Abstract
Although it is known that mTOR complex 2 (mTORC2) functions upstream of Akt, the role of this protein kinase complex in cancer is not well understood. Through an integrated analysis of cell lines, in vivo models and clinical samples, we demonstrate that mTORC2 is frequently activated in glioblastoma (GBM), the most common malignant primary brain tumor of adults. We show that the common activating epidermal growth factor receptor (EGFR) mutation (EGFRvIII) stimulates mTORC2 kinase activity, which is partially suppressed by PTEN. mTORC2 signaling promotes GBM growth and survival, and activates NF-κB. Importantly, this mTORC2-NF-κB pathway renders GBM cells and tumors resistant to chemotherapy in a manner independent of Akt. These results highlight the critical role of mTORC2 in GBM pathogenesis, including through activation of NF-κB downstream of mutant EGFR, leading to a previously unrecognized function in cancer chemotherapy resistance. These findings suggest that therapeutic strategies targeting mTORC2, alone or in combination with chemotherapy, will be effective in cancer.
Keywords: EGFRvIII, mTORC2, Rictor, NF-κB, and chomotherapy resistance
INTRODUCTION
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase that is implicated in a variety of diseases including cancer. mTOR exists in two multi-protein complexes, which differ in regulation, function and response to the allosteric mTOR inhibitor rapamycin (1). mTORC1 consists of mTOR in association with Raptor and other core regulatory components. Downstream of phosphoinositide-3 kinase (PI3K), mTORC1 is activated by Akt, at least in part, through inhibitory phosphorylation of the TSC1-TSC2 complex. mTORC1 links PI3K signaling with the control of protein synthesis, metabolism, and cell growth (2, 3).
mTORC2 is composed of mTOR in association with unique regulatory proteins, including Rictor and SIN1 (1). In contrast to mTORC1, mTORC2 functions upstream of Akt, and the mechanism by which it is regulated is poorly understood (1, 4). PI3K catalyzes formation of phosphatidylinositol (3,4,5)-trisphosphate (PIP3), bringing Akt to the cell membrane where it is phosphorylated by phosphoinositide dependent protein kinase 1 (PDK1) on T308 and by mTORC2 on S473, to promote maximal Akt activity (5–9). mTORC2 has been shown to be required for proper Akt signaling in vivo and its loss is lethal during embryogenesis (4). Akt activation is thought to be the critical function of mTORC2. However, mTORC2 also phosphorylates other protein kinases related to Akt, including serum- and glucocorticoid-induced protein kinase 1 (SGK1) and some members of the PKC family (10–13), raising the possibility that mTORC2 may have important cellular functions independent of Akt.
mTOR signaling is frequently deregulated in cancer (7, 14). Amplifications and activating mutations affecting receptor tyrosine kinases, mutation of PI3K and its regulatory subunits, and loss of the PTEN tumor suppressor protein lead to elevated and growth factor-independent activation of PI3K accompanied by downstream activation of mTOR signaling (15). mTORC1 promotes cell growth and proliferation, activates hypoxia-inducible factor-1-dependent glycolysis (HIF1) (16) and stimulates angiogenesis in many types of cancer (17, 18). Therefore, mTORC1 is well established as a cancer drug target. In contrast to mTORC1, the role of mTORC2 in cancer is not well understood. mTORC2 is required for the development of PTEN loss-induced prostate cancer in mice, suggesting a central role in mediating PI3K-dependent carcinogenesis (19). However, the impact of targeting mTORC2 in the clinic is not currently known. The allosteric mTOR inhibitor rapamycin does not directly bind and inhibit mTORC2, unlike the case for mTORC1 (1). This is critical, because rapamycin has failed as a treatment for a variety of PI3K-hyperactivated cancers (20), calling into question the validity of mTOR2 as a drug target. It is likely that the new generation of mTOR kinase inhibitors possessing activity against both mTOR complexes will provide new insights into the importance of mTORC2 signaling in cancer (21).
Glioblastoma (GBM), the most common malignant primary brain cancer of adults, presents an important cancer in which to examine the impact of mTORC2 signaling in tumor pathogenesis and response to treatment. PI3K signaling is hyperactivated in nearly 90% of GBMs, most frequently in association with epidermal growth factor (EGFR) amplification and mutation, and loss of the PTEN tumor suppressor protein. We have previously shown that mTOR is a critical effector of downstream signaling in EGFR-mutated, PTEN deficient GBMs, mediating resistance to EGFR tyrosine kinase inhibitors (22). The elevated Akt S473 phosphorylation was associated with significantly shorter time to tumor progression, suggesting the importance of negative feedback loops to PI3K signaling is evident from the clinical trial (20). S6K-mediated negative feedback after mTORC1 activation phosphorylates Rictor to inhibit mTORC2, which is not through insulin receptor substrate 1 (IRS-1), and additional feedback mechanisms likely exist (23, 24). Therefore mTORC1 inhibition is likely to be insufficient to suppress tumor growth, potentially implicating mTORC2 as a critical mediator of PI3K signaling. Consistent with this clinical observation, a recent study found that the fly ortholog of mTORC2 is required for the growth of a Drosophila model of glioma featuring activation of EGFR and PI3K (25).
NF-κB, typically the p50-RelA/p65 heterodimer, is activated in multiple types of cancers and functions to control expression of genes associated with proliferation and suppression of apoptosis (26, 27). NF-κB is negatively regulated through interactions with IκB family proteins and is activated through IKK, which phosphorylates IκB leading to its proteasome-dependent degradation. The activation of NF-κB is strongly associated with cancer therapy resistance (26). Interestingly, most gliomas with EGFR expression exhibit monoallelic loss of NFKBIA encoding IκBα, the major negative regulator of NF-κB (28). These results suggests that NF-κB activation is important in glioma downstream of EGFR-dependent signaling under conditions where EGFR is not amplified or mutated (29). Recent work indicates that point mutated EGFR in lung cancer can lead to the activation of NF-κB and that NF-κB is important for cancer cell growth/survival in this setting (30), although the underlying mechanism of its activation is not well understood.
To address these issues, we performed integrated analyses of GBM cell lines, in vivo xenograft models and clinical samples to examine the importance of mTORC2 signaling in cancer. Here, we demonstrate that EGFRvIII promotes mTORC2 activation and that PTEN suppresses it. mTORC2 promotes tumor growth and survival, independent of mTORC1. We demonstrate that dual inhibition of mTORC1 and mTORC2 inhibits tumor growth and leads to tumor cell death. Surprisingly, we show that mTORC2 promotes Akt-independent resistance to chemotherapy through NF-κB, and that cisplatin resistance can be reversed in vivo by inhibition of mTORC2. These results demonstrate the importance of mTORC2 signaling in GBM and point to a previously unrecognized function of mTORC2 in mediating cancer chemotherapy resistance, indicating the need for mTORC2 inhibition alone or in combination with chemotherapy.
RESULTS
EGFRvIII stimulates mTORC2 kinase activity and signaling
The mechanisms of mTORC2 activation are not well understood (21). Growth factor signaling through PI3K (10, 31–34), potentially through enhanced association with ribosomes (34), and upregulation of mTORC2 regulatory subunits (19, 35, 36) have been proposed as mechanisms of mTORC2 activation (21). To determine whether oncogenic EGFR affects mTORC2, we employed an isogenic set of GBM-derived cell lines that represent the most common genetic events driving GBM: PTEN loss in the presence or absence of EGFR overexpression or activating mutation (EGFRvIII) (22, 37). Phosphorylation of Akt S473 is the best-characterized mTORC2 activity (10). However, mTORC2 also activated SGK1, and phosphorylation of the SGK1-specific substrate NDRG1 on T346 has emerged as a reliable biomarker for mTORC2 signaling (11, 38).
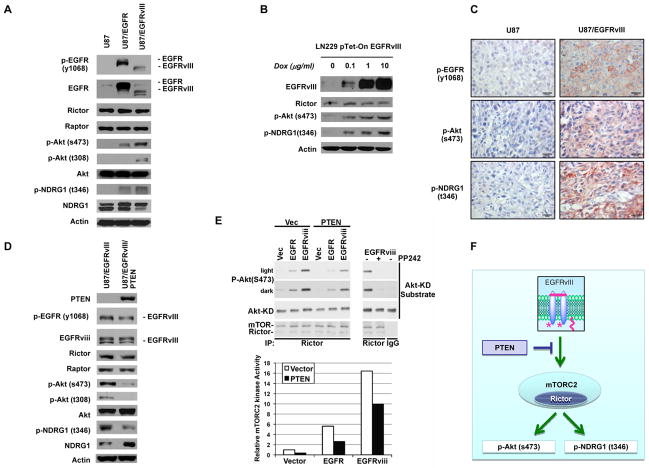
EGFRvIII and, to a lesser extent, wild type EGFR increased Akt S473 and NDRG1 T346 phosphorylation (Fig. 1A; Supplementary Fig. S1A). EGFRvIII, when placed under a doxycycline-regulatable promoter in a different GBM cell line, LN229, similarly increased Akt S473 and NDRG1 T346 phosphorylation in a dose-dependent fashion (Fig. 1B), thus confirming EGFRvIII-mediated mTORC2 signaling in different cell line models, although Rictor expression was not changed (Fig. 1A and B). EGFRvIII expression was similarly associated with elevated mTORC2 signaling when the tumor cells were implanted in a xenograft model (Fig. 1C). Hepatocyte growth factor (HGF) stimulation of GBM cells expressing MET, another PI3K-activating receptor tyrosine kinase commonly detected in GBMs, resulted in Akt S473 and NDRG1 T346 phosphorylation. However, in contrast to the sustained mTORC2 signaling detected in EGFRvIII-expressing tumor cells, the signaling was transient (Supplementary Fig. S1B).
Figure 1. EGFRvIII stimulates mTORC2 activation in vitro and in vivo.
(A) Biochemical analysis of EGFRvIII/EGFR signaling on mTORC2 biomarkers using U87 isogenic cells. Cell lines were cultured in serum-free media for 24 hours.
(B) Immunoblot analysis of LN229 GBM cells in which EGFRvIII was placed under a doxycycline regulatable promoter.
(C) Representative immunohistochemical images demonstrating p-EGFR(Y1068), p-Akt(S473) and p-NDRG1(T346) to assess EGFRvIII-mediated mTORC2 signaling. Scale bar, 20 μm.
(D) Effect of PTEN reconstitution on EGFRvIII-mediated mTORC2 signaling.
(E) Lysates form U87 isogenic cells were subjected to immunoprecipitation with either Rictor antibody or control IgG. Rictor immunoprecipitate was divided into equal fractions for separate kinase reactions using Akt1 purified from insect cells as the substrate. PP242 was added to the kinase reaction in EGFRvIII cells. mTORC2 in vitro kinase activity was assessed by Akt S473 phosphorylation.
(F) The schema of EGFRvIII-stimulated mTORC2 signaling.
See also Supplementary Figure S1.
In light of the demonstrated requirement for mTORC2 in PTEN-loss-dependent prostate cancer initiation (19), we examined the effect of PTEN reconstitution on mTORC2 signaling. Exogenous PTEN re-expression suppressed EGFRvIII-mediated or EGF-stimulated mTORC2 signaling (Fig. 1D; Supplementary Fig. S1C). Therefore, EGFRvIII promoted mTORC2 signaling in GBM cells, which was partially suppressed by PTEN.
To determine whether the effects of oncogenic EGFR signaling and PTEN loss on downstream targets of mTORC2 described above reflect direct increases in mTORC2 activation, we measured the basal mTORC2 kinase activity in Rictor immunoprecipitates from U87 GBM cells or their isogenic counterparts expressing EGFRvIII. Consistent with these differences between wild-type and oncogenic EGFR and the inhibitory effects of PTEN, EGFRvIII expression promoted a 16-fold increase in mTORC2 kinase activity, which was partially suppressed by reconstitution of PTEN and completely abrogated by the mTOR kinase inhibitor PP242 (Fig. 1E). Overexpression of wild-type EGFR activated mTORC2 kinase activity to a lesser degree and was similarly suppressed by PTEN (Fig. 1E). These results suggest that EGFRvIII stimulates mTORC2 activation, which is partially suppressed by PTEN (Fig. 1F). Taken together, these results indicate that EGFRvIII is associated with increased mTORC2 activity and downstream signaling in GBM cells in vitro and in vivo.
mTORC2 signaling promotes GBM growth and survival
To determine the functional significance of mTORC2 in GBM, we examined the effect of Rictor knockdown and overexpression. Rictor knockdown inhibited the proliferation of all GBM cells tested (U87MG, U87/EGFRvIII, U251, T98G), with enhanced anti-proliferative effects in EGFRvIII-expressing tumor cells (Fig. 2A; Supplementary Fig. S2A). The decrease in tumor cell proliferation was associated with increased G1 cell cycle fraction (Supplementary Fig. S2B). Conversely, Rictor overexpression resulted in 2.5-fold increase in tumor cell proliferation (p<0.001; Fig. 2B), and exogenous myc-Rictor made a complex with mTOR in U87 cells. Taken together, these results demonstrate that mTORC2 signaling promotes GBM proliferation.
Figure 2. mTORC2 promotes GBM cell growth, is resistant to rapamycin treatment and its inhibition has anti-tumor efficacy.
(A) U87 and U87/EGFRvIII cells were transfected with Rictor siRNA or scrambled control siRNA constructs for 48 hours in 96-well plates. Relative cell growth was calculated with the cell proliferation assay. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.05, NS; not significant).
(B) U87 cells were transfected with myc-Rictor expressing or empty vectors for 48 hours in 96-well plates. Relative cell growth was calculated with the cell proliferation assay. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.001). IP; Immunoprecipitation assay, IB; Immunoblot assay
(C) Immunohistochemical images of p-Akt(S473), p-NDRG1(T346) and p-S6(S235/236) staining (reddish brown) in tumor samples of GBM patient who received rapamycin treatment. Tissue is counterstained with hematoxylin. Scale bar, 50 μm.
(D) Immunoblot analysis of p-Akt(S473), p-NDRG1(T346), and p-S6(S235/236) in U87/EGFRvIII cells with siRNA Rictor and/or rapamycin treatment.
(E) Immunoblot analysis using indicated antibodies of U87/EGFRvIII and U251MG cells with the siRNA against Raptor, Rictor or scrambled control.
(F) U87/EGFRvIII cells were transfected with siRNA constructs against Raptor, Rictor, or scrambled control for 24 hours in 6-well plates, and changed to 1% FBS medium for 3 days. Cell death was measured by trypan blue exclusion. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.05, **p<0.01).
(G) U251MG cells were transfected with siRNA constructs against Raptor, Rictor, or scrambled control for 24 hours in 6-well plates, and changed to 1% FBS medium for 3 days. Cell death was measured by trypan blue exclusion. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.05, **p<0.01, #p<0.05, ##p<0.01).
See also Supplementary Figure S2, 3 and 4.
Rapamycin is a highly specific allosteric mTOR inhibitor that blocks mTORC1 activity and has variable effects on mTORC2 (39). mTORC1 signaling is known to exert negative feedback effects on Akt activation through a variety of mechanisms (23). We previously observed a more rapid clinical progression in GBM patients whose tumors showed inhibition of S6K1 phosphorylation with concomitant increase in Akt S473 phosphorylation (20). The finding that mTORC2 can support GBM proliferation raised the possibility that the mTORC2 signaling could potentially underlie clinical resistance to rapamycin. To determine whether mTORC2 signaling could be detected during rapamycin treatment, we analyzed tumor tissue from a GBM patient before and after 10 days of treatment. Following rapamycin treatment, phospho-S6 (S235/236) immunostain ing, a marker of mTORC1 activity, was decreased, whereas markers of mTORC2 activity, including the phosphorylation of Akt (S473) and NDRG1 (T346) were elevated relative to baseline (Fig. 2C). In EGFRvIII-expressing GBM cells, rapamycin treatment for 16 hours similarly inhibited mTORC1 signaling, as measured by decreased S6 (S235/236) phosphorylation (Fig. 2D). In contrast, markers of mTORC2 signaling were concomitantly increased, the effects of which were abrogated by Rictor knockdown (Fig. 2D). These results suggest that dual inhibition of mTORC1 and mTORC2 could be more effective.
Therefore, we analyzed the effect of Rictor and Raptor knockdown, alone or in combination, on signal transduction, tumor cell proliferation and survival. Similar to rapamycin treatment, Raptor knockdown increased mTORC2 signaling in U87/EGFRvIII, U251 and A172 cells (Fig. 2E; Supplementary Fig. S3). In contrast, Rictor knockdown decreased mTORC2 signaling (Fig. 2E; Supplementary Fig. S3). Combined Raptor and Rictor knockdowns significantly decreased cell proliferation in U87/EGFRvIII and U251 models and increased cell death in the U251 cells (Fig. 2F and G). These results suggest the potential therapeutic utility of mTOR kinase domain inhibitors, which target both signaling complexes. Consistent with this model, inhibition of both mTORC1 and mTORC2 signaling with the mTOR kinase inhibitor PP242 (40) significantly suppressed GBM cell proliferation in a dose-dependent manner (Supplementary Fig. S4A and B).
EGFRvIII activates NF-κB through mTORC2
Given our finding that mTORC1 inhibition is not sufficient to block GBM growth (20), we examined additional pathways that might be activated in GBM. Included in our candidate downstream pathways was NF-κB, which we found to be robustly activated by the EGFRvIII mutant, as indicated by phosphorylation of p65 and IκBα, decreased level of total IκBα, and expression of NF-κB target genes Bcl-xL and cyclin D1 (Fig. 3A). In an electrophoretic mobility gel shift assay (EMSA), EGFRvIII markedly increased the NF-κB DNA-binding activity (Fig. 3B), increased NF-κB luciferase reporter activity 4-fold (p<0.01; Fig. 3C) and increased expression of NF-κB-target genes cyclin D1 (10-fold, p<0.01); Bcl2 (10-fold, p<0.01) and Bcl-xL (30-fold, p<0.001; Supplementary Fig. S5A). These activities were EGFR kinase dependent (Supplementary Fig. S5A) and could be suppressed by re-expression of PTEN in these cells (Fig. 3C). NF-κB activation was also associated with EGFR signaling in a tumor xenograft model, as indicated by an increase in the phosphorylation of p65 (p<0.001; Supplementary Fig. S5B), and EGF-stimulated NF-κB activation was suppressed by reconstitution of PTEN (Supplementary Fig. S5C).
Figure 3. EGFRvIII activates NF-κB through mTORC2.
(A) Immunoblot analysis of p-p65(S536), p-IκBα(S32/36), and NF-κB target genes such as Bcl-xl and cyclinD1 in whole cell extracts of U87 isogenic cells.
(B) EMSAs were performed using nuclear extracts from U87 isogenic cells lysed 48 h after they were cultured in serum free medium. Arrow denotes the DNA/protein EMSA complex. Immunoblot analysis of p65 and TBP which was used to normalize protein loading for nuclear extracts.
(C) Luciferase reporter assays targeting NF-κB signal transduction using U87 isogenic cells (measured as relative luciferase/luminescence units). Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.01, **p<0.05).
(D) Biochemical analysis of Rictor knockdown on NF-κB signaling in U87/EGFRvIII cells. Cell lines were cultured in serum-free media for 24 hours after transfection of siRNA against Rictor and scramble.
(E) Assessment of changes in mRNA levels of NF-κB target gene expression in U87/EGFRvIII cells with knockdown of Rictor and scramble using RT–PCR method. Data represent the mean +/− SEM of three independent experiments.
(F) Biochemical analysis of Rictor over-expression on NF-κB signaling in U87 cells. Cell lines were cultured in serum-free media for 24 hours after transfection of myc-Rictor expressing or empty vectors.
(G) EMSAs using nuclear extracts from U87 cells transfected with myc-Rictor expressing vector and adenovirus encoding IκB-super repressor (IκBα-SR; a dominant negative mutant of human IκBα). Arrow denotes the DNA/protein EMSA complex, P; positive control, C; control LacZ.
(H) Luciferase reporter assays targeting NF-κB signaling in U87 cells transfected with myc-Rictor expressing vector and adenovirus encoding IκBα-SR. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.001, **p<0.001), C; control LacZ
(I) The schema of EGFRvIII-stimulated NF-κB through mTORC2.
See also Supplementary Figure S5, 6, 7, 8 and 9.
Given a recent study in lymphocytes suggesting that NF-κB can be activated downstream of mTORC2 (41), we tested the effects of knocking down the core mTORC2 component Rictor on EGFRvIII-mediated activation of NF-κB. Rictor siRNA knockdown inhibited mTORC2 signaling and abrogated NF-κB activity, as detected by diminished IκBα S32/36 phosphorylation (Fig. 3D). Rictor knockdown also decreased the NF-κB DNA-binding activity (Supplementary Fig. S6A) and abrogated EGFRvIII-dependent upregulation of NF-κB target gene expression, such as cyclin D1, Bcl-2, Bcl-xL, and IL-6 (Fig. 3E).
Rictor overexpression, which has been demonstrated to activate mTORC2 signaling in other settings (35), resulted in dose-dependent increases in mTORC2 signaling and IκBα S32/36 phosphorylation, and decreases in total IκBα expression in U87MG cells (Fig. 3F). This activation of mTORC2 also led to markedly increased NF-κB DNA-binding activity (Fig. 3G) and increased NF-κB luciferase reporter activity (Fig. 3H). NF-κB target gene expression was also upregulated (Supplementary Fig. S6B) and was suppressed by expression of an activated mutant of IκBα (IκBα-super repressor, IκBα-SR; Fig. 3G and H; Supplementary Fig. S6B) (42). These findings indicated that EGFRvIII activates NF-κB through mTORC2 (Fig. 3I).
We have previously shown that Akt can activate NF-κB through mTORC1 in PTEN null prostate cancer cells (43) raising the possibility that NF-κB activity was also mediated through mTORC1. Interestingly, Raptor knockdown modestly increased, while Rictor knockdown significantly inhibited, NF-κB reporter activity (Supplementary Fig. S7A) and IκBα S32/36 phosphorylation (Supplementary Fig. S7B). Therefore, mTORC1 inhibition alone cannot suppress NF-κB activation in GBM cells. In addition, pharmacological inhibition of Akt did not attenuate NF-κB signaling in these cells (Supplementary Fig. S8A). Therefore, we determined whether the well-described mTORC2 effector SGK1 is required for NF-κB activity. SGK1 siRNA knockdown greatly attenuated NF-κB signaling (Supplmentary Fig S8B). Taken together, these data demonstrate that EGFRvIII promotes NF-κB activation through mTORC2 by an SGK1 dependent pathway that does not require Akt, or mTORC1.
mTORC2 mediates EGFRvIII-dependent cisplatin resistance through NF-κB, independent of Akt
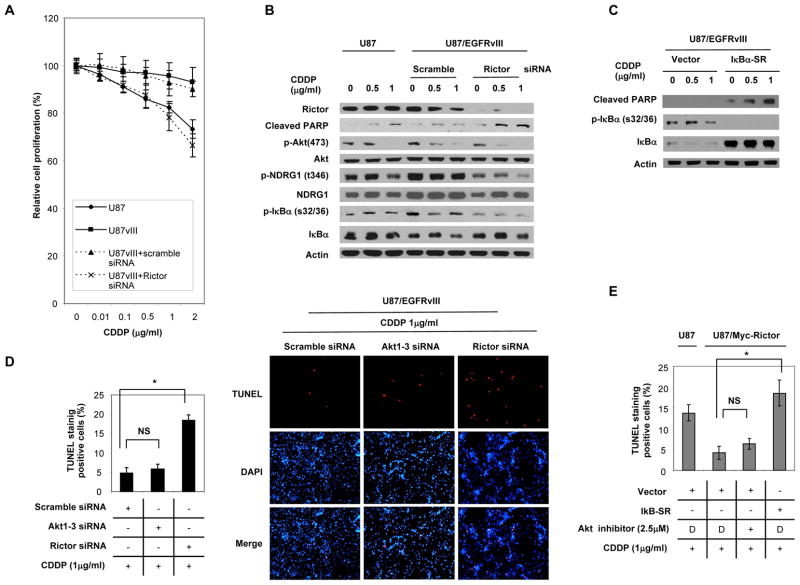
The emerging role for NF-κB in mediating chemotherapy resistance in GBM downstream of EGFR (28, 29), prompted us to investigate the role of mTORC2 in cisplatin resistance. EGFRvIII rendered GBM cells strikingly resistant to cisplatin (CDDP), (Fig. 4A), as previously reported (44). Rictor siRNA knockdown significantly reversed CDDP resistance, effectively sensitizing U87-EGFRvIII cells to CDDP-mediated cell death, as indicated by cleaved PARP and increased TUNEL-positive cells (Fig. 4B and D). To determine the downstream mechanism by which mTORC2-mediates CDDP resistance, we examined the involvement of downstream targets, including Akt and NF-κB. Interestingly, expression of the activated mutant of IκBα (IκBα-SR) sensitized GBM cells to CDDP-mediated apoptosis, as indicated by cleaved PARP expression (Fig. 4C), suggesting that apoptotic resistance is mediated through NF-κB. Unlike Rictor knockdown, siRNA-mediated knockdown of all three Akt isoforms did not sensitize GBM cells to CDDP-mediated cell death in TUNEL staining assay (Fig. 4D; Supplementary Fig. S9A and B). Like EGFRvIII, activation of mTORC2 signaling by Rictor over-expression also conferred CDDP resistance to U87MG cells, which was reversed by inhibition of NF-κB but not by inhibition of Akt in TUNEL staining assays (Fig. 4E; Supplementary Fig. S10). Taken together, these results demonstrate a previously unknown role for mTORC2 in mediating cisplatin resistance through NF-κB, in an Akt-independent manner.
Figure 4. mTORC2 mediates EGFRvIII-dependent chemotherapy resistance through NF-κB, independent of Akt.
(A) Relative cell proliferation of U87, U87/EGFRvIII, U87/EGFRvIII+scrambled control siRNA and U87/EGFRvIII+Rictor siRNA cells with the treatment of varying amounts of cisplatin (CDDP) for 48 hours. Data represent the mean +/− SEM of three independent experiments.
(B) Immunoblot analysis using indicated antibodies of U87MG and U87/EGFRvIII transfected with siRNA against Rictor and scrambled control treated with CDDP or normal saline.
(C) Immunoblot analysis using indicated antibodies of U87/EGFRvIII infected with adenovirus encoding IκBα-SR treated with CDDP or normal saline.
(D) TUNEL staining in U87/EGFRvIII cells transfected with siRNA against Akt1-3, Rictor and scrambled control treated with CDDP (1 μg/ml) or normal saline. The percentage of apoptotic cells was calculated as the percentage of TUNEL-positive cells out of 400 cells for each group using NIH image. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.01, NS; not significant). Images are magnified x100.
(E) TUNEL staining in myc-Rictor expressing U87 cells treated with Akt inhibitor (2.5 μM) or transfected adenovirus encoding IκBα-SR under CDDP treatment (1 μg/ml). D; DMSO. The percentage of apoptotic cells was calculated as the percentage of TUNEL-positive cells out of 400 cells for each group using NIH image. Data represent the mean +/− SEM of three independent experiments (Statistically significant with *p<0.01, NS; not significant).
See also Supplementary Figure S9, 10, 11 and 12.
To assess the possibility that pharmacological inhibition of mTOR kinase inhibitor could be used to sensitize GBMs to cisplatin, and potentially other DNA-damaging chemotherapies, we tested the effect of the mTOR kinase inhibitor, PP242 on mediating cellular response to CDDP, and other DNA damaging agents (temozolomide and etoposide). PP242 significantly enhanced CDDP-mediated cell death of U87-EGFRvIII-expressing GBM cells (Supplementary Fig. S11A and B), as did the IKK inhibitor BMS-345541 (Supplementary Fig. S12A and B). PP242 also increased PARP cleavage of EGFRvIII-expressing GBM cells treated with temozolomide or etoposide (Supplementary Fig. S11A and C), suggesting a potentially broader role for mTOR kinase inhibitors in sensitizing GBMs to DNA damaging chemotherapies through IKK/NF-κB signaling.
mTORC2 inhibition reverses cisplatin resistance in xenograft tumors
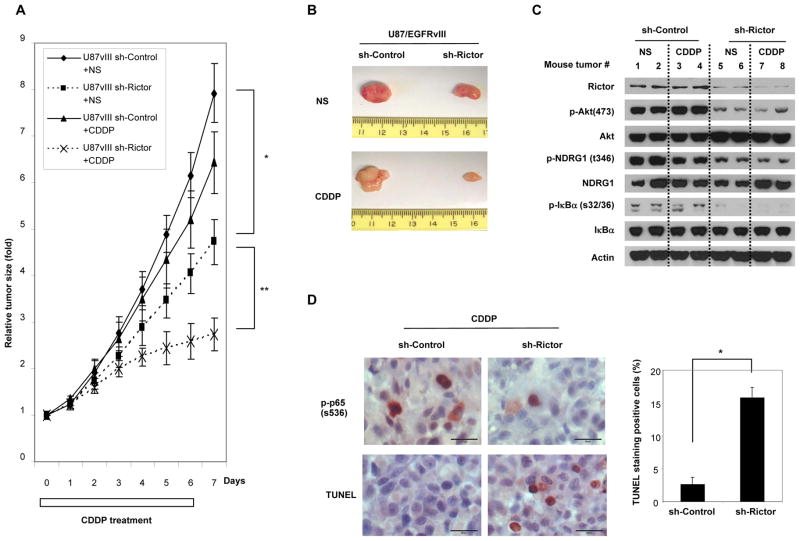
To determine whether mTORC2 inhibition sensitizes EGFRvIII-expressing GBM cells to cisplatin in vivo, we generated stable cell lines with shRNA-mediated knockdown of Rictor. We used this genetic approach, as opposed to pharmacological inhibition of the mTOR kinase, to unambiguously identify the importance of mTORC2 signaling on chemotherapy resistance in vivo, without any direct suppression of mTORC1 signaling. We confirmed stable knockdown of Rictor and suppression of mTORC2 and NF-κB signaling in U87 and U87/EGFRvIII cells, which also resulted in decreased cell proliferation (Supplementary Fig. S13A and B). Rictor knockdown remarkably inhibited mTORC2 and NF-κB signaling in xenograft tumors and decreased tumor size by about 50% (p<0.05; Fig. 5A, B and C), without significant induction of apoptosis. Importantly, Rictor knockdown reversed CDDP resistance, resulting in about 80% tumor shrinkage (p<0.01; Fig. 5A and B). In immunohistochemical analysis, Rictor knockdown led to decrease in p-p65 (S536)-positive tumor cells and a 5-fold increase in apoptotic cells (p<0.001; Fig. 5D) in the treatment of cisplatin. Therefore, mTORC2 inhibition can reverse chemotherapy resistance in vivo and acts synergistically with cisplatin to induce tumor cell death.
Figure 5. mTORC2 inhibition reverses chemotherapy resistance in vivo.
(A) Each mouse was s.c. injected with 3×105 U87/EGFRvIII shRNA control cells (on the left flank) and with 3×105 U87/EGFRvIII shRNA Rictor cells (on the right flank). Mice bearing tumors were treated with i.p. injections of cisplatin (CDDP) (3mg/kg body weight). The control group received equal volume (100 μl) of normal saline (NS). Treatment started 10 days after implantation. Tumor volume was measured, at indicated time points, from xenografts derived from each of 6 mice. Tumor volumes (fold) are measured as indicated in Materials and Methods. Data represent the mean +/− SEM (Statistically significant with **p<0.01, *p<0.05).
(B) Representative images of tumor with shRNA Rictor and control treated with CDDP or normal saline.
(C) Immunoblot analysis using indicated antibodies of tumor lysates from U87/EGFRvIII shRNA control and shRNA Rictor cells in mice treated with CDDP or normal saline.
(D) Representative images demonstrating p-p65(S536) and TUNEL staining (brown cells) to assess apoptotic effects. Quantification of TUNEL staining was performed using NIH image. Data represent the mean +/− SEM of three independent images (Statistically significant with *p<0.001). Scale bar, 20 μm. See also Supplementary Figure S13.
mTORC2 signaling is hyperactivated and associated with NF-κB and phospho-EGFR in the majority of clinical GBM samples
To determine whether the mTORC2-NF-κB pathway defined above is active in human GBM, we examined surrogate biomarkers of mTORC2 and NF-κB in tumor tissue samples and adjacent normal brain from 140 patients arrayed on two tissue microarrays (Fig. 6A). Elevated phosphorylation of EGFR (Y1068) and Akt (S473) were detected in 44% and 77% of GBMs respectively, as previously reported (37). These numbers are consistent with the independent findings of EGFR mutation and/or amplification in 45% and PI3K pathway activating mutations in 87% of GBMs, reported in the Cancer Genome Atlas studies (45). Importantly, elevated levels of Rictor (64%) and phosphorylated NDRG1 (T346; 58%), and p65 (S536; 59%) were frequently detected in tumor samples relative to normal brain tissue (Fig. 6A and B; Supplementary Table S1A). The detection of Rictor, phospho-Akt, phospho-NDRG1 and phospho-EGFR were all significantly correlated with phospho-p65 (p<0.001, OR 2.78 for Rictor; p<0.05, OR 2.32 for p-Akt S473; p<0.05, OR 1.91 for p-NDRG1 T346; p<0.05, OR 2.01 for p-EGFR Y1068, Chi-square test; Fig. 6A and C). The detection of phospho-Akt and phospho-NDRG1 were significantly correlated with Rictor (p<0.01 OR 3.24 for p-Akt S473; p<0.05 OR 1.81 for p-NDRG1 T346, Chi-square test; Supplementary Table S1B). Therefore, in an analysis of a large number of clinical samples, elevated mTORC2 signaling can be detected in nearly 60% of GBMs and is associated with EGFR phosphorylation and NF-κB activation. Finally, immunoblot analysis of GBM autopsy lysates confirmed coordinate increases in mTORC2 and NF-κB signaling in tumor tissue relative to normal brain (Fig. 6D and E). In summary, we showed that EGFRvIII stimulates mTORC2 activity which is partially suppressed by PTEN, and mTORC2 mediates EGFRvIII-stimulated NF-κB activation promoting tumor growth, survival and chemotherapy resistance (Fig. 7).
Figure 6. mTORC2 signaling is hyperactivated in the majority of clinical GBM samples, in association with NF-κB and phospho-EGFR.
(A) The schema of each correlation of EGFR/mTORC2/NF-κB signaling on Tissue microarray (TMA) analysis. P-value and Odds Ratio (OR) were determined by Chi- square for independence test.
(B) Immunohistochemical images of p-EGFR(Y1068), p-Akt(S473), Rictor, p-NDRG1(T346) and p-p65(S536) staining (reddish brown) in two TMAs comprising 252 tumor cores from 140 primary GBM patients. Tissue is counterstained with hematoxylin. Scale bar, 20 μm.
(C) Immunohistochemical analysis of TMAs based on correlation of p-p65(S536) with p-Akt(S473), p-NDRG1(T346) and p-EGFR(Y1068). Numbers may not add up to 252 because of missing cores. P-value was determined by Chi-square for independence test (Statistically significant with **p<0.001, *p<0.05).
(D) Representative gross and microscopic pictures of tumor tissue (T) and contralateral normal brain tissue (N) from the brain of a GBM patient obtained at autopsy. Scale bar, 100 μm.
(E) Immunoblot analysis of Rictor, p-Akt(S473), p-NDRG1(T346), and p-p65(S536) staining in tumor (T) and contralateral normal brain tissue (N) from three GBM patients obtained at autopsy. See also Supplementary Table S1.
Figure 7. EGFRvIII stimulates NF-κB activity through mTORC2.
(A) EGFRvIII stimulates mTORC2 activity; PTEN suppressed it
(B) mTORC2 promotes NF-κB activity.
(C) mTORC2 mediates EGFRvIII-stimulated NF-κB activation promoting tumor growth, survival and chemotherapy resistance.
DISCUSSION
The relative frequency of mTORC2 activation in human cancer including GBM, and its association with EGFR mutations has not, until now, been analyzed. We show that mTORC2 activation is a common event in GBM, particularly in tumors harboring EGFR activating lesions (Fig. 6). Interestingly, EGFRvIII was significantly more potent than wild type EGFR at promoting mTORC2 kinase activity relative to the level of EGFR phosphorylation (Fig. 1). This is consistent with prior studies that show that EGFRvIII preferentially activates PI3K signaling despite lower levels of receptor phosphorylation, leading to differential activation of downstream effectors (46). These results also suggest an essential role for PI3K in mediating mTORC2 activation (1). EGFRvIII-dependent mTORC2 activity in GBM cells was suppressed by reconstitution of PTEN (Fig. 1). Importantly, these data raise the possibility that mTORC2 could function downstream of other PI3K-activating mutations to promote chemotherapy resistance in additional cancer types.
These results also suggest a potential mechanism underlying rapamycin resistance, at least in some GBM patients. Rapamycin is a potent mTORC1 inhibitor, at least with regard to its inhibition of S6K/S6 signaling, but is not a general mTORC2 inhibitor, exhibiting mTORC2 complex formation in some, but not all cancer cell lines (39). Rapamycin treatment in GBM patients is strongly associated with feedback activation of Akt and more rapid clinical progression (20). We have also previously shown that mTORC1 negatively regulates mTORC2 through another negative feedback loop involving S6K-1 dependent phosphorylation of Rictor (24). Here we demonstrated that rapamycin (or genetic mTORC1 inhibition by raptor knockdown) promoted Akt S473 and NDRG1 T346 phosphorylation; this feedback activation could be suppressed by mTORC2 inhibition (Fig. 2). Further, in a clinical sample from a GBM patient analyzed before, and 10 days after, treatment with rapamycin, mTORC2 signaling was elevated concomitant with significant mTORC1 inhibition, as measured by decreased S6 phosphorylation (Fig. 2). NF-κB signaling was also upregulated in GBM cell lines and clinical samples treated with rapamycin (Data not shown). These data suggest the possibility that failure to suppress mTORC2 signaling, including NF-κB signaling, may underlie rapamycin resistance and the poor clinical outcome associated with it in some GBM patients. Combined mTORC1 and mTORC2 genetic inhibition by Raptor and Rictor knockdown potently inhibited GBM cell growth and induced tumor cell death (Fig. 2), strongly arguing for the use of mTOR kinase inhibitors to block both signaling complexes and their downstream effectors, including NF-κB.
These results also delineate a new function for mTORC2 as a potent activator of NF-κB and as a mediator of chemotherapy resistance in cancer. mTORC2 was recently shown to promote NF-κB activation in lymphocytes (41), but until now, mTORC2-mediated regulation of NF-κB in cancer has not been appreciated. The recent demonstration that NF-κB is a critical downstream effector of mutant EGFR in lung cancer (30), taken together with our findings that NF-κB activation is mediated downstream of EGFRvIII through mTORC2, raises the possibility that mutant EGFR-mTORC2-NF-κB signaling may have an important role in other cancer types. We studied whether mTORC2/NF-kB signaling contributed to EGFRvIII-mediated resistance to cisplatin because we (44) have previously shown that EGFRvIII promotes resistance to cisplatin, a form of which, carboplatin, is still used in GBM treatment. Our finding that the mTOR kinase inhibitor, PP242 sensitizes EGFRvIII-expressing tumors to cisplatin-mediated cell death, and potentially to other chemotherapies, has important implications for combining mTOR kinase inhibitors with chemotherapy in the clinic. Future studies will be needed to better understand the potential role of mTORC2/NF-κB signaling in mediating resistance to a range of chemotherapies in GBM, and potentially in other cancers.
Akt is commonly thought to be the most important mTORC2 effector and a primary mediator of chemotherapy resistance (47). Surprisingly, mTORC-2 mediated chemotherapy resistance did not require Akt (Fig. 4), but was dependent on NF-κB. These results suggest that glioma cells have developed additional routes toward chemotherapy resistance and that Akt inhibition alone will not be sufficient to chemosensitize tumors. These results suggest that EGFRvIII may promote an mTORC2 function which renders chemotherapy resistance through NF-κB, highlighting the importance of Akt-independent signaling downstream of mTORC2. We show that the well-described mTORC2 effector SGK1 is required for NF-κB activity downstream of EGFRvIII, underlying the Akt-independence of this pathway. These data are also consistent with the recent observation in xenopus that SGK1 functions downstream of PI3K to regulate NF-κB (48). Future studies will be needed to further explore the potential role of SGK1 as a mediator of chemotherapeutic drug resistance.
NF-κB is required for Ras-induced and, potentially, PI3K-induced tumorigenesis under certain cancer cell contexts (42, 49). The results of this study confirm the concept that NF-κB may be an important effector in PI3K-activated cancers, placing it downstream of EGFR mutations in GBM. EGFR mutation has recently been shown to activate the NF-κB pathway in lung cancer (30). The results reported here provide a potential mechanism for mutant EGFR-mediated NF-κB activation in GBM and other cancer types. The results also suggest that EGFR tyrosine kinase inhibitor resistance could also potentially be abrogated by targeting mTORC2-mediated NF-κB activation. These results also suggest a molecular explanation for the mutual exclusivity of monoallelic loss of NFKBIA encoding IκBα and EGFR amplification and/or mutation that has recently been identified in GBM (28). IκBα binds to NF-κB, promotes its cytoplasmic localization, and blocks DNA binding. NFKBIA deletion has been shown to be deleted in 24% of clinical samples. Remarkably, two copy loss of NFKBIA was not detected in any of the 790 samples studied (28), suggesting that GBM cells need to retain some degree of control over the inducibility of NF-κB in order to remain viable (29). Therefore, the observed mutual exclusivity of EGFR mutation/amplification and NFKBIA monoallelic deletion and the similar phenotype of chemotherapy resistance and short survival, could be a consequence of NF-κB activation being downstream of EGFRvIII (29).
EGFR mutations do not occur in isolation in GBM; they are part of a constellation of molecular lesions that dysregulate “core pathways” such as RAS/PI3K, p53 and pRB signaling, among others (45). Similarly, many factors can contribute to NF-κB activation in cancer. Therefore, it is likely that multiple factors contribute to chemotherapy resistance, as has been demonstrated for the role of MGMT promoter methylation in determining response to alkylating agents in GBM (50, 51). mTOR, because of its critical role in integrating diverse cellular inputs including growth factor signaling, nutritional and energy status with an array of cellular functions including protein translation, cell proliferation and cellular metabolism, may be a critical signaling nexus for cancer cells serving as a potential node of convergence of multiple core pathways regulating tumor growth survival and chemotherapy resistance. These results point to mTORC2 as an integrator of two canonical signaling networks that are commonly altered in cancer, EGFR/PI3K and NF-κB. These results also validate the importance of mTORC2 as a cancer target, and provide new insights into its role in mediating chemotherapy resistance, suggesting new treatment strategies.
METHODS
Detailed protocols are found in the Supplemental Experimental Procedures.
Cell lines
U87 and U87-EGFRvIII, U87-EGFR, U87-EGFRvIIII-PTEN, U87-EGFRvIIII-KD isogenic GBM cell lines obtained as described previously (52), and U251, LN229, T98 and A172 GBM cell lines were cultured in Dulbecco’s modified Eagle’s medium (Cellgro) supplemented with 10% FBS (Omega Scientific) and 100U/mL penicillin and streptomycin (Gibco) in a humidified 5% CO2 incubator at 37°C
RNA extraction and Real time PCR
Total RNA from cell lines was extracted using RNeasy Plus Mini Kit (Qiagen). First- strand cDNA was synthesized from 500ng of total RNA using SuperScript III transcriptase (Invitrogen). Real-time PCR was performed with 5 μl of diluted cDNA using iQ™ SYBR Green Supermix (Bio-Rad) on an iCycler (Bio-Rad) following the manufacturer’s instructions. All reactions were performed in triplicate. Primers used for real-time PCR are described in the Supplemental Information (Supplementary Table S2). Relative quantification was performed for each sample and normalized with GAPDH expression for comparison.
Tissue microarrays
Tissue microarrays (TMAs) were performed to analyze Rictor, p-EGFR Tyr1068, p-Akt Ser473, p-NDRG1 Thr346, p-p65 Ser536 immunohistochemical staining in 140 GBM patient samples as described previously (53, 54).
Statistical analysis
Results are shown as mean ± standard errors of the mean (SEM). Chi-square for independence test was used to assess correlations between various molecular markers on TMAs. Mann-Whitney’s U test was used to examine tumor volumes. Other comparisons in cell proliferation assays, tumor volumes, luciferase reporter assay, and cell death (TUNEL staining) were performed with Student’s t-test, as well as by analysis of variance, as appropriate. P < 0.05 was considered as statistically significant.
Supplementary Material
SIGNIFICANCE.
This study demonstrates that mTORC2 signaling promotes GBM proliferation, survival and chemotherapy resistance through Akt-independent activation of NF-κB. We demonstrate that this pathway is activated in the majority of GBMs, including by EGFRvIII, suggesting a critical role in GBM pathogenesis. These results highlight the role of mTORC2 as an integrator of two canonical signaling networks that are commonly altered in cancer, EGFR/PI3K and NF-κB. These results also validate the importance of mTORC2 as a cancer target, and provide new insights into its role in mediating chemotherapy resistance, suggesting new treatment strategies.
Acknowledgments
We thank the members of the Mischel laboratory, Daisuke Kuga and Akio Iwanami for helping in the TMA analysis; David Shackelford for providing shRNA plasmids. We thank the UCLA Brain Tumor Translational Resource for biospecimen and biorepository support. We also thank Kei Hiraoka and Akihito Inagaki (Kasahara laboratory, UCLA) for helping cell cycle analysis; Hiroki Takahashi and Hideyuki Ishiguro (Hirshberg Pancreatic cancer Research laboratory, UCLA) for helping TUNEL staining analysis; Takashi Sasayama (Neurosurgery, Kobe University in Japan) for discussion throughout the course of these studies.
Grant Support
This work was supported by NIH CA119347 (PSM), Accelerate Brain Cancer Cure (PSM), STOP Cancer (PSM), The Fred Miller Family (PSM), The John W Carson Foundation (PSM), The Lya and Harrison Latter endowed Chair (PSM), Ziering Family Foundation in memory of Sigi Ziering (TFC and PSM), The Ben and Catherine Ivy Foundation Fund (WHY and TFC), Art of the Brain Fund (WHY and TFC), Henry Singleton Brain Cancer Fund (WHY and TFC), NIH P01-CA95616 (WKC), NIH CA122617 (BDM), NIH CA75080 and CA73756 (ASB), Samuel Waxman Cancer Research Foundation (ASB), and Uehara Memorial Foundation (KT).
References
- 1.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 2.Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10:307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- 3.Duvel K, Yecies JL, Menon S, Raman P, Lipovsky AI, Souza AL, et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol Cell. 2010;39:171–83. doi: 10.1016/j.molcel.2010.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, et al. Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell. 2006;11:859–71. doi: 10.1016/j.devcel.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- 6.Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51. [PMC free article] [PubMed] [Google Scholar]
- 7.Guertin DA, Sabatini DM. Defining the role of mTOR in cancer. Cancer Cell. 2007;12:9–22. doi: 10.1016/j.ccr.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 8.Hanada M, Feng J, Hemmings BA. Structure, regulation and function of PKB/AKT--a major therapeutic target. Biochim Biophys Acta. 2004;1697:3–16. doi: 10.1016/j.bbapap.2003.11.009. [DOI] [PubMed] [Google Scholar]
- 9.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sarbassov DD, Ali SM, Kim DH, Guertin DA, Latek RR, Erdjument-Bromage H, et al. Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr Biol. 2004;14:1296–302. doi: 10.1016/j.cub.2004.06.054. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–85. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 12.Facchinetti V, Ouyang W, Wei H, Soto N, Lazorchak A, Gould C, et al. The mammalian target of rapamycin complex 2 controls folding and stability of Akt and protein kinase C. EMBO J. 2008;27:1932–43. doi: 10.1038/emboj.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ikenoue T, Inoki K, Yang Q, Zhou X, Guan KL. Essential function of TORC2 in PKC and Akt turn motif phosphorylation, maturation and signalling. EMBO J. 2008;27:1919–31. doi: 10.1038/emboj.2008.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menon S, Manning BD. Common corruption of the mTOR signaling network in human tumors. Oncogene. 2008;27 (Suppl 2):S43–51. doi: 10.1038/onc.2009.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Majumder PK, Febbo PG, Bikoff R, Berger R, Xue Q, McMahon LM, et al. mTOR inhibition reverses Akt-dependent prostate intraepithelial neoplasia through regulation of apoptotic and HIF-1-dependent pathways. Nat Med. 2004;10:594–601. doi: 10.1038/nm1052. [DOI] [PubMed] [Google Scholar]
- 17.Inoki K, Corradetti MN, Guan KL. Dysregulation of the TSC-mTOR pathway in human disease. Nat Genet. 2005;37:19–24. doi: 10.1038/ng1494. [DOI] [PubMed] [Google Scholar]
- 18.Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med. 2011;89:221–8. doi: 10.1007/s00109-011-0726-6. [DOI] [PubMed] [Google Scholar]
- 19.Guertin DA, Stevens DM, Saitoh M, Kinkel S, Crosby K, Sheen JH, et al. mTOR complex 2 is required for the development of prostate cancer induced by Pten loss in mice. Cancer Cell. 2009;15:148–59. doi: 10.1016/j.ccr.2008.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, et al. Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med. 2008;5:e8. doi: 10.1371/journal.pmed.0050008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sparks CA, Guertin DA. Targeting mTOR: prospects for mTOR complex 2 inhibitors in cancer therapy. Oncogene. 2010;29:3733–44. doi: 10.1038/onc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mellinghoff IK, Wang MY, Vivanco I, Haas-Kogan DA, Zhu S, Dia EQ, et al. Molecular determinants of the response of glioblastomas to EGFR kinase inhibitors. N Engl J Med. 2005;353:2012–24. doi: 10.1056/NEJMoa051918. [DOI] [PubMed] [Google Scholar]
- 23.Efeyan A, Sabatini DM. mTOR and cancer: many loops in one pathway. Curr Opin Cell Biol. 2010;22:169–76. doi: 10.1016/j.ceb.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dibble CC, Asara JM, Manning BD. Characterization of Rictor phosphorylation sites reveals direct regulation of mTOR complex 2 by S6K1. Mol Cell Biol. 2009;29:5657–70. doi: 10.1128/MCB.00735-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Read RD, Cavenee WK, Furnari FB, Thomas JB. A drosophila model for EGFR-Ras and PI3K-dependent human glioma. PLoS Genet. 2009;5:e1000374. doi: 10.1371/journal.pgen.1000374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Basseres DS, Baldwin AS. Nuclear factor-kappaB and inhibitor of kappaB kinase pathways in oncogenic initiation and progression. Oncogene. 2006;25:6817–30. doi: 10.1038/sj.onc.1209942. [DOI] [PubMed] [Google Scholar]
- 27.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–6. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 28.Bredel M, Scholtens DM, Yadav AK, Alvarez AA, Renfrow JJ, Chandler JP, et al. NFKBIA deletion in glioblastomas. N Engl J Med. 2011;364:627–37. doi: 10.1056/NEJMoa1006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rinkenbaugh AL, Baldwin AS. Monoallelic deletion of NFKBIA in glioblastoma: when less is more. Cancer Cell. 2011;19:163–5. doi: 10.1016/j.ccr.2011.01.045. [DOI] [PubMed] [Google Scholar]
- 30.Bivona TG, Hieronymus H, Parker J, Chang K, Taron M, Rosell R, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–6. doi: 10.1038/nature09870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Frias MA, Thoreen CC, Jaffe JD, Schroder W, Sculley T, Carr SA, et al. mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr Biol. 2006;16:1865–70. doi: 10.1016/j.cub.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 32.Jacinto E, Facchinetti V, Liu D, Soto N, Wei S, Jung SY, et al. SIN1/MIP1 maintains rictor-mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell. 2006;127:125–37. doi: 10.1016/j.cell.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 33.Yang Q, Inoki K, Ikenoue T, Guan KL. Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 2006;20:2820–32. doi: 10.1101/gad.1461206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zinzalla V, Stracka D, Oppliger W, Hall MN. Activation of mTORC2 by Association with the Ribosome. Cell. 2011;144:757–68. doi: 10.1016/j.cell.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 35.Masri J, Bernath A, Martin J, Jo OD, Vartanian R, Funk A, et al. mTORC2 activity is elevated in gliomas and promotes growth and cell motility via overexpression of rictor. Cancer Res. 2007;67:11712–20. doi: 10.1158/0008-5472.CAN-07-2223. [DOI] [PubMed] [Google Scholar]
- 36.Gulhati P, Cai Q, Li J, Liu J, Rychahou PG, Qiu S, et al. Targeted inhibition of mammalian target of rapamycin signaling inhibits tumorigenesis of colorectal cancer. Clin Cancer Res. 2009;15:7207–16. doi: 10.1158/1078-0432.CCR-09-1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guo D, Prins RM, Dang J, Kuga D, Iwanami A, Soto H, et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci Signal. 2009;2:ra82. doi: 10.1126/scisignal.2000446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Huang J, Wu S, Wu CL, Manning BD. Signaling events downstream of mammalian target of rapamycin complex 2 are attenuated in cells and tumors deficient for the tuberous sclerosis complex tumor suppressors. Cancer Res. 2009;69:6107–14. doi: 10.1158/0008-5472.CAN-09-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sarbassov DD, Ali SM, Sengupta S, Sheen JH, Hsu PP, Bagley AF, et al. Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol Cell. 2006;22:159–68. doi: 10.1016/j.molcel.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 40.Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, et al. Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol. 2009;7:e38. doi: 10.1371/journal.pbio.1000038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee K, Gudapati P, Dragovic S, Spencer C, Joyce S, Killeen N, et al. Mammalian target of rapamycin protein complex 2 regulates differentiation of Th1 and Th2 cell subsets via distinct signaling pathways. Immunity. 2010;32:743–53. doi: 10.1016/j.immuni.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Meylan E, Dooley AL, Feldser DM, Shen L, Turk E, Ouyang C, et al. Requirement for NF-kappaB signalling in a mouse model of lung adenocarcinoma. Nature. 2009;462:104–7. doi: 10.1038/nature08462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dan HC, Cooper MJ, Cogswell PC, Duncan JA, Ting JP, Baldwin AS. Akt-dependent regulation of NF-{kappa}B is controlled by mTOR and Raptor in association with IKK. Genes Dev. 2008;22:1490–500. doi: 10.1101/gad.1662308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nagane M, Levitzki A, Gazit A, Cavenee WK, Huang HJ. Drug resistance of human glioblastoma cells conferred by a tumor-specific mutant epidermal growth factor receptor through modulation of Bcl-XL and caspase-3-like proteases. Proc Natl Acad Sci U S A. 1998;95:5724–9. doi: 10.1073/pnas.95.10.5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang PH, Mukasa A, Bonavia R, Flynn RA, Brewer ZE, Cavenee WK, et al. Quantitative analysis of EGFRvIII cellular signaling networks reveals a combinatorial therapeutic strategy for glioblastoma. Proc Natl Acad Sci U S A. 2007;104:12867–72. doi: 10.1073/pnas.0705158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Abedini MR, Muller EJ, Bergeron R, Gray DA, Tsang BK. Akt promotes chemoresistance in human ovarian cancer cells by modulating cisplatin-induced, p53-dependent ubiquitination of FLICE-like inhibitory protein. Oncogene. 2010;29:11–25. doi: 10.1038/onc.2009.300. [DOI] [PubMed] [Google Scholar]
- 48.Endo T, Kusakabe M, Sunadome K, Yamamoto T, Nishida E. The kinase SGK1 in the endoderm and mesoderm promotes ectodermal survival by down-regulating components of the death-inducing signaling complex. Sci Signal. 2011;4:ra2. doi: 10.1126/scisignal.2001211. [DOI] [PubMed] [Google Scholar]
- 49.Basseres DS, Ebbs A, Levantini E, Baldwin AS. Requirement of the NF-kappaB subunit p65/RelA for K-Ras-induced lung tumorigenesis. Cancer Res. 2010;70:3537–46. doi: 10.1158/0008-5472.CAN-09-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 51.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 52.Wang MY, Lu KV, Zhu S, Dia EQ, Vivanco I, Shackleford GM, et al. Mammalian target of rapamycin inhibition promotes response to epidermal growth factor receptor kinase inhibitors in PTEN-deficient and PTEN-intact glioblastoma cells. Cancer Res. 2006;66:7864–9. doi: 10.1158/0008-5472.CAN-04-4392. [DOI] [PubMed] [Google Scholar]
- 53.Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A, et al. Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res. 2003;63:2742–6. [PubMed] [Google Scholar]
- 54.Lu KV, Zhu S, Cvrljevic A, Huang TT, Sarkaria S, Ahkavan D, et al. Fyn and SRC are effectors of oncogenic epidermal growth factor receptor signaling in glioblastoma patients. Cancer Res. 2009;69:6889–98. doi: 10.1158/0008-5472.CAN-09-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.