Abstract
Most estimates of the cost of informal caregiving in patients with Alzheimer’s disease (AD) remain cross-sectional. Longitudinal estimates of informal caregiving hours and costs are less frequent and are from assessments covering only short periods of time. The objectives of this study were to estimate long-term trajectories of the use and cost of informal caregiving for patients with AD and the effects of patient characteristics on the use and cost of informal caregiving. The sample is drawn from the Predictors Study, a large, multicenter cohort of patients with probable AD, prospectively followed annually for up to 7 years in three university-based AD centers in the United States (n = 170). Generalized linear mixed models were used to estimate the effects of patient characteristics on use and cost of informal caregiving. Patients’ clinical characteristics included cognitive status (Mini-Mental State Examination), functional capacity (Blessed Dementia Rating Scale (BDRS)), comorbidities, psychotic symptoms, behavioral problems, depressive symptoms, and extrapyramidal signs. Results show that rates of informal care use and caregiving hours (and costs) increased substantially over time but were related differently to patients’ characteristics. Use of informal care was significantly associated with worse cognition, worse function, and higher comorbidities. Conditional on receiving informal care, informal caregiving hours (and costs) were mainly associated with worse function. Each additional point on the BDRS increased informal caregiving costs 5.4%. Average annual informal cost was estimated at $25,381 per patient, increasing from $20,589 at baseline to $43,030 in Year 4.
Keywords: informal care, costs, Alzheimer’s disease, longitudinal study
Informal caregivers provide the majority of care for patients with Alzheimer’s disease (AD) living in the community. As patients with AD become progressively less capable of self-care over time and rely on others to manage and supervise the most basic mental and physical tasks, informal care becomes increasingly more time consuming. Eventually, patients with AD reach a level of disability that requires constant care and supervision. Existing estimates of informal caregiving hours that patients receive range from 13 to 107 hours per week, with associated costs of between $2,019 and $19,688 per year.1 Most of these studies are cross-sectional. Longitudinal estimates of informal caregiving hours and cost are less frequent and are from assessments covering only short time periods.2,3
In the Predictors Study, a large, multicenter cohort of patients with probable AD was followed from early stages of the disease in three university-based AD centers in the United States. The goals of this study are to estimate empirically long-term trajectories of informal caregiving hours and costs of AD and relate them to patient characteristics. The longitudinal design offers several advantages. The most important one is that patients were recruited at early stages of the disease and followed for long periods of time so that the cohort encompasses the full range of disease progression. Longer-term effects on informal caregiving hours and costs are therefore more easily interpreted. By estimating informal caregiving hours and cost trajectories, it is hoped that useful data can be provided for future evaluations of the effects of AD on informal care.
METHODS
Sample
The sample was drawn from the Predictors 2 cohort, and consisted of 204 patients with probable AD recruited between 1998 and 2004 from Columbia University Medical Center, Johns Hopkins School of Medicine, and Massachusetts General Hospital. Each local institutional review board approved the study. The inclusion and exclusion criteria are fully described elsewhere.4–6 Briefly, subjects met Diagnostic and Statistical Manual of Mental Disorders, Third Edition, Revised, criteria for primary degenerative dementia of the Alzheimer type and National Institute for Neurological Disorders and Stroke/Alzheimer’s Disease and Related Disorder Association criteria for probable AD. Enrollment required a modified Mini-Mental State Examination score of 30 or higher, equivalent to a score of approximately of 16 or higher on the Folstein Mini-Mental State Examination (MMSE).7,8 Because subjects were followed at academic AD centers, there is a high degree of certainty in their AD diagnosis. Clinical diagnosis of AD has been confirmed in 93% of those with postmortem evaluation.6
Recruitment of patients began in 1998. Baseline data were collected for 13.3% of patients in 1998, 8.3% in 1999, 24.3% in 2000, 26.0% in 2001, 15.5% in 2002, 11.1% in 2003, and 1.1% in 2004. After the baseline visit, all patients were followed semiannually, with annual assessments of informal care use. At the time of data analysis, 82.4% had at least one follow-up assessment.
Because patterns of informal care utilization differ substantially for patients living in the community and those living in institutions,9,10 information on patients’ living arrangements was collected at each visit. Of the 204 patients in the sample, 48 (23.5%) reported living in an institutional setting (nursing homes, assisted living facilities, retirement homes) at some point during the study. Another 20 patients entered the study living at home and moved to an institutional setting at a later time. Four patients reported changing living environments more than once during the study. Visits during which the patient was living in an institutional setting were excluded from the analysis sample. Seven patients with missing information on informal care were also excluded. For the present study, the analysis sample consisted of 409 observations from 170 patients who lived at home. Of these 170 patients, 42 had one assessment only (at baseline), 56 had two assessments (baseline and 1 follow-up visit), 37 had three, 23 had four, 10 had five, and two had six assessments. Median follow-up for the cohort was 2.5 years; maximum was 6 years. Patients who did not respond at a particular visit could respond at a subsequent visit.
Measures
Informal Caregiving Time and Cost
Informal caregiving time was asked for up to three caregivers on basic and instrumental activities of daily living. Basic activities of daily living included eating, dressing, and personal care. Instrumental activities of daily living included shopping, chores, personal business, and transportation. Hours of informal care provided per day for each caregiving task were asked in the following categories: 0, <3, 3 to 6, >6 to 9, >9 to 12, & >12. The mean value of each category was used to estimate caregiving hours (>12 was top coded to 12). Hours provided for each task were summed to obtain an estimate of the total caregiving hours. Following the literature, total caregiving hours were top coded at 16 hours.11 Top coding affected six (1%) observations. The national average hourly earning for all private industries for each year was used as the hourly wage rate to estimate unpaid caregiving costs.12 Because 12.0% to 23.5% of the patients did not receive any informal care at each visit, a dichotomous variable was constructed to measure informal care utilization.
Clinical and Demographic Characteristics
Disease progression was characterized using MMSE score.7 Higher MMSE scores indicate better cognitive status. For ease of presenting descriptive results, MMSE scores were categorized into two groups at a cutpoint of 20, because it marked a transition from mild (MMSE >20) to moderate dementia (MMSE ≤20). Blessed Dementia Rating Scale (BDRS) Parts I and II were used to assess patients’ functional capacity.13 Higher BDRS scores indicate worse functional status. For ease of presenting descriptive results, the BDRS scores were categorized into high- and low-functioning groups at the baseline median score, because there are no established cutoff points for this scale.
The Columbia University Scale for Psychopathology in Alzheimer’s Disease was used to measure the presence or absence of psychotic, behavioral, and depressive symptoms.14,15 Following previous work,16,17 the presence of delusions, hallucinations, or illusions was considered to indicate the presence of psychotic symptoms. The presence of any of the following five symptoms (wandering away from home or caregiver, verbal outbursts, physical threats or violence, agitation or restlessness, or sundowning (more confusion at night or evening than during the day)) was considered to indicate the presence of behavioral problems. Depressed mood (feeling sad, depressed, blue) and difficulty sleeping or a change in appetite were considered to indicate the presence of depressive symptoms.
A modified Unified Parkinson’s Disease Rating Scale was used to measure the presence or absence of extrapyramidal signs (EPS).15,18,19 Following previous work,6,20 a dichotomous indicator was constructed for the presence of EPS if any of the following 11 items was rated 2 or higher (0 being normal and 4 indicating maximum impairment): speech, facial expression, tremor at rest, neck rigidity, right arm rigidity, left arm rigidity, right leg rigidity, left leg rigidity, posture, gait, and bradykinesia.
Patients’ medical histories were used to construct a modified version of the Charlson index of comorbidities.17,20,21 Comorbidities included myocardial infarction, congestive heart failure, peripheral vascular disease, hypertension, chronic obstructive pulmonary disease, arthritis, gastrointestinal diseases, liver disease, diabetes mellitus, chronic renal disease, and systemic malignancy. Patients’ age, ethnicity, sex, and highest level of education were recorded at the baseline visit, and marital status was recorded at each visit.
Analysis
Informal care utilization and hours were compared using the following clinical characteristics: functional status (high or low); cognition (mild or moderate); comorbidities (0, 1, ≥2); and the presence or absences of psychotic, EPS, depressive, and behavioral problems. Group comparisons of categorical variables (utilization) were performed using chi-square tests, and comparisons of continuous variables (hours) were performed using nonparametric Wilcoxon rank sum tests.
Generalized linear mixed models were used to estimate utilization and hours of informal care.22,23 The first part of the model estimates the probability of receiving any informal care. The second part estimates the continuous amount of informal care received, conditional on receiving any. Because hours of informal care were highly skewed to the right (skewness = 1.91), log hours were examined as the dependent variable.
Within- and between-person change in the trajectories of informal care utilization and hours over time were estimated as follows. A simple model that included an intercept and time as fixed effects and a random intercept term was first estimated. Time was measured in years from baseline (time 0). Time (year) squared was then included in the estimation model. The coefficient on time squared was statistically insignificant and was dropped in subsequent models. Potential nonlinearity was also tested for using squared-root and cubic terms. Next a random slope was included to allow for differences between patients in their overall rate of increase. Likelihood ratio tests suggested that including a random slope did not improve the model, and it was dropped in subsequent models. Finally, patient characteristics were included as fixed effects to control for any systematic differences in the sample on these variables. All clinical variables were entered as time-variant covariates, and all demographic variables except marital status were entered as time-invariant covariates.
For the first part of the model, informal care utilization, odds ratios are reported for ease of interpreting the results. For the second part of the model, because the dependent variable was log-transformed, interpretation of the coefficient estimates requires some care. For continuous explanatory variables, a coefficient of β estimates the proportional change in caregiving hours for a unit change in the explanatory variable, holding all other variables constant. That is, a unit increase in the explanatory variable increases caregiving hours by 100 β percent. For dichotomous explanatory variables, the corresponding proportional change on caregiving hours of the explanatory variable from the reference group is estimated as 100(e(β−1/2V(β)) − 1).24 All analyses were performed using Stata 9.0 (StataCorp., College Station, TX).
RESULTS
Sociodemographic and Clinical Characteristics
Because the sample used for the present analyses included only patients living in the community, patients’ baseline characteristics were first compared according to living arrangement: lived at home throughout the study period, lived in an institutional setting throughout the study period, and changed living arrangement during the study. Patients who lived in an institutional setting throughout the study period were older (85.3 vs 75.2, P<.001), more likely to be female (79.2% vs 56.3%, P = .03), and less likely to be married (12.5% vs 66.7%, P<.001) than patients living in other settings. Patients who lived in nursing homes throughout the study period had lower MMSE scores (20.3 vs 22.1, P = .04), higher BDRS scored (4.5 vs 3.5, P = .04), and marginally more comorbidities (1.3 vs 0.7, P = .08). Patients who lived in nursing homes throughout the study period were less likely to receive informal care (56.3% vs 77.5%, P = .05) and, of those who received care, received fewer caregiving hours (12.9 vs 14.0 h/wk, P = .01). Differences between patients who lived in retirement homes and assisted living facilities throughout the study period were not statistically significant, possibly because the number of patients living in these environments was small.
Longitudinal patterns of patient characteristics are shown in Table 1. The average patient in the sample was aged 74.9. Half were women (50.1%). Patients in the sample were largely non-Hispanic white (95.8%), well educated (average 14.7 years of schooling), and married (71.4%) or widowed (22.0%). Because of the study inclusion criteria, all patients were initially at the early stages of AD. As expected, patients’ cognition and function declined over time. Number of comorbidities (mean 0.7) remained stable over time.
Table 1.
Demographic and Clinical Characteristics of the Sample
| Characteristic | All Years (N = 409) | Baseline (n = 170) | Year 1 (n = 102) | Year 2 (n = 71) | Year 3 (n = 41) | Year 4 (n = 25) |
|---|---|---|---|---|---|---|
| Sociodemographic variables | ||||||
| Age at baseline, means ± SD | 74.9 ± 7.7 | 75.0 ± 7.6 | 75.2 ± 7.9 | 75.3 ± 8.3 | 74.0 ± 5.9 | 73.6 ± 8.6 |
| Female, % | 50.1 | 55.3 | 47.1 | 52.1 | 34.1 | 48.0 |
| Race, % | ||||||
| White | 95.8 | 95.3 | 97.1 | 94.4 | 95.1 | 100.0 |
| Other | 4.2 | 4.7 | 3.0 | 5.6 | 4.9 | 0.0 |
| Years of schooling, mean ± SD | 14.7 ± 3.3 | 14.5 ± 3.3 | 15.0 ± 3.1 | 14.8 ± 3.4 | 15.2 ± 3.4 | 13.6 ± 3.9 |
| Marital status, % | ||||||
| Married | 71.4 | 68.2 | 70.6 | 70.4 | 82.9 | 80.0 |
| Widowed | 22.0 | 24.7 | 21.6 | 22.5 | 14.6 | 16.0 |
| Other | 5.9 | 7.1 | 6.9 | 5.6 | 0.0 | 4.0 |
| Clinical characteristics | ||||||
| Mini-Mental State Examination score, mean ± SD (range 0–30) | 20.5 ± 5.5 | 22.1 ± 3.8 | 20.0 ± 5.8 | 19.5 ± 5.7 | 18.3 ± 7.6 | 15.6 ± 8.3 |
| Blessed Dementia Rating Scale score, mean ± SD (range 0–17) | 4.5 ± 3.0 | 3.4 ± 2.2 | 4.8 ± 3.1 | 5.0 ± 3.0 | 5.5 ± 3.3 | 8.0 ± 3.9 |
| Modified comorbidity index | 0.7 ± 0.9 | 0.7 ± 0.9 | 0.7 | 0.7 ± 0.9 | 0.7 ± 0.9 | 0.8 ± 0.9 |
| Behavioral problems, % | 49.4 | 42.4 | 49.0 | 62.0 | 53.7 | 56.0 |
| Extrapyramidal signs, % | 17.4 | 14.1 | 19.6 | 16.9 | 24.4 | 20.0 |
| Depressive symptoms, % | 19.6 | 19.4 | 25.5 | 16.9 | 9.8 | 20.0 |
| Psychotic symptoms, % | 35.0 | 31.2 | 34.3 | 36.6 | 48.8 | 36.0 |
| Site, % | ||||||
| Columbia | 51.3 | 51.8 | 42.2 | 56.3 | 58.5 | 50.0 |
| Johns Hopkins | 21.5 | 22.4 | 22.5 | 15.5 | 19.5 | 32.0 |
| Massachusetts General Hospital | 27.1 | 25.9 | 35.3 | 28.2 | 22.0 | 18.0 |
SD = standard deviation.
Unadjusted Utilization and Caregiving Hours Received
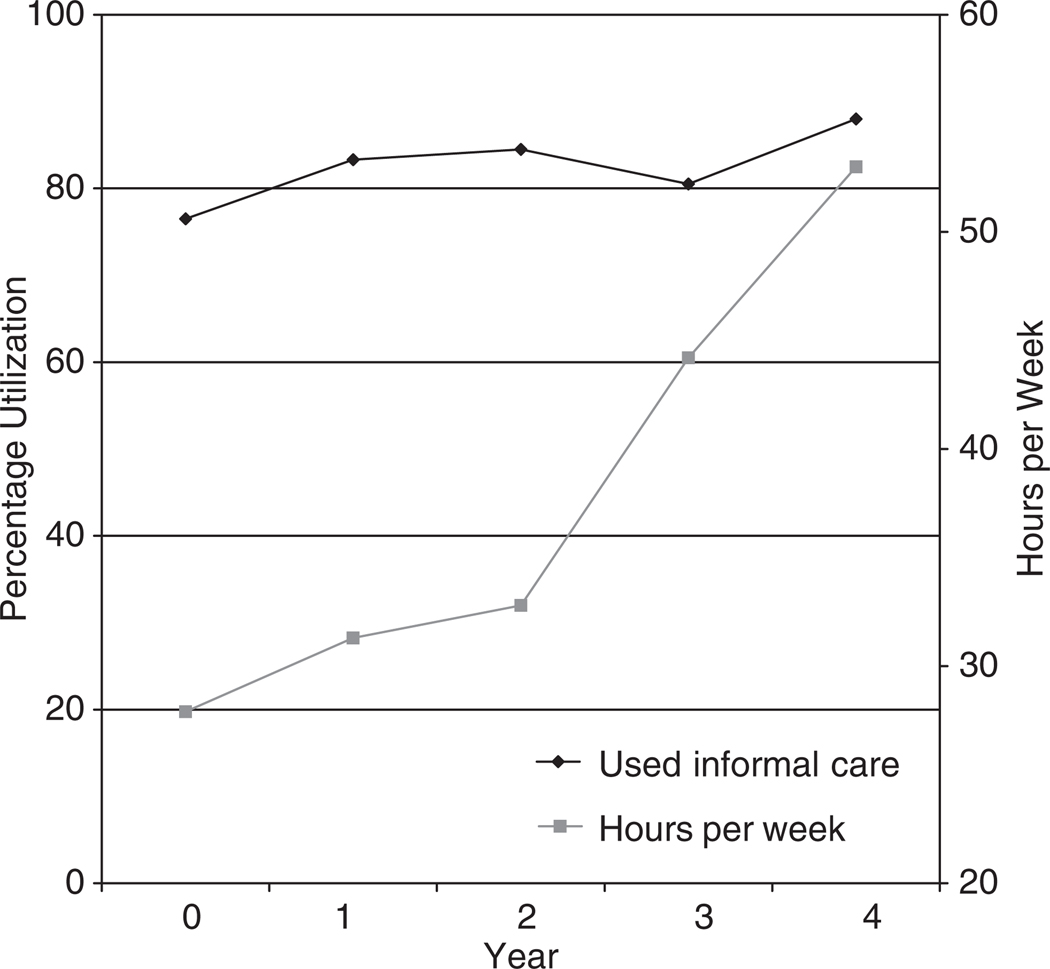
Figure 1 presents data on informal care utilization rates over time and, of those who received care, average caregiving hours received per week. Usage of informal care increased slightly from 76.5% at baseline to 88.0% in Year 4 (average utilization rate across years 80.7%). Of patients receiving care, average hours received was 33.1 per week (4.7 per day). Over time, average hours received per week increased from 27.9 at baseline to 53.0 in Year 4. Per user average annual cost of informal care was estimated at $25,381, increasing from $20,589 at baseline to $43,030 in Year 4.
Figure 1.
Utilization and hours of informal care received over time.
Table 2 presents data on informal care utilization rates and average weekly caregiving hours received over time according to patients’ clinical characteristics. Except for depressive symptoms, patients with worse clinical characteristics were more likely to receive informal care. Differences in utilization rates according to clinical characteristics were almost always consistent in each year and were significant according to BDRS score, MMSE score, and number of comorbidities (all P<.001). Of those who received care, differences in hours of informal care received were significantly different according to presence or absence of psychotic symptoms, EPS, depressive symptoms, BDRS score, and MMSE score (all P<.05).
Table 2.
Utilization and Hours of Informal Care Received over Time by Clinical Characteristic
| Utilization Rates per Patient |
Average Hours per Week per User |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Characteristic | All Years (n = 409) |
Baseline (n = 170) |
Year 1 (n = 102) |
Year 2 (n = 71) |
Year 3 (n = 41) |
Year 4 (n = 25) |
All Years (n = 330) |
Baseline (n = 130) |
Year 1 (n = 85) |
Year 2 (n = 60) |
Year 3 (n = 33) |
Year 4 (n = 22) |
| All patients | 80.7 | 76.5 | 83.3 | 84.5 | 80.5 | 88.0 | 33.1 | 27.9 | 31.3 | 32.8 | 44.2 | 53.0 |
| Psychotic symptoms | ||||||||||||
| Not present | 78.9 | 76.1 | 79.1 | 86.7 | 71.4 | 87.5 | 30.0* | 25.8 | 27.7 | 31.0 | 46.2 | 45.0 |
| Present | 83.9 | 77.4 | 91.4 | 80.8 | 90.0 | 88.9 | 38.5 | 32.3 | 41.0 | 32.0 | 42.6 | 66.9 |
| Extrapyramidal signs | ||||||||||||
| Not present | 79.3 | 76.0 | 80.5 | 84.7 | 74.2 | 90.0 | 31.1* | 27.2 | 27.8 | 31.3 | 47.0 | 46.7 |
| Present | 87.3 | 79.2 | 95.0 | 83.3 | 100.0 | 80.0 | 41.3 | 31.5 | 49.7 | 31.5 | 37.8 | 81.4 |
| Depressive symptoms | ||||||||||||
| Not present | 82.4 | 78.1 | 84.2 | 89.8 | 81.1 | 85.0 | 31.8* | 26.7 | 30.0 | 31.7 | 46.9 | 44.5 |
| Present | 73.8 | 69.7 | 80.8 | 58.3 | 75.0 | 100.0 | 38.8 | 33.3 | 41.0 | 28.5 | 17.5 | 81.9 |
| Behavioral problems | ||||||||||||
| Not present | 77.3 | 72.4 | 80.8 | 81.5 | 84.2 | 81.8 | 30.5 | 25.4 | 29.5 | 32.0 | 40.7 | 53.7 |
| Present | 84.2 | 81.9 | 86.0 | 86.4 | 77.3 | 92.9 | 35.5 | 30.8 | 36.0 | 30.9 | 47.6 | 52.5 |
| Blessed Dementia Rating Scale | ||||||||||||
| Higher function | 73.5† | 70.8 | 75.5 | 82.4 | 75.0 | 33.3 | 27.8† | 24.5 | 27.0 | 30.3 | 46.4 | 52.5 |
| Lower function | 89.6 | 90.0 | 91.8 | 86.5 | 84.0 | 95.5 | 38.4 | 34.3 | 37.9 | 32.2 | 43.0 | 53.0 |
| Mini-Mental State Examination score | ||||||||||||
| >20 | 74.0† | 73.5 | 78.8 | 72.7 | 70.6 | 50.0 | 28.3† | 27.3 | 27.7 | 21.9 | 49.0 | 31.5 |
| ≤20 | 89.0 | 83.9 | 91.3 | 94.1 | 83.3 | 100.0 | 36.6 | 28.8 | 36.9 | 36.4 | 46.2 | 58.8 |
| Number of comorbidities | ||||||||||||
| 0 | 72.5† | 65.1 | 74.5 | 80.0 | 73.9 | 91.7 | 34.5 | 28.2 | 33.4 | 29.9 | 47.6 | 60.1 |
| 1 | 89.5 | 86.4 | 94.3 | 91.3 | 80.0 | 100.0 | 30.7 | 27.4 | 30.5 | 31.0 | 39.4 | 47.3 |
| ≥2 | 87.5 | 89.3 | 87.5 | 84.6 | 100.0 | 71.4 | 34.2 | 28.1 | 36.3 | 35.3 | 42.0 | 44.1 |
Differences by clinical characteristics significant at
5% and
1% levels.
Adjusted Usage and Weekly Caregiving Hours
The first two columns of Table 3 present regression results of the effects of patients’ clinical and sociodemographic characteristics on informal care utilization. Results show that, controlling for other covariates, utilization of informal care remained stable over time. Each additional point in MMSE score decreased the probability of receiving informal care 9%. Each additional point in BDRS score increased the probability of receiving informal care 29.5%. Each additional comorbidity increased the probability of receiving informal care 62.2%. Table 3 also presents estimation results of the effects of each variable on informal caregiving hours for patients who received care. For patients who received some informal care, average informal caregiving hours increased 9.9% per year, and each additional point in BDRS score increased informal caregiving hours 5.4%. Differences in hours of informal care according to the presence of psychotic symptoms, EPS, or depressive symptoms found in bivariate analyses were no longer statistically significant.
Table 3.
Random Effect Model of Utilization and Costs of Informal Caregiving
| Utilization Rates per Patient (n = 170) |
Average Hours per Week per User (n = 152) |
||
|---|---|---|---|
| Variable | Coefficient (Standard Error) |
Odds Ratio | Coefficient (Standard Error) |
| Year | 0.148 (0.150) | 0.862 | 0.099 (0.037)† |
| Mini-Mental State Examination score | 0.094 (0.047)* | 0.910 | 0.011 (0.009) |
| Blessed Dementia Rating Scale score | 0.295 (0.106)† | 1.343 | 0.054 (0.020)† |
| Number of comorbidities | 0.622 (0.253)* | 1.862 | 0.017 (0.047) |
| Behavioral problems (1 = present, 0 = absent) | 0.044 (0.392) | 0.957 | 0.017 (0.090) |
| Extrapyramidal signs (1 = present, 0 = absent) | 0.101 (0.531) | 0.904 | 0.045 (0.113) |
| Depressive symptoms (1 = present, 0 = absent) | 0.539 (0.444) | 0.584 | 0.160 (0.109) |
| Psychotic symptoms (1 = present, 0 = absent) | 0.760 (0.439) | 0.467 | 0.128 (0.093) |
| Women (1 = yes, 0 = no) | 0.369 (0.438) | 0.691 | 0.056 (0.094) |
| Aged <65 (1 = yes, 0 = no) | 0.466 (0.647) | 1.594 | 0.102 (0.128) |
| Marital status (reference = other) | |||
| Married (1 = yes, 0 = 0) | 1.316 (0.768) | 3.728 | 0.212 (0.197) |
| Widowed (1 = yes, 0 = 0) | 0.941 (0.710) | 2.563 | 0.078 (0.190) |
| Site (reference = Columbia) | |||
| Johns Hopkins | 1.957 (1.794) | 7.078 | 0.300 (0.209) |
| Massachusetts General Hospital | 0.171 (0.431) | 0.843 | 0.466 (0.300) |
| Constant | 1.847 (1.450) | 9.894 (0.326)† | |
| Total number of observations | 409 | 306 | |
| Log likelihood | −158.5 | −315.6 | |
P<
.05,
.01.
DISCUSSION
These results extend the literature on informal caregiving for patients with AD in a number of ways. Most existing studies on informal caregiving costs for patients with AD are cross-sectional and include patients at various stages of the disease.1 This study prospectively followed a large cohort of patients from the early stages of the disease and examined patterns of informal caregiving utilization and costs longitudinally. It was estimated that, on average, 80% of AD patients living in the community received some informal care. Those receiving care received an average of 33.1 hours of care per week, with an estimated cost of $25,381 per patient per year. The consistency of the results with existing studies indicates the validity of the data collection process and costing methods used in the Predictors Study.
Little can be said in cross-sectional studies about informal caregiving trajectories. Results from a longitudinal study show that rates of informal care utilization and caregiving hours (and costs) increased substantially at each subsequent follow-up. Informal cost of AD was estimated at $20,589 per patient per year at baseline (27.9 h/wk), when all patients were at the early stages of the disease, to $43,030 in Year 4 (53.0 h/wk). These results are consistent with two studies that reported an average increase of 7 hours per week in informal caregiving time over a year.2,3
The multivariate analyses suggest that longitudinal trajectories of informal care utilization and intensity relate differently to patients’ clinical characteristics. Patients with worse cognition, lower function, and more comorbidities were significantly more likely to receive informal care. Alternatively, in patients who received care, informal caregiving hours (and cost) were significantly associated only with patients’ function. It is estimated that, with a baseline informal caregiving cost at $20,589 per patient per year, each additional point in BDRS score increased informal cost by $1,112 a year.
The effects of psychotic and behavioral problems and EPS on the cost of caring for patients with AD are not well understood. Similar to the baseline study25 but contrary to several cross-sectional studies that examined the effects of behavioral problems26–28 and EPS29 on costs, the presence of psychotic symptoms, behavior problems, or EPS were not found to be significantly associated with higher informal caregiving costs. Differences in the results may be due to several factors. First, informal caregiving hours reported in this study are total hours spent caring for patients with AD and not incremental caregiving hours, as used in previous studies. Second, the nonsignificant result found could be partly due to the roughness of the measures used in this study. For example, subcategories of EPS and psychotic symptoms were grouped together, and only dichotomous gradations of severity were used. In addition, behavioral and psychiatric symptoms in AD fluctuate over time, and particular symptoms can occur any time during the course of AD.17 Persistence of these symptoms also differs from symptom to symptom.30 The effects on hours of finer gradations of subtype and severity of each symptom will be examined in detail in future studies.
The decreasing number of patients included in the analysis over time reflects the staggered nature of the sample recruitment and patient deaths (7%). During the period in which each subject was followed, missed visits were rare; 15.6% missed one, 2.5% missed two, and 1% missed three visits. Baseline characteristics of the patients who had complete 4-year follow-up data were compared with those of patients who did not. There were no differences in sociodemographic and clinical characteristics between the two groups except that the completers were marginally more likely to be married at baseline (P = .07). Utilization and cost trajectories were also examined for the patients who had complete 4-year follow-up data. Utilization and cost trajectories for this subsample were similar to those of the entire sample, suggesting that the cost increases that were reported were not specific to those with long-term follow-up.
There are several limitations to this study. Patients were selected from tertiary care university hospitals and specialized diagnostic and treatment centers and thus represent a nonrandom sample of those affected by AD in the population. Patients in the sample also were predominantly white and highly educated. Caution is needed in generalizing the results of this study to patients of other ethnicities and lower levels of education and income and to patients with AD living in the community. The relative homogeneity of the sample may mask differences in clinical measures and patterns of informal caregiving. For example, black and Latino patients with moderate to severe dementia have been shown to have higher prevalence of dementia-related behavioral problem than whites.31 Sociodemographic and cultural differences between different racial/ethnic groups also may influence patterns of informal care and modify the effects of the clinical variables. Although no effects on informal caregiving of several important clinical variables (e.g., depression, behavioral problems, EPS) were found in this sample, these characteristics may be more pronounced in more-diverse samples. Because informal caregiving patterns may be different for individuals living in different environments, only patients living in the community were included. Future research will need to examine AD cost trajectories in samples that are more representative of the general population.
In general, several factors support confidence in these findings. A major contribution lies in the careful diagnosis and clinical follow-up that patients received. Clinical diagnosis took place in university hospitals with specific expertise in dementia and was based on uniform application of widely accepted criteria via consensus diagnostic conference procedures. Patients were followed prospectively, eliminating potential biases inherent in retrospective chart reviews. Evaluations were performed annually, which provides multiple assessments of cost and permits more-accurate coefficient estimates. The cohort had high rates of follow-up participation with few missing data. Clinical signs were ascertained and coded in a standardized fashion at each visit. Finally, patients were recruited at early stages of the disease and followed for long periods of time. The cohort describes the full range of progression over time, making longer-term effects on costs more easily interpreted.
ACKNOWLEDGMENTS
The Predictors Study is supported by Grants R01-AG07370 and U01AG010483 from the National Institute on Aging, National Institute of Health (NIH) and RR00645 from the National Center for Research Resources, NIH. Drs. Zhu and Sano also are supported by the Department of Veterans Affairs, Veterans Health Administration.
Footnotes
Financial Disclosure: The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs. The authors all certify that they have no relevant financial interests in this manuscript.
Author Contributions: Carolyn W. Zhu: conception and design, analysis and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content. Nikolaos Scarmeas and Yaakov Stern: conception and design, acquisition of data, critical revision of manuscript for important intellectual content. Rebecca Torgan, Marilyn Albert, Jason Brandt, Deborah Blacker, and Mary Sano: acquisition of patients and data, critical revision of manuscript for important intellectual content.
Sponsor’s Role: The sponsors had no role in executing or publishing this project.
REFERENCES
- 1.Bloom BS, de Pouvourville N, Straus WL. Cost of illness of Alzheimer’s disease: How useful are current estimates. Gerontologist. 2003;43:158–164. doi: 10.1093/geront/43.2.158. [DOI] [PubMed] [Google Scholar]
- 2.Albert SM, Sano M, Bell K, et al. Hourly care received by people with Alzheimer’s disease: Results from an urban, community survey. Gerontologist. 1998;38:704–714. doi: 10.1093/geront/38.6.704. [DOI] [PubMed] [Google Scholar]
- 3.Feldman HH, van Baelen B, Kavanagh SM, et al. Cognition, function, and caregiving time patterns in patients with mild-to-moderate Alzheimer disease: A 12-month analysis. Alzheimer Dis Assoc Disord. 2005;19:29–36. doi: 10.1097/01.wad.0000157065.43282.bc. [DOI] [PubMed] [Google Scholar]
- 4.Stern Y, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the ‘predictors study’). I. Study design, cohort description, and intersite comparisons. Alzheimer Dis Assoc Disord. 1993;7:3–21. doi: 10.1097/00002093-199307010-00002. [DOI] [PubMed] [Google Scholar]
- 5.Richards M, Folstein M, Albert M, et al. Multicenter study of predictors of disease course in Alzheimer disease (the ‘predictors study’). II. Neurological, psychiatric, and demographic influences on baseline measures of disease severity. Alzheimer Dis Assoc Disord. 1993;7:22–32. doi: 10.1097/00002093-199307010-00003. [DOI] [PubMed] [Google Scholar]
- 6.Scarmeas N, Hadjigeorgiou GM, Papadimitriou A, et al. Motor signs during the course of Alzheimer disease. Neurology. 2004;63:975–982. doi: 10.1212/01.wnl.0000138440.39918.0c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Folstein MF, Folstein SE, McHugh PR. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 8.Stern Y, Sano M, Paulson J, et al. Modified mini-mental state examination. Validity and reliability. Neurology. 1987;37:179. [Google Scholar]
- 9.Leon J, Cheng CK, Neumann PJ. Alzheimer’s disease care: Costs and potential savings. Health Aff (Millwood) 1998;17:206–216. doi: 10.1377/hlthaff.17.6.206. [DOI] [PubMed] [Google Scholar]
- 10.Menzin J, Lang K, Friedman M, et al. The economic cost of Alzheimer’s disease and related dementias to the California Medicaid program (‘Medi-Cal’) in 1995. Am J Geriatr Psychiatry. 1999;7:300–308. [PubMed] [Google Scholar]
- 11.Penrod JD, Kane RL, Finch MD, et al. Effects of post hospital Medicare home health and informal care on patient functional status. Health Serv Res. 1998;33:513–529. [PMC free article] [PubMed] [Google Scholar]
- 12.Economic Report of the President. Washington, DC: Council of Economic Advisers; 2003. [Google Scholar]
- 13.Blessed G, Tomlinson BE, Roth M. The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry. 1968;114:797–811. doi: 10.1192/bjp.114.512.797. [DOI] [PubMed] [Google Scholar]
- 14.Devanand DP, Miller L, Richards M, et al. The Columbia University Scale for Psychopathology in Alzheimer’s disease. Arch Neurol. 1992;49:371–376. doi: 10.1001/archneur.1992.00530280051022. [DOI] [PubMed] [Google Scholar]
- 15.Stern Y, Tang MX, Albert MS, et al. Predicting time to nursing home care and death in individuals with Alzheimer disease. JAMA. 1997;277:806–812. [PubMed] [Google Scholar]
- 16.Scarmeas N, Brandt J, Albert M, et al. Association between the APOE genotype and psychopathologic symptoms in Alzheimer’s disease. Neurology. 2002;58:1182–1188. doi: 10.1212/wnl.58.8.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarmeas N, Brandt J, Albert M, et al. Delusions and hallucinations are associated with worse outcome in Alzheimer disease. Arch Neurol. 2005;62:1601–1608. doi: 10.1001/archneur.62.10.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern MB, Hurting HI. The Comprehensive Management of Parkinson’s Disease. New York: PMA Corp; 1978. The clinical characteristics of Parkinson’s disease and parkinsonian syndromes: Diagnosis and assessment; pp. 3–50. [Google Scholar]
- 19.Richards M, Marder K, Bell K, et al. Interrater reliability of extrapyramidal signs in a group assessed for dementia. Arch Neurol. 1991;48:1147–1149. doi: 10.1001/archneur.1991.00530230055021. [DOI] [PubMed] [Google Scholar]
- 20.Scarmeas N, Albert M, Brandt J, et al. Motor signs predict poor outcomes in Alzheimer disease. Neurology. 2005;64:1696–1703. doi: 10.1212/01.WNL.0000162054.15428.E9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Rabe-Hesketh S, Skrondal A, Pickles A. Reliable estimation of generalized linear mixed models using adaptive quadrature. Stata J. 2002;2:1–21. [Google Scholar]
- 23.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. College Station, TX: Stata Press; 2005. [Google Scholar]
- 24.Kennedy P. Estimation with correctly interpreted dummy variables in semi-logarithmic equations. Am Econ Rev. 1981;71:801. [Google Scholar]
- 25.Zhu CW, Scarmeas N, Torgan R, et al. Clinical features associated with costs in early AD. Baseline data from the predictors study. Neurology. 2006;66:1021–1028. doi: 10.1212/01.wnl.0000204189.18698.c7. [DOI] [PubMed] [Google Scholar]
- 26.Beeri MS, Werner P, Davidson M, et al. The cost of behavioral and psychological symptoms of dementia (BPSD) in community dwelling Alzheimer’s disease patients. Int J Geriatr Psychiatry. 2002;17:403–408. doi: 10.1002/gps.490. [DOI] [PubMed] [Google Scholar]
- 27.Zhu CW, Moore M, Clipp E. Dementia problem behavior and the production of informal caregiving services. J Econ Household. 2003;1:59–76. [Google Scholar]
- 28.Murman DL, Chen Q, Powell MC, et al. The incremental direct costs associated with behavioral symptoms in AD. Neurology. 2002;59:1721–1729. doi: 10.1212/01.wnl.0000036904.73393.e4. [DOI] [PubMed] [Google Scholar]
- 29.Murman DL, Kuo SB, Powell MC, et al. The impact of parkinsonism on costs of care in patients with AD and dementia with Lewy bodies. Neurology. 2003;61:944–949. doi: 10.1212/wnl.61.7.944. [DOI] [PubMed] [Google Scholar]
- 30.Devanand DP, Jacobs DM, Tang MX, et al. The course of psychopathologic features in mild to moderate Alzheimer disease. Arch Gen Psychiatry. 1997;54:257–263. doi: 10.1001/archpsyc.1997.01830150083012. [DOI] [PubMed] [Google Scholar]
- 31.Sink KM, Covinsky KE, Newcomer R, et al. Ethnic differences in the prevalence and pattern of dementia-related behaviors. J Am Geriatr Soc. 2004;52:1277–1283. doi: 10.1111/j.1532-5415.2004.52356.x. [DOI] [PubMed] [Google Scholar]