-4660 used is a pure extract of 98.16% isoflavones from soybeans consisting of 83.3% genistein, 14.6% daidzein and 0.26% glycitein (manufactured by Organic Technologies and obtained from NIH). The soy isoflavones mixture was dissolved in 0.1 mol/L Na2CO3 and mixed with sesame seed oil at a 2:1 ratio just prior to treatment to facilitate gavage and avoid irritation of the esophagus by Na2CO3 [10–13]. Mice were orally treated with 1mg per day of soy isoflavones (50 mg/kg body weight/day) by gavage. Control mice received the vehicle alone and no effect on tumor growth was observed in different tumor models [10–13].
Tumor-bearing lung irradiation
Three anesthetized mice, in jigs, were positioned under a 6.4 mm lead shield with 3 cut-outs in an aluminum frame mounted on the X-ray machine to permit selective irradiation of the left tumor-bearing lungs in 3 mice at a time, as previously described [16]. The radiation dose to the lung and the scattered dose to areas of the mouse outside of the radiation field were carefully monitored. To minimize backscattering of radiation, the bottom of the aluminum frame that holds the jigs was hollowed out and the backplate of the jig was thinned to 1.6mm thickness. Under these conditions and the lead shielding, the X ray dose to the shielded regions was reduced to 1% of the tumor dose. The dose rate was 101.0 cGy/min and HVL was 2 mm Cu. Photon irradiation was performed at a dose of 12 Gy with a Siemens Stabilipan X-ray set (Siemens Medical Systems, Inc) operated at 250 kV, 15 mA with 1 mm copper filtration at a distance of 47.5 cm from the target.
Treatment of A549 Lung Tumor Nodules with Soy Isoflavones and Radiation
To monitor tumor establishment in the lungs of mice, preliminary kinetics experiments were performed and mice were sacrificed at different time points after i. v. injection of A549 cells. Lungs were resected and processed for histological staining with H&E. Established tumor nodules of about 100–300 μm in diameter were detected by day 20 in the midst of the lung tissue, therefore this time point was selected to initiate treatment with soy isoflavones. In each experiment, 2 mice were killed at day 20 to validate the formation of tumor nodules. On day 20, mice were pre-treated with 1mg/day oral soy isoflavones each day for 3 days. Then, the left lung was selectively irradiated with 12 Gy. Soy treatment was continued on a daily basis for 4 more weeks. To assess the therapeutic response of lung tumors to soy and radiation, 6 mice per experimental group were treated. By day 49, the tumor nodules in untreated lungs were very large up to 1200 μm in diameter. Therefore this time point was selected for termination of our experiment so that the tumor nodules in treatment groups could be compared to those in control group prior to mouse death from tumor burden. This experiment was repeated twice.
Lung tissue preparation for histology
At completion of experiments, mice were killed and the irradiated left lung and the non-irradiated right lung were resected and processed separately for histology. The lungs were fixed in 10% buffered formalin, embedded in paraffin and sectioned. Sections were stained with hematoxylin-eosin (H&E) or with Masson-Trichome. Quantitation of histological findings were performed by evaluation of lung tissues from 5 representative mice per treatment group. Because only the left lung was irradiated, to differentiate between the effects of radiation and soy, the left and right lung were analyzed separately using a Nikon E-800 microscope. The number of nodules in each lobe was enumerated. Morphometric measurements of each tumor nodule were performed using Image-ProPlus version 6.2 software (MediaCybernetics). The two largest diameters of each nodule were measured to calculate the nodule surface area. The percent viable cells and degenerating giant tumor cells as well as the extent of inflammatory infiltrates in tumor nodules were evaluated and scored. The response of the normal lung tissue in left and right lung was analyzed separately. The extent of inflammatory infiltrates and hemorrhages in the lung tissues was scored in H&E stained slides. The extent of fibrosis was evaluated from Masson-Trichome stained slides.
Statistical analysis
For histological data analysis, differences in the number and size of tumor nodules among the various treatments groups were analyzed by two-tailed unpaired Student’s t test.
RESULTS
Greater destruction of lung tumor nodules by soy isoflavones combined with radiation
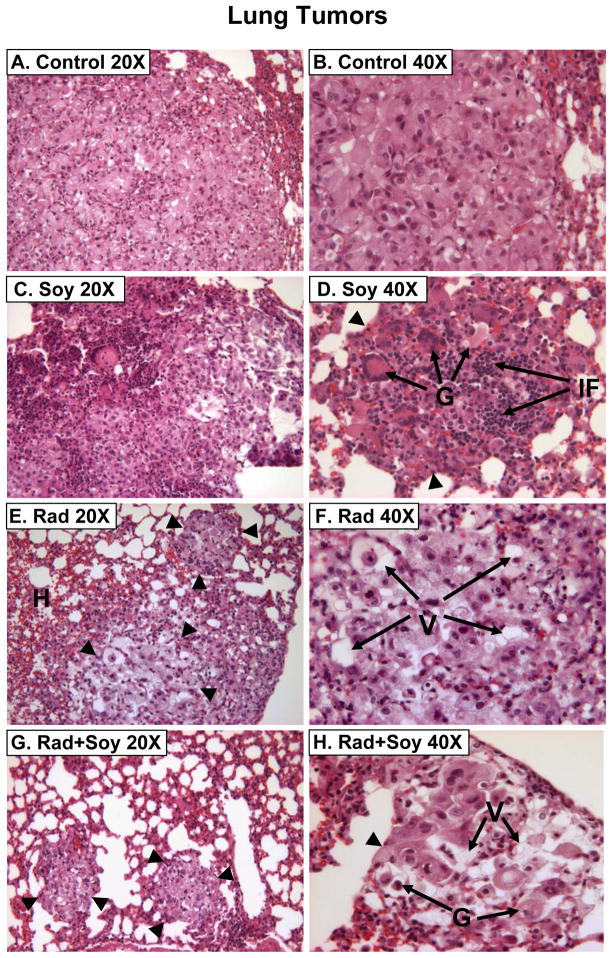
By day 20, following A549 cell i. v. injection, lung tumor nodules were detected histologically. Mice bearing established A549 lung tumors were either treated with vehicle only (control), soy alone, left lung irradiation alone or with soy combined with left lung irradiation. Soy was orally administered at 1 mg/day for 3 days on day 20–22 after cell injection (Fig. 1). Then, the left lung was selectively irradiated with 12 Gy and soy treatment was continued on a daily basis for 4 more weeks. Mice were killed on day 49 after cell injection (Fig. 1). The irradiated left lung and the non-irradiated right lung were processed separately for histology. In control untreated tumors, large tumor nodules were observed in lung tissue sections and presented with morphological features of adenocarcinoma consisting of large pleomorphic tumor cells, cytoplasmic vacuoles, large nuclei and prominent nucleoli (Fig. 2A,B). Soy treatment caused the formation of giant abnormal multinucleated tumor cells and a significant infiltration of inflammatory cells, comprising of mostly lymphocytes in tumor nodules (Fig. 2C,D). Left lung irradiated tumor nodules were smaller and showed marked degenerative changes in the cytoplasm (multiple vacuoles) and nuclei of tumor cells with inflammatory infiltrates (Fig. 2E,F). In left lungs irradiated and treated with soy, most mice had no detectable tumor nodules or only residual very small nodules with degenerating tumor cells including large vacuoles (Fig. 2G,H). The right non-irradiated lungs in these mice showed only changes induced by systemic soy treatment in lung tumor nodules. These findings were reproduced in a second independent experiment.
Figure 1.
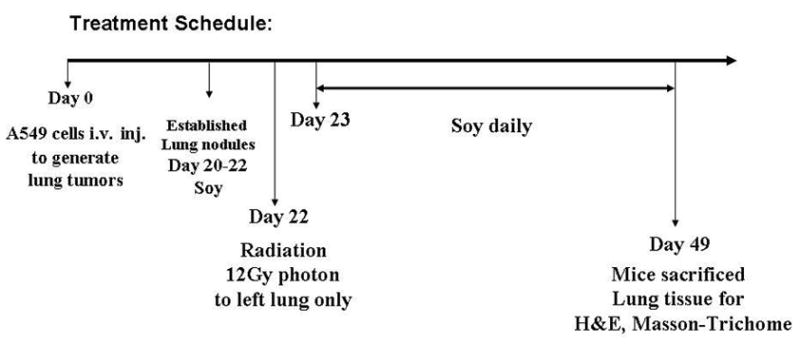
Treatment schedule of soy and radiation for established A549 lung tumors
Figure 2. Histology of A549 lung tumors from mice treated with radiation and soy.
A, B. Lung tumors from control mice showing a large tumor nodule composed of cells arranged in clusters and nodular aggregates. The cells are large, pleomorphic with abundant eosinophilic cytoplasm containing a few vacuoles. C, D. Lung tumors from soy-treated mice showing tumor nodules (arrowheads) with many multinucleated tumor giant cells [G arrows] infiltrated with inflammatory cells [IF arrows]. E, F. Lung tumors from radiation treated mice showing small tumor nodules (arrowheads) consisting mostly of cells with degenerative changes in the nuclei and cytoplasm with multiple large vacuoles [V arrows], hemorrhages [H arrows] and scattered inflammatory infiltrates. G, H. Lung tumors from radiation and soy treated mice showing very small residual tumor nodules (arrowheads) consisting of degenerating giant tumor cells [G arrows] with large vacuoles [V arrows] and few scattered lymphocytes.
A summary of quantitation of the main histological findings observed in lung tumor nodules is presented in Table 1, comparing the left irradiated lung and the right non-irradiated lung in mice treated with single modality soy isoflavones or radiation and both combined. The number of tumor nodules was reduced by radiation and soy in the lungs. The size of the tumor nodules was significantly smaller in the irradiated left lung (p<0.05) and was further reduced by combination with soy isoflavones compared to mice treated with radiation or soy only and to control mice (p<0.05). Tumor nodules treated with radiation and soy showed a dramatic decrease in cell viability concomitant with an increase in degenerating giant tumor cells (Table 1). These findings were also found in nodules treated with radiation or soy only but at a lower degree. Radiation increased infiltration of inflammatory cells in tumor nodules (Table 1). Tumor nodules from mice treated with soy isoflavones showed extensive inflammatory infiltrates as observed in Fig. 2C and 2D.
Table 1.
Quantitation of histological observations in lung tumor nodules following treatment with radiation and soy
| # Tumor Nodulesa mean±SE | Nodule Areab mean±SE×104μm2 (Range) | %Viablec Tumor Cells | % Giantd Tumor Cells | IFe Cells | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RL | LL | RL | LL | RL | LL | RL | LL | RL | LL | |
| Control | 8 ± 2 | 4 ± 1 | 28.1 ± 5.7 (12 – 48) | 19.9± 7.6 (4 – 47) | 90 | 90 | 5–10 | 5–10 | ± | ± |
| Radiation | 9 ± 1 | 2 ± 1 | 32.6 ± 3.1 (25 – 41) | *5.1 ± 1.0 (1.7 – 7.8) | 80 | 40–50 | 5–10 | 40–60 | ± | ++ |
| Soy isoflavones | 7 ± 2 | 4 ± 2 | *12.5 ± 1.3 (8 – 15) | 8.9 ± 2.1 (2.5 –14) | 50–60 | 50–60 | 25–50 | 25–50 | +++ | +++ |
| Rad + Soy | *3 ± 1 | *1 ± 0 | *7.6 ± 2.4 (1.6 – 15.2) | *1.5 ± 0.6 (0.0 –2.9) | 50–60 | 10–20 | 50 | 80 | ++ | ++ |
Histological findings, from the experiment presented in Figure 2 were quantified. Histology slides of H&E stained lung sections of the left lung (LL) and right lung (RL) were separately analyzed because only the left lung was irradiated. All data are reported as the mean of five mice per treatment group.
The tumor nodules were enumerated and the mean number of tumor nodules ± SE is reported.
The nodule area was estimated by morphometric measurements of the 2 largest diameters of each tumor nodule and the mean ± SE of all nodules as well as the range are presented.
The percentage of
viable tumor cells in the tumor nodules or
giant degenerating tumor cells was estimated.
The extent of inflammatory infiltration (IF) was scaled from mild (±) to heavy (+++).
Statistically significant values (p<0.05) compared to values from control mice.
Beneficial effect of soy isoflavones on irradiated normal lung tissue
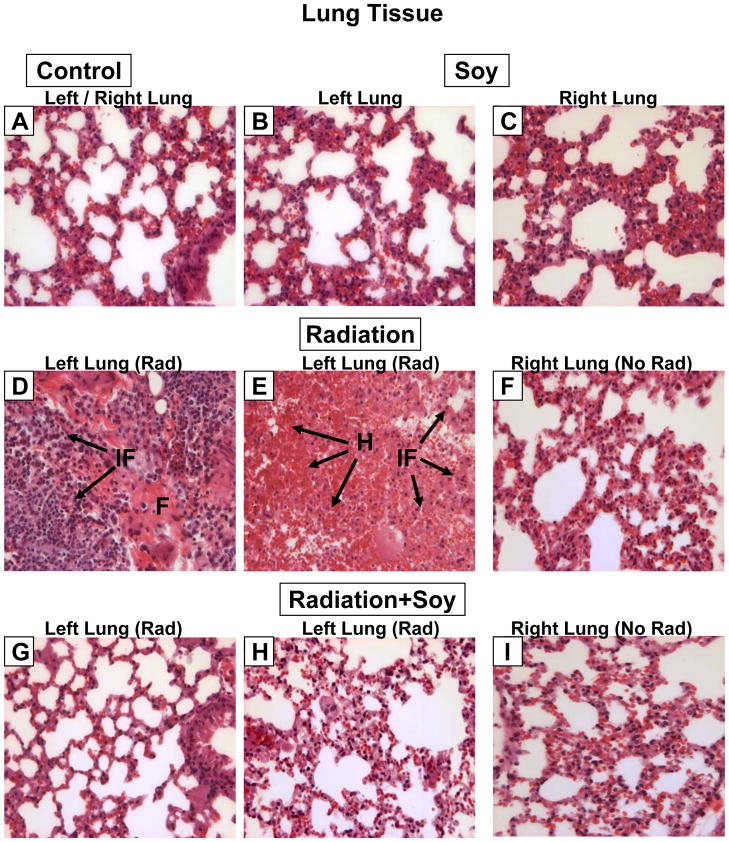
The lung tissue surrounding the tumor nodules was evaluated for changes induced by single and combined treatments (Fig. 3). In control mice, the lung tissue showed normal lung alveoli but also some areas of thick alveolar septae with interstitial fibrosis and hemorrhages were observed in the vicinity of tumor nodules (Fig. 3A, 4A,B). Soy treatment alone caused no changes in the morphology of the lung alveoli compared to control mice (Fig. 3B,C). Radiation to the left lung caused a heavy infiltration of inflammatory cells (lymphocytes and neutrophils) and fibrous tissue (Fig. 3D). Areas of extensive hemorrhages were observed (Fig. 3E). The right non-irradiated lung showed normal morphology, as could be expected (Fig. 3F), indicative of proper lead shielding from radiation. Following radiation to the left lung combined with soy, the morphology of the lung tissue resembled normal alveoli (Fig. 3G, H), comparable to that seen in the right non-irradiated lung (Fig 3. I).
Figure 3. Histology of lung tissue from A549 lung tumor-bearing mice treated with radiation and soy.
A. Lung tissue from control mice showing normal lung alveoli. B, C. Lung tissue from left and right lung from mice treated with soy showing no changes in the morphology of lung alveoli compared to control mice. D, E. Irradiated left lung showing heavy infiltration of inflammatory cells [IF arrows] consisting mostly of lymphocytes and a few neutrophils. Fibrous tissue [F arrows] is observed in the midst of inflammatory infiltrates [IF arrows]. In panel E areas of extensive hemorrhages [H arrows] with inflammatory infiltrates are observed in irradiated lung tissue. F. Non-irradiated right lung from radiation (left lung) treated mice showing normal lung tissue morphology. G, H. Irradiated left lung from mice treated with left lung irradiation and systemic soy. The morphology of lung tissue resembles normal alveoli as seen in control mice. I. Right lung non-irradiated from mice treated with left lung irradiation and systemic soy showing normal morphology of lung tissue. All magnifications 40X.
Figure 4. Masson Trichome staining of fibrosis in A549 lung tumor-bearing mice treated with radiation and soy.
A. Lung tissue from control untreated mice showing typical blue staining of collagen lining surrounding the basement membrane of vessels and bronchioles (arrows) but no staining in lung alveoli. B. Tumor nodule from control untreated mice showing blue peripheral staining of vessels. C, D. Lung tissue from soy treated mice with no significant collagen staining. E, F. Lung tissue from irradiated left lung showing extensive fibrotic tissue staining and hemorrhages (F). G, H. Lung tissue from irradiated left lung of mice treated with left lung irradiation and systemic soy showing lung morphology and limited or no staining of fibrotic tissue and remaining degenerative large tumor cells [arrowheads] with inflammatory infiltrates (H). All magnifications 40X.
Masson Trichome staining for collagen was used to detect further radiation-induced fibrosis (Fig. 4). In lung tissues from control mice, blue staining of collagen was seen around vessels and bronchioles (Fig. 4A) and in vessels of tumor nodules (Fig. 4B). In soy-treated lung, thinning of alveolar septa was observed with only scattered focal areas of thicker septae stained by interstitial collagen staining (Fig. 4C,D). However, the irradiated left lung had areas of extensive collagen staining, suggesting fibrotic tissue (Fig. 4E) and also showed hemorrhages (Fig. 4F). Radiation caused thicker alveoli septae, more intense collagen staining and thick perivascular collagen staining. In contrast, irradiated left lung treated with soy showed only limited fibrosis and thin alveoli septae (Fig. 4G), and remaining degenerating large tumor cells (Fig. 4H).
A summary of quantitation of the main histological findings in the normal lung tissue surrounding the tumor nodules is presented in Table 2, comparing the left irradiated lung and the right non-irradiated lung in mice treated with soy isoflavones or radiation as a single modality and both combined. A dramatic infiltration of inflammatory cells and extensive hemorrhages were observed in the irradiated left lung but not in the left lung treated with radiation combined with soy (Table 2). The extent of fibrosis evaluated by Masson-Trichome showed a strong increase of collagen staining in the alveoli septae of irradiated lungs but only weak collagen staining in lungs treated with combined radiation and soy. These findings were consistently reproduced.
Table 2.
Quantitation of histological observations of normal lung tissue following treatment with radiation and soy
| Inflammatory Infiltrates | Hemorrhages | Fibrosis | ||||
|---|---|---|---|---|---|---|
| RL | LL | RL | LL | RL | LL | |
| Control | ± | ± | ± | ± | ± | ± |
| Radiation | ± | +++ | ± | +++ | ± | ++ |
| Soy isoflavones | ± | ± | ± | ± | ± | ± |
| Rad + Soy | ± | ± | ± | ± | ± | ± |
Histological findings of normal lung tissue surrounding the tumor nodules, from the experiment presented in Figures 3 and 4 were quantified and the extent of inflammatory infiltrates, hemorrhages and fibrosis were scaled from weak (±), moderate (+), strong (++) to heavy (+++). The fibrotic score was obtained from analysis of lung sections stained with Masson-Trichome as shown in Figure 4. Lung tissue sections of the left and right lung were separately analyzed because only the left lung was irradiated.
DISCUSSION
Our pre-clinical and clinical studies, and others have shown that soy isoflavones are safe natural compounds, which not only can act as anti-cancer agents, but also act as anti-oxidants in normal tissues and protect them from treatment toxicity [reviewed in 9]. Moreover, we have demonstrated that soy isoflavones can act as radiosensitizers by enhancing radiation-induced cell killing in various tumor cell lines in vitro and in orthotopic pre-clinical models of prostate and kidney tumors in vivo [5–13]. Soy isoflavones could be an ideal complementary approach for RT for NSCLC, with the potential to increase the response of the tumor whilst simultaneously attenuating radiation-induced injury to normal lung tissue.
In the current study, using a lung cancer pre-clinical orthotopic model in mice, we have investigated the use of soy isoflavones as a new biological strategy to favorably modify clinical responses to radiotherapy for NSCLC. A dose of 12 Gy X rays radiation was selected based on previous rodent studies reporting a lethal dose (LD50) of 11–15 Gy [17]. Radiation was administered only to the left lung to discriminate between the radiation effect and the soy effect in the same mouse when given as single or combined modalities. Soy induced aberrations in the morphology of tumor cells in A549 lung tumor nodules including the formation of multinucleated tumor giant cells. A significant inflammatory infiltrate was observed in the tumor nodules. Radiation caused degenerative changes in the nuclei and cytoplasm of tumor cells, including multiple vacuoles. Radiation also recruited inflammatory cells into the tumors and lung tissue. The alterations induced by soy isoflavones or radiation on lung tumors and their microenvironment were comparable to those previously reported both in murine xenograft or syngeneic prostate tumors [10–12]. Following combination of systemic soy treatment with localized left lung irradiation in mice bearing A549 lung tumor nodules, differential effects were observed on the left irradiated lung compared to the right non-irradiated lung. In the left irradiated lung, very few residual and small tumor nodules were detectable which consisted mainly of degenerating tumor cells and large empty vacuoles whereas the right non-irradiated lung had tumor nodules with aberrations consistent with the soy effect only, as could be expected. These data confirm that soy augments the therapeutic effect of radiotherapy for lung tumors in a pre-clinical lung tumor model. The effect of genistein/soy isoflavones in potentiating radiotherapy administered to the tumor-bearing organ was also demonstrated in additional xenograft models of prostate cancer and renal cancer [10,12,13]. In addition, we have shown that genistein enhances the effect of tumor irradiation in a syngeneic model of RM-9 prostate cancer in normal immunocompetent C57BL/6 mice [11]. Therefore, the radio-enhancement mediated by soy isoflavones on the tumor is independent of the mouse strain or their immune status, and is not associated with a T cell response as nude mice have defective immature T cells.
In NSCLC patients, RT induced injury to normal lung tissue results in an inflammatory process caused by damage to capillary endothelial and epithelial lung cells leading to pneumonitis and fibrosis [3, 4]. Radiation-induced pneumonitis was also documented following single dose or fractionated radiation by 2–4 months after radiation in immunocompetent naïve mice and rats not-bearing lung tumors [17–19]. End-points to assess pneumonitis included measurement of DNA damage in lung cells by micronucleus assay, macrophage activation, cytokine expression and lung function by measuring breating rate [18,19]. In our studies, using athymic nude mice bearing A549 lung tumor nodules, observations of the normal lung tissue surrounding tumor nodules showed effects of radiation-induced injury as early as 4 weeks after radiation. We found that radiation caused hemorrhages which are indicative of disruption of vessels in the lung tissue. A heavy inflammatory infiltrate was also observed. These findings were previously documented in normal immunocompetent mice and do not reflect a peculiar radiation response of lung tissues from athymic T cell immunodeficient mice [20]. Using a syngeneic model of lung tumor nodules generated by i. v. injection of the murine Renca renal adenocarcinoma in immunocompetent Balb/c mice, we had previously demonstrated that left lung irradiation with 8 Gy caused marked injury in the lung tissue as early as 7–15 days after irradiation [20]. These effects included vascular damage resulting in multifocal hemorrhages and inflammatory infiltration with mononuclear cells including macrophages that could be reflective of early effects of radiation on the lung tissue [20]. Although nude mice have defective immature T cells, they still have other functional cellular components of the immune system including granulocytes, natural killer (NK) cells, B lymphocytes and macrophages. These cells could all contribute to the inflammatory response induced by radiation damage to the normal tissue and to the tumor cells that was observed in the human A549/nude mouse xenograft model. Furthermore, Masson Trichome staining confirmed the presence of fibrotic collagen tissue in the normal lung surrounding the tumor nodules that could result from radiation-induced lung tissue damage. Interestingly, radiation injury in the normal lung tissues was markedly reduced by combining RT with continuous soy isoflavones treatment. Our data indicate that soy isoflavones decrease the hemorrhages, inflammation and fibrosis caused by radiation in lung tissue, suggesting protection of normal lung tissue. Previous studies performed in naïve mice or rats also showed that pure genistein reduced the extent of fibrosis induced by radiation injury to lung tissue [18,19]. In these studies, the findings of radioprotection by genistein seemed to be controversial and reported partial protection of lung tissue, although a decrease in inflammatory cytokines, collagen content, oxidative damage and fibrosis were documented [18,19]. We have shown that the effects of a natural mixture of isoflavones extracted from soybeans are more potent and safer than synthetized pure genistein [12,21]. Whether radioprotection is more effective with the mixture of soy isoflavones than pure genistein remains to be clarified. Because our studies were performed in mice bearing tumor nodules, it is conceivable that tumor cell damage induced by radiation could influence the normal surrounding tissue response probably by cytokine release from inflammatory cells recruited at the tumor site after initial radiation-induced damage to the tumor cells and the tumor microenvironment including the vasculature and stroma. Ongoing studies are addressing these issues in naïve normal mice as well as in athymic T cell immunodeficient mice bearing xenograft lung tumor nodules at early and late time points after radiation. In addition, the effect of soy isoflavones on hypoxia and inhibition of HIF-1α induction by radiation are under investigation in relation to tissue radioprotection as HIF-1α activity in irradiated lungs was associated with radiation-induced inflammation and fibrosis [8, 22].
The findings in the pre-clinical lung tumor model and previous radioprotection studies discussed in this manuscript suggest that a complimentary approach could be applied to the treatment of patients with unresectable stage III locally advanced NSCLC in which radiation dose-limiting toxicity could be mitigated by the protective effects of soy isoflavones on normal lung tissue. In particular, patients with already compromised lung functions, as COPD in smokers, could particularly benefit from this complementary approach [3].
Acknowledgments
These studies were supported by the American Institute for Cancer Research and Wayne State University Department of Radiation Oncology. The project described was also supported in part by Grant Number R21CA155518 from the National Cancer Institute. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute or National Institutes of Health. We thank Sanket Gujarathi for excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No financial disclosures.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Auperin A, Le PC, Rolland E, Curran WJ, Furuse K, Fournel P, et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:2181–90. doi: 10.1200/JCO.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 3.Jin H, Tucker SL, Liu HH, Wei X, Yom SS, Wang S, et al. Dose-volume thresholds and smoking status for the risk of treatment-related pneumonitis in inoperable non-small cell lung cancer treated with definitive radiotherapy. Radiother Oncol. 2009;91:427–32. doi: 10.1016/j.radonc.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kong FM, Hayman JA, Griffith KA, Kalemkerian GP, Arenberg D, Lyons S, et al. Final toxicity results of a radiation-dose escalation study in patients with non-small-cell lung cancer (NSCLC): predictors for radiation pneumonitis and fibrosis. Int J Radiat Oncol Biol Phys. 2006;65:1075–86. doi: 10.1016/j.ijrobp.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Raffoul JJ, Wang Y, Kucuk O, Forman JD, Sarkar FH, Hillman GG. Genistein inhibits radiation-induced activation of NF-kappaB in prostate cancer cells promoting apoptosis and G2/M cell cycle arrest. BMC Cancer. 2006;6:107. doi: 10.1186/1471-2407-6-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raffoul JJ, Banerjee S, Singh-Gupta V, Knoll ZE, Fite A, Zhang H, et al. Down-regulation of apurinic/apyrimidinic endonuclease 1/redox factor-1 expression by soy isoflavones enhances prostate cancer radiotherapy in vitro and in vivo. Cancer Res. 2007;67:2141–9. doi: 10.1158/0008-5472.CAN-06-2147. [DOI] [PubMed] [Google Scholar]
- 7.Singh-Gupta V, Joiner MC, Runyan L, Yunker CK, Sarkar FH, Miller S, et al. Soy Isoflavones Augment Radiation Effect by Inhibiting APE1/Ref-1 DNA Repair Activity in Non Small Cell Lung Cancer. J Thoracic Oncol. 2011;4:688–98. doi: 10.1097/JTO.0b013e31821034ae. [DOI] [PubMed] [Google Scholar]
- 8.Singh-Gupta V, Zhang H, Banerjee S, Kong D, Raffoul JJ, Sarkar FH, et al. Radiation-induced HIF-1alpha cell survival pathway is inhibited by soy isoflavones in prostate cancer cells. Int J Cancer. 2009;124:1675–84. doi: 10.1002/ijc.24015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hillman GG, Singh-Gupta V. Soy Isoflavones Sensitize Cancer Cells to Radiotherapy: From Bench to Clinic. Free Radical Biology and Medicine. 2011;51:289–98. doi: 10.1016/j.freeradbiomed.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 10.Hillman GG, Wang Y, Kucuk O, Che M, Doerge DR, Yudelev M, et al. Genistein potentiates inhibition of tumor growth by radiation in a prostate cancer orthotopic model. Mol Cancer Ther. 2004;3:1271–9. [PubMed] [Google Scholar]
- 11.Wang Y, Raffoul JJ, Che M, Doerge DR, Joiner MC, Kucuk O, et al. Prostate cancer treatment is enhanced by genistein in vitro and in vivo in a syngeneic orthotopic tumor model. Radiat Res. 2006;166:73–80. doi: 10.1667/RR3590.1. [DOI] [PubMed] [Google Scholar]
- 12.Raffoul JJ, Banerjee S, Che M, Knoll ZE, Doerge DR, Abrams J, et al. Soy isoflavones enhance radiotherapy in a metastatic prostate cancer model. Int J Cancer. 2007;120:2491–8. doi: 10.1002/ijc.22548. [DOI] [PubMed] [Google Scholar]
- 13.Hillman GG, Wang Y, Che M, Raffoul JJ, Yudelev M, Kucuk O, et al. Progression of renal cell carcinoma is inhibited by genistein and radiation in an orthotopic model. BMC Cancer. 2007;7:4. doi: 10.1186/1471-2407-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Messina M, Kucuk O, Lampe JW. An overview of the health effects of isoflavones with an emphasis on prostate cancer risk and prostate-specific antigen levels. J AOAC Int. 2006;89:1121–34. [PubMed] [Google Scholar]
- 15.Ahmad IU, Forman JD, Sarkar FH, Hillman GG, Heath E, Vaishampayan U, et al. Soy isoflavones in conjunction with radiation therapy in patients with prostate cancer. Nutr Cancer. 2010;62:996–1000. doi: 10.1080/01635581.2010.509839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Younes E, Haas GP, Dezso B, Ali E, Maughan RL, Kukuruga MA, et al. Local tumor irradiation augments the response to IL-2 therapy in a murine renal adenocarcinoma. Cell Immunol. 1995;165:243–51. doi: 10.1006/cimm.1995.1211. [DOI] [PubMed] [Google Scholar]
- 17.Hill RP. Radiation effects on the respiratory system. BJR Suppl. 2005;27:75–81. [Google Scholar]
- 18.Para AE, Bezjak A, Yeung IW, Van Dyk J, Hill RP. Effects of genistein following fractionated lung irradiation in mice. Radiother Oncol. 2009;92:500–10. doi: 10.1016/j.radonc.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 19.Calveley VL, Jelveh S, Langan A, Mahmood J, Yeung IW, Van Dyk J, et al. Genistein can mitigate the effect of radiation on rat lung tissue. Radiat Res. 2010;173:602–11. doi: 10.1667/RR1896.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dezso B, Haas GP, Hamzavi F, Kim S, Montecillo EJ, Benson PD, et al. The mechanism of local tumor irradiation combined with Interleukin 2 therapy in murine renal carcinoma: histological evaluation of pulmonary metastases. Clin Cancer Res. 1996;2:1543–52. [PubMed] [Google Scholar]
- 21.Singh-Gupta V, Zhang H, Yunker CK, Ahmad Z, Zwier D, Sarkar FH, Hillman GG. Daidzein effect on hormone refractory prostate cancer in vitro and in vivo compared to genistein and soy extract: potentiation of radiotherapy. Pharm Res. 2010;27:1115–27. doi: 10.1007/s11095-010-0107-9. [DOI] [PubMed] [Google Scholar]
- 22.Rabbani ZN, Mi J, Zhang Y, Delong M, Jackson IL, Fleckenstein K, et al. Hypoxia inducible factor 1alpha signaling in fractionated radiation-induced lung injury: role of oxidative stress and tissue hypoxia. Radiat Res. 2010;173:165–74. doi: 10.1667/RR1816.1. [DOI] [PMC free article] [PubMed] [Google Scholar]