Abstract
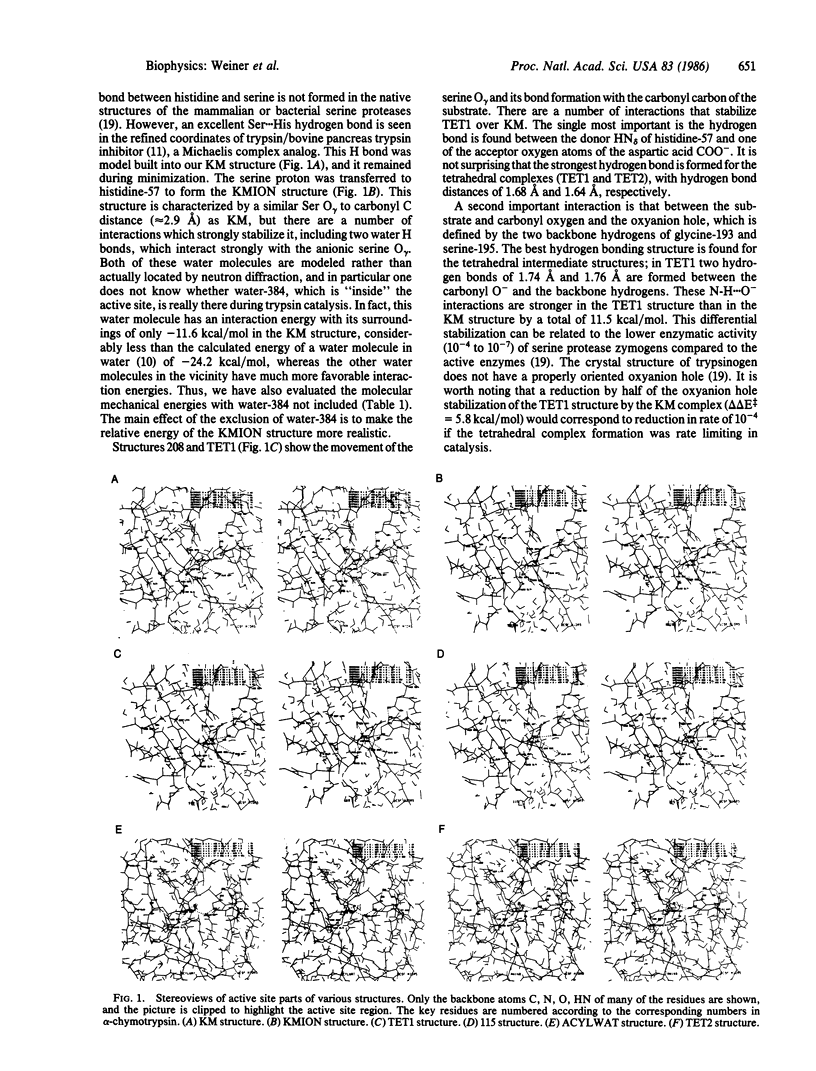
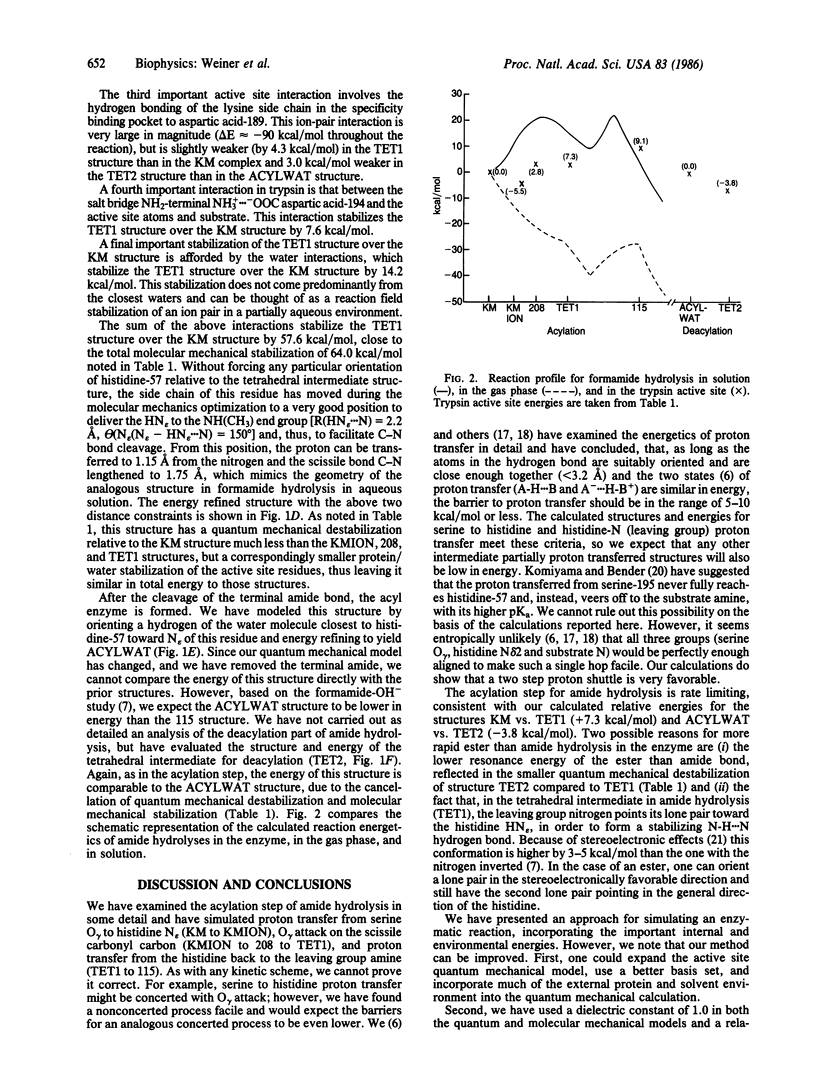
We present a combined quantum/molecular mechanical study of the trypsin-catalyzed hydrolysis of a specific tripeptide substrate, including the entire enzyme in the calculation, as well as 200 H2O molecules. The results illustrate how the enzyme and nearby H2O molecules stabilize the ionic intermediates in peptide hydrolysis, such that the reaction is calculated to have a barrier that is significantly smaller than the calculated and experimental base-catalyzed barrier of formamide hydrolysis in aqueous solution. This enables us to understand how serine proteases increase the rates for reactions that take place in their active sites, compared to the corresponding rates for analogous solution reactions.
Full text
PDF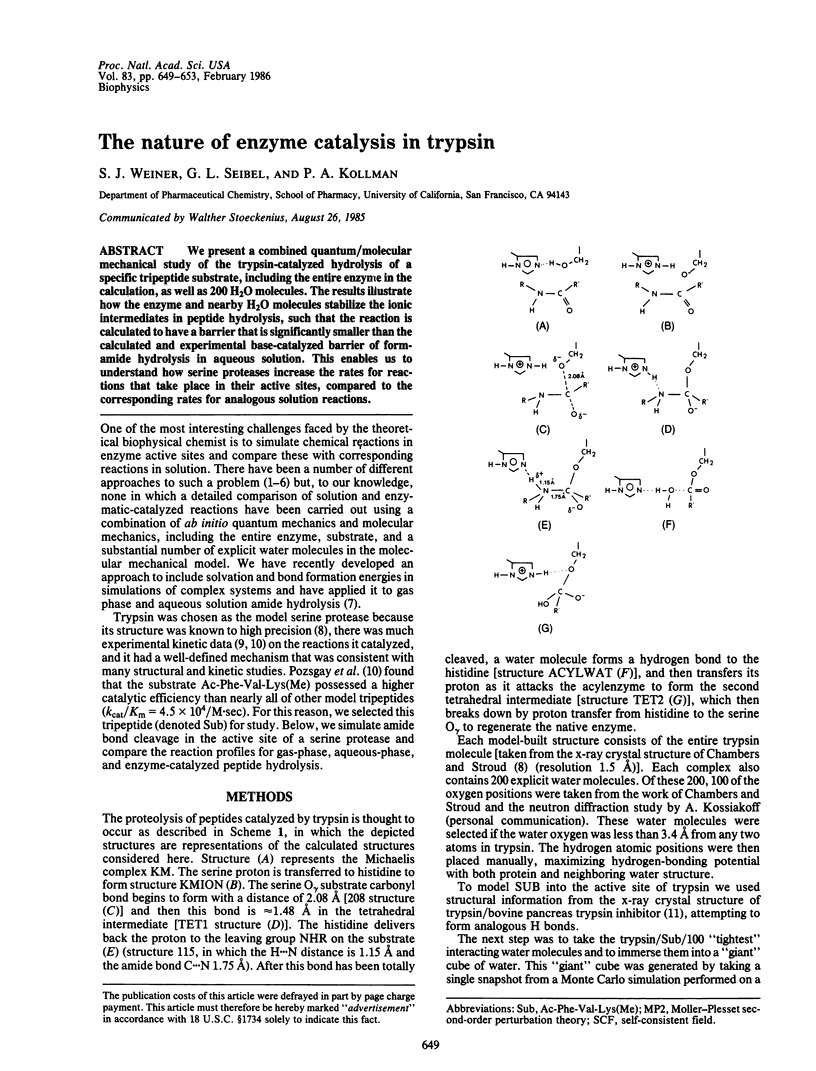


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen L. C. The catalytic function of active site amino acid side chains in well-characterized enzymes. Ann N Y Acad Sci. 1981;367:383–406. doi: 10.1111/j.1749-6632.1981.tb50580.x. [DOI] [PubMed] [Google Scholar]
- Craik C. S., Largman C., Fletcher T., Roczniak S., Barr P. J., Fletterick R., Rutter W. J. Redesigning trypsin: alteration of substrate specificity. Science. 1985 Apr 19;228(4697):291–297. doi: 10.1126/science.3838593. [DOI] [PubMed] [Google Scholar]
- Dewar M. J., Storch D. M. Alternative view of enzyme reactions. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2225–2229. doi: 10.1073/pnas.82.8.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 A resolution. J Mol Biol. 1974 Oct 15;89(1):73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Komiyama M., Bender M. L. Do cleavages of amides by serine proteases occur through a stepwise pathway involving tetrahedral intermediates? Proc Natl Acad Sci U S A. 1979 Feb;76(2):557–560. doi: 10.1073/pnas.76.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut J. Serine proteases: structure and mechanism of catalysis. Annu Rev Biochem. 1977;46:331–358. doi: 10.1146/annurev.bi.46.070177.001555. [DOI] [PubMed] [Google Scholar]
- Pozsgay M., Szabó G., Bajusz S., Simonsson R., Gáspár R., Elödi P. Investigation of the substrate-binding site of trypsin by the aid of tripeptidyl-p-nitroanilide substrates. Eur J Biochem. 1981 Apr;115(3):497–502. doi: 10.1111/j.1432-1033.1981.tb06230.x. [DOI] [PubMed] [Google Scholar]
- Scheiner S., Hillenbrand E. A. Modification of pK values caused by change in H-bond geometry. Proc Natl Acad Sci U S A. 1985 May;82(9):2741–2745. doi: 10.1073/pnas.82.9.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz T. A., Shulman R. G. Crystallographic and NMR studies of the serine proteases. Annu Rev Biophys Bioeng. 1982;11:419–444. doi: 10.1146/annurev.bb.11.060182.002223. [DOI] [PubMed] [Google Scholar]
- Umeyama H., Hirono S., Nakagawa S. Charge state of His-57-Asp-102 couple in a transition state analogue-trypsin complex: a molecular orbital study. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6266–6270. doi: 10.1073/pnas.81.20.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warshel A., Levitt M. Theoretical studies of enzymic reactions: dielectric, electrostatic and steric stabilization of the carbonium ion in the reaction of lysozyme. J Mol Biol. 1976 May 15;103(2):227–249. doi: 10.1016/0022-2836(76)90311-9. [DOI] [PubMed] [Google Scholar]
- Warshel A., Russell S. T. Calculations of electrostatic interactions in biological systems and in solutions. Q Rev Biophys. 1984 Aug;17(3):283–422. doi: 10.1017/s0033583500005333. [DOI] [PubMed] [Google Scholar]
- van Duijnen P. T., Thole B. T., Hol W. G. On the role of the active site helix in papain, an ab initio molecular orbital study. Biophys Chem. 1979 Mar;9(3):273–280. doi: 10.1016/0301-4622(79)85010-3. [DOI] [PubMed] [Google Scholar]


