Abstract
Structural damage to the prefrontal-cingulate network has been implicated in cognitive and neurobehavioral deficits associated with traumatic brain injury (TBI). Forty-six children who had sustained moderate-to-severe TBI and 43 children with extracranial injury were imaged using diffusion tensor imaging (DTI). Decreased fractional anisotropy (FA) and increased apparent diffusion coefficient (ADC) values were found in the cingulum bundles bilaterally in the TBI group. Cingulum ADC was related to frontal lesion volume, injury severity, and injury mechanism. Finally, cingulum DTI parameters were related to cognitive control measures. DTI detects TBI-related injury to the cingulum, which may facilitate advances in assessment and treatment.
Traumatic brain injury (TBI) is the leading cause of injury-related morbidity and mortality among children and young adults (J. F. Kraus, Rock, & Hemyari, 1990; Thurman, Coronado, & Selassie, 2007). Every year in the United States alone, an estimated half a million children under the age of 14 sustain a TBI (Langlois et al., 2003). Deficits frequently associated with TBI include cognitive impairment, affecting memory and executive functioning. Behavioral disturbance and psychosocial maladjustment are also common and persistent sequelae of moderate to severe TBI (Mateer & Sira, 2006). Furthermore, injury to the developing brain may alter subsequent maturation and impact neurobehavioral and cognitive development (Catroppa & Anderson, 2005; Suskauer & Huisman, 2009).
TBI, particularly at the moderate-to-severe level, results in both focal and diffuse injury in the human brain (Gennarelli, 1993; Povlishock & Katz, 2005). Although conventional magnetic resonance imaging (MRI) techniques readily detect focal lesions, the correlation of neuropathological changes to functional outcomes in TBI is hampered by the insensitivity of conventional MRI to diffuse axonal injury (DAI) (Azouvi, 2000; Goetz et al., 2004). More advanced MRI techniques, including diffusion tensor imaging (DTI), may lead to more accurate assessment of the extent and degree of pathological changes in brain white matter (WM) including DAI and its relationship to outcome. DTI allows for a determination of WM fiber integrity in specific areas of the brain through common metrics such as fractional anisotropy (FA) or apparent diffusion coefficient (ADC; also referred to as mean diffusivity) (Alexander, Lee, Lazar,&Field, 2007; Huisman et al., 2004). FA is dependent on directionality of WM fibers as well as anisotropic diffusion, or the tendency of water molecules to move faster in parallel to nerve fibers rather than perpendicular to them. A high degree of anisotropic diffusion correlates with homogeneity in fiber orientation, increased fiber density or axonal diameter, and the ratio of intracellular/extracellular space. ADC represents the average diffusivity of free water movement within tissue, which also enables inferences regarding the microstructure in tissue; generally higher average diffusivity is indicative of decreased fiber density, axonal diameter, decreased myelination and/or increased extracellular space. The validity of DTI metrics in the study of DAI has already been established through significant correlation of FA and ADC (or mean diffusivity) values as well as other DTI-related metrics with injury indicators such as the Glasgow Coma Scale (GCS) and outcome measures in adult patients with head trauma (Benson et al., 2007; Huisman et al., 2004; M. F. Kraus et al., 2007; Kumar et al., 2009; Perlbarg et al., 2009; Sidaros et al., 2008). Additionally, this has been established in limited studies involving children with TBI using FA (Wozniak et al., 2007; Yuan et al., 2007) as well as both FA, ADC, and other DTI metrics (Ewing-Cobbs et al., 2008; Levin, Wilde et al., 2008; Wilde et al., 2006; Wozniak et al., 2007; Yuan et al., 2007). Other investigators have also shown that DTI can detect WM injury independent of T1- and T2-weighted image findings in TBI patients (Goetz et al., 2004; Lipton et al., 2008). In addition, FA reductions, ADC increases, and/or changes in other DTI-derived metrics following TBI are demonstrated in WM pathways including, but not necessarily limited to, the corpus callosum, posterior limb of the internal capsule, and the external capsule (Arfanakis et al., 2002; Ewing-Cobbs et al., 2008; Huisman et al., 2004; Inglese et al., 2005; Wilde et al., 2006).
The few DTI studies that have addressed pediatric TBI are limited, and are generally based on relatively small numbers of subjects. Wilde et al. (2006) reported significantly reduced FA in the genu, body, and splenium of the corpus callosum in the TBI group, with higher FA related to increased cognitive processing speed and faster interference resolution on an inhibition task. Yuan et al. (2007) reported significantly reduced FA values in the TBI group in genu of corpus callosum, posterior limb of internal capsule, superior longitudinal fasciculus, superior fronto-occipital fasciculus, and centrum semiovale, and a correlation of GCS scores with FA in several of these white matter areas. Wozniak et al. (2007) also reported lower FA in inferior frontal, superior frontal, and supracallosal white matter in a TBI group, where FA in the frontal and supracallosal regions was correlated with executive functioning, and supracallosal FA was correlated with motor speed and behavioral. Ewing-Cobbs et al. (2008) reported that TBI was associated with significant reduction in FA and increased diffusivity in the posterior third and genu of the corpus callosum. IQ, working memory, motor, and academic skills correlated significantly with radial diffusion and/or FA from the isthmus and splenium of the corpus callosum in the TBI group. Knowledge of the effects of pediatric TBI on WM integrity and its relation to cognitive and functional outcome should be expanded with DTI concurrent with follow-up assessments.
The anterior cortex of the cingulate gyrus, a part of the limbic system, has extensive WM connectivity with the prefrontal cortex and has been associated with several aspects of cognition, emotion, and motor processing, while the posterior portion is linked primarily with memory and pain (Nielson & Bryant, 2005; Paus, Petrides, Evans, & Meyer, 1993). Structural damage to the prefrontal-cingulate network has been offered as an explanation for the cognitive and neurobehavioral deficits associated with TBI (Azouvi, 2000). Morphometric MRI studies demonstrate significant atrophy of the cingulate following TBI (Yount et al., 2002), and TBI patients suffering cognitive deficits show functional damage of the cingulate in fMRI and PET studies (Fontaine, Azouvi, Remy, Bussel,& Samson, 1999; Y. H. Kim et al., 2009; Scheibel et al., 2009; Soeda et al., 2005).
Although the cingulate is likely involved in several of the neurobehavioral and cognitive sequelae commonly associated with TBI, the anterior cingulate cortex has been presumed a necessary neural substrate for cognitive control, or the ability to guide information processing and behavior in the service of a goal, which has been considered central to many higher level cognitive functions such as attention, working memory, and executive functioning (Miller & Cohen, 2001). Functional imaging studies have supported the role of the anterior cingulate cortex in detecting cognitive conflict and signaling the need for increased allocation of attentional resources to resolve conflict and to prevent future performance decrements (M. Botvinick, Nystrom, Fissell, Carter,&Cohen, 1999; M. M. Botvinick, Cohen,&Carter, 2004; Carter et al., 1998; Carter & van Veen, 2007; Kerns et al., 2004; A. W. MacDonald, 3rd, Cohen, Stenger, & Carter, 2000). However, studies specifically examining the relation between integrity of the cingulum bundle (fiber bundle passing longitudinally in the white matter of the cingulate gyrus) as measured by DTI and measures cognitive control in patients with TBI have not been performed despite the frequency of TBI-induced deficits in cognitive domains implicating cognitive control.
Using DTI-derived metrics, our aim was to determine whether TBI-related injury affected the structural integrity of the cingulum bundle, and to examine the extent to which this structural injury related to cognitive performance on tasks of cognitive control. We hypothesized first that TBI induces damage to WM fiber connectivity and integrity in the cingulate region, resulting in decreased FA and increased ADC of the right and left cingulum bundle in TBI patients as compared to a demographically similar group of orthopedically injured (OI) children. We also hypothesized that TBI injury severity (i.e., initial Glasgow Coma Scale score) would relate to DTI-derived parameters in the region, further implicating injury as the cause of the structural changes in this region. Third, since no focal injury (i.e., lesions visible on conventional imaging sequences) was evident in the TBI group, we hypothesized that focal injury (i.e., lesion volume) in other brain regions such as the frontal, temporal and parietal lobes that connect via the cingulum bundle would relate to DTI-derived metrics in the cingulum itself, given that axonal disconnection and downstream axonal degeneration are mechanisms thought to be involved in TBI. Finally, we hypothesized that DTI-derived changes in the cingulum bundle reflecting decreased structural integrity would affect aspects of cognition thought to be mediated by the cingulate such as cognitive control (i.e., measured by the Flanker and Sternberg tasks).
METHODS
Participants
Forty-six children (32 M, 14 F) aged 7.1 to 17.2 years (mean = 13.5 ± 2.9) who had sustained closed head trauma (primarily due to motor vehicle–related or pedestrian–motor vehicle injuries) comprised the TBI group and as part of the study design, neuroimaging, outcome, and cognitive assessments were planned for three months post-injury. Post-resuscitation GCS scores recorded in the emergency center ranged from 3–15 with a mean of 7.7 ± 4.3. Consistent with convention, severe TBI was defined by the lowest postresuscitation GCS (Teasdale & Jennett, 1974) score of 3–8 indicative of coma, whereas a moderate TBI was defined by a lowest postresuscitation GCS score of 9–12, which reflects impaired consciousness but not coma (Teasdale & Jennett, 1974) or a GCS score of 13–15 associated with an acute brain lesion seen on the CT scan within 24 hours after injury—often termed “complicated mild” TBI. The rationale for including patients with brain lesions despite mild impairment of consciousness is based on a recent finding (Levin, Hanten et al., 2008) that children in this category exhibit significant long-term cognitive deficit at 12 months post-injury. Based on these criteria, the TBI group was comprised of 26 children with severe TBI, 8 children with moderate TBI, and 9 children with complicated mild TBI. Eligibility criteria for TBI patients included a score less than 4 on an Abbreviated Injury Scale (AIS) (Committee on Injury Scaling, 1990) for areas of the body other than the head and absence of post-resuscitation hypoxia or hypotension exceeding 30 minutes in duration.
The comparison group comprised 43 children (31 M, 12 F) aged 7.6 to 16.6 years (mean 12.1 ± 2.4) who had sustained orthopedic injury (OI) and were admitted to the hospital for at least an overnight hospitalization. In this study, orthopedic injury was defined by any traumatic bone fracture or other extracranial injury requiring at least an overnight hospitalization provided that the AIS score was 1–3, indicating relatively mild injury. The modal injury for participants in this study was a fracture to an upper or lower extremity. The OI comparison group controls for risk factors (Bijur & Haslum, 1995; Stancin et al., 2001; Stancin et al., 1998) that predispose to injury, including preexisting behavioral problems, subtle learning disabilities, and family variables. The absence of significant previous head trauma in the OI group was confirmed through a detailed developmental questionnaire administered to the parent or legal guardian, and the absence of concurrent head injury was confirmed through medical records and/or physician report of relevant history and physical examination findings and, when available, clinical imaging results (i.e., negative CT).
For both groups, all participants were recruited as consecutive admissions to the trauma centers of the participating hospitals, generally in the emergency department. All children included in the study were right-handed, English-speaking, and had no pre-existing head injury involving loss of consciousness or post-concussive symptoms, neurologic disorder associated with cerebral dysfunction and/or cognitive deficit (e.g., cerebral palsy, mental retardation, epilepsy), diagnosed learning disability, psychiatric disorder, or history of child abuse. Additionally, minimum birth weight of 2500 grams (5 lbs, 8 oz) and a minimum of 37 weeks of gestation were required for inclusion, and this was verified by parent report on a detailed developmental questionnaire. Of the eligible patients that were approached (in both groups), an estimated 30–50% agreed to participate at each site. Patients and parents of patients who declined to participate most frequently stated time constraints and scheduling difficulties as reasons for not wishing to participate. There was no apparent systematic bias in injury severity or age for subjects who elected to participate versus those who did not. Demographic and injury-related characteristics for each group, including age at injury, ethnicity, gender, handedness, socioeconomic index, time post-injury, injury severity as measured by GCS scores appear in Table 1.
TABLE 1.
Demographic and Injury Characteristics of TBI and OI Groups
| TBI (N = 46) >Mean (SD) | OI (N = 43) Mean (SD) | |
|---|---|---|
| Age at injury (years) | 13.5 (2.9) range 7–17 | 12.1 (2.4) range 7–16 |
| Time postinjury (years) | 4.1 (1.2) range 1.9–7.7 | 4.1 (1.0) range 2.7–7.1 |
| Gender | 32 M / 14 F | 31 M / 12 F |
| Race/Ethnicity | 20 C / 19 H / 5 AA /1 N Am / 1 Biracial | 15 C / 14 H / 11 AA / 1 Asian / 2 Biracial |
| SCI (z-score) | −0.1 (0.8) range −1.9 to 1.4 | 0.1 (0.9) range −1.3 to 1.9 |
| Handedness | 46 R / 0 L | 43 R / 0 L |
| Mechanism of injury | 18 MVA / 7 motorcycle / 4 RV-ATV / 2 bicycle / 8 fall / 1 sports-play / 5 hit by motor vehicle / 1 other | 2 MVA / 5 motorcycle / 1 RV-ATV / 2 bicycle / 7 fall / 1 hit by falling object / 22 sports-play / 1 hit by motor vehicle / 2 other |
| GCS score | 7.7 (4.3) range 3–15 | N/A |
TBI = traumatic brain injury; OI = orthopedically injured; C = Caucasian; H = Hispanic; AA = African American; N Am = Native American; SCI = Socioeconomic Composite Index; MVA = Motor vehicle accident, RV-ATV = Recreation vehicle or all-terrain vehicle accident; GCS = Glasgow Coma Scale.
MRI Acquisition
All subjects underwent MRI without sedation on Philips 1.5 T Intera scanners (Philips, Cleveland, OH) at Texas Children’s Hospital (Houston), the Rogers MRI Center, University of Texas South-western Medical Center (Dallas), Jackson Memorial Hospital (Miami), or Miami Children’s Hospital (Miami) using identical protocols.
Lesion analysis
A coronal T2-w fluid attenuated inversion recovery (FLAIR) sequence was used (1100 msec TR, 140 msec TE, 5.0 mm slices). For this sequence, a 220 mm FOV was used with a reconstructed voxel size of 0.86 × 0.86 × 5.0 mm.
DTI fiber tracking analysis
Transverse multislice spin echo, single shot, echo planar imaging (EPI) sequences were used (10150.5 msec TR, 90 msec TE, 2.7 mm slices, 0 mm gap). A 256 FOV (RFOV = 100%) was used with a measured voxel size of 2.67 × 2.69 × 2.70 mm. Diffusion was measured along 15 directions (number of b-value = 2, low b-value = 0 and high b-value = 860 sec/mm2). To improve signal to noise ratio, each high b-value image was acquired twice and averaged, but the low b image was only acquired once. The acquisition time was approximately 5:45 minutes, and 55 slices were acquired.
Lesion analysis
Areas of signal abnormality were identified and traced by a board-certified neuroradiologist using FLAIR imaging as previously described (Wilde et al., 2005). No lesions were identified in the cingulate gyri or the underlying WM of the cingulum bundle of either TBI or the OI children.
DTI analysis
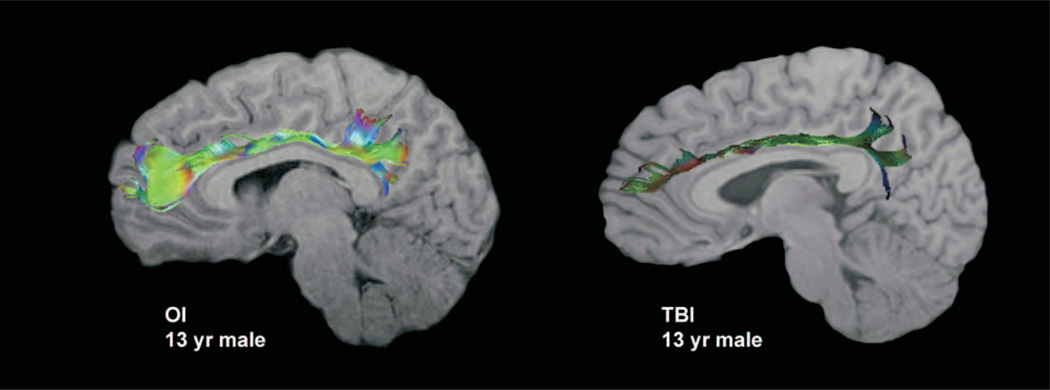
The Philips PRIDE-registration tool (Netsch, 2001) was used to remove shear and eddy current distortion and head motion prior to calculating FA maps with Philips fiber tracking 4.1v3 Beta 2 software. Regions of interest (ROIs) were created on the right and left parasagittal images using the protocol described later. After ROIs were created, automated Philips three-dimensional fiber tracking tool (Hoogenraad, 2002) was utilized to determine fiber tracks passing through the cingulum bundle; mean FA of the fiber systems identified was used as the quantitative measure for all DTI variables. For example, Figure 1 illustrates the fiber system that emerged from the left cingulum bundle in both an orthopedically injured child and an age- and gender-equivalent child with TBI overlaid on a T1-weighted image. The algorithm for fiber tracking is based on the fiber assignment by continuous tracking (FACT) method (Mori, Crain, Chacko, & van Zijl, 1999). Tracking terminated if the FA in the voxels decreased below 0.2 or if the angle between adjacent voxels along the track was larger than 7 degrees.
FIGURE 1.
Left cingulum bundle in a child with orthopedic injury (OI) and traumatic brain injury (TBI). Diffusion tensor imaging (DTI) tractography overlaid on a sagittal T1-weighted image for a 13-year-old child with OI compared with an age- and gender-equivalent child with TBI. Despite the lack of focal or diffuse injury in the TBI child at this level, DTI tractography illustrates the marked difference in the density, length, and brightness of the “fiber” streamlines (indications of decreased fractional anisotropy [FA] and/or increased apparent diffusion coefficient [ADC]) in this participant
Cingulum Region of Interest
ROIs in DTI fiber tracking regions originating from the cingulum bundle were created on FA color maps in the left and right parasagittal planes. Using the automatic 3D ROI algorithm function, two to three seed points were placed linearly along the cingulum bundle in the right parasagittal plane. The multi-ROI fiber tracking function was used to select fibers that were common to the three ROIs, and the software provided the statistics including the mean FA and ADC for the selected fibers. This procedure was repeated for the left side.
Interoperator Reliability
Two experienced raters independently measured FA twice for both the right and left cingulum bundles in a subset of 11 TBI patients and 11 OI subjects. Intra-rater and inter-rater reliabilities were calculated using Shrout-Fleiss reliability statistics to obtain intraclass correlation coefficients (ICCs), which were all above 0.90 (range 0.94 to 0.99; mean = 0.975 ± 0.02.)
Socioeconomic Composite Index
The SCI provides a measure of a family’s socioeconomic status and has been shown to moderate the effects of severe TBI on long-term outcome (Yeates et al., 1997). The index is derived by computing z-scores based on the combined distributions of the OI and TBI groups for three variables including: (1) an 8-point scale rating family income, (2) a 7-point scale of parent/guardian education, and (3) a rating of occupational prestige using the Total Socioeconomic Index (TSI) (Hauser &Warren, 1999). The z-scores for these variables were summed and standardized (mean = 0, SD = 1) based on the aggregate sample of participants (OI and TBI groups) to form the SCI score.
Cognitive Testing Procedures
We indexed two processes fundamental to cognitive control: working memory and inhibition. To avoid effects of TBI on language, we selected tasks that are relatively language-free, are stable, and that rely on reaction time. Our measure of inhibition, the Eriksen Flanker Task, has been used with fMRI to study cognitive control in children (Bunge, Dudukovic, Thomason, Vaidya, & Gabrieli, 2002). To measure working memory, we used the Sternberg Item Recognition Task, which has been used with fMRI to examine the relationship between working memory and inhibition under demands of cognitive control (Bunge, Ochsner, Desmond, Glover, & Gabrieli, 2001). The tasks are further detailed next.
Sternberg Item Recognition Task
Processing speed (reaction time) on a working memory task with varying memory loads was investigated with a modification of the Sternberg Item Recognition Task (Bunge et al., 2001; Sternberg, 1966) for 89 subjects (data was missing for 9 participants on this task, including 3 from the OI group and 6 from the TBI group). Missing data was due to technical problems with the recording mechanism of this computerized task, the child’s refusal to complete the task, or time constraints of the parent or guardian that precluded administration. There did not appear to be any source of systematic bias for the subjects with missing data such as greater injury severity or cognitive impairment, and missing data was distributed across the three sites. For each of 128 trials, children viewed a memory set of 1, 4, or 6 uppercase alphabetic letters and, after a 3-second delay, used “yes” (responding with right hand) and “no” (responding with left hand) key presses to indicate whether a single probe letter had been in the memory set. There were equal numbers of target and non-target trials. Memory load condition (1, 4, or 6), target presence, and position of the target in the memory set were varied randomly by trial. Subjects were instructed to respond as quickly as possible without making errors. Variables used in this study were mean reaction time (RT) on memory loads 1, 4, and 6.
Flanker Task of Cognitive Processing Speed and Interference
To measure cognitive processing speed and visual interference, we used the Flanker Task (Eriksen & Eriksen, 1974), which was administered to 73 subjects (data was missing for 16 subjects, including 5 OI subjects and 9 TBI subjects). As with the Sternberg task, missing data was due to technical problems with this computerized task, the child’s refusal to complete the task, or time constraints of the parent or guardian that precluded administration. There did not appear to be any source of systematic bias for the subjects with missing data such as greater injury severity or cognitive impairment, and data was missing from all three sites. In this task, a horizontal central arrow pointing to the left or to the right appeared on each trial. The child was asked to quickly press the button on the right or the left consistent with the direction that the arrow was pointing. Task conditions included in this study were baseline, facilitation, and interference. Under the baseline condition, the arrow was flanked by horizontal dashes that provided no cue to the child as to which button to press. The interference condition consisted of flanker arrows that pointed in the direction opposite to the central arrow. In the facilitation condition, the flanker arrows pointed in the same direction as the central arrow. There were 112 trials, including 28 trials of each task condition which were randomly interspersed. RT in each condition was the performance measure.
Design and Statistical Analysis
Demographic and injury severity data were tested using chi-square analysis for gender, t-test for age at injury, time post-injury, and Socioeconomic Composite Index (SCI) score, and Fisher’s exact test for mechanism of injury and race/ethnicity. A general linear model (GLM) analysis approach was used to examine group differences in mean FA between both the right and left cingulum bundles, with age at testing included as a covariate. Where appropriate, age was controlled in each subsequent model. Critical assumptions of GLM analysis were examined and no violations were noted. To determine whether there was a difference between right and left hemispheric FA in the two groups, a paired t-test was employed within each group, using the difference between right and left sides as a dependent variable (right FA – left FA). Correlation analyses were used to examine the relation of lesion volumes in the right and left (1) frontal, (2) temporal, and (3) parietal lobes to both FA and ADC in the ipsilateral cingulum bundle (e.g., right frontal lobe with right cingulum bundle), yielding a total of twelve comparisons. Furthermore, the TBI group was subdivided into subgroups of children with and without lesions in the area of interest (e.g., frontal, temporal, parietal and occipital), and t-tests were used to compare mean FA and ADC for these subgroups. Spearman correlation analysis was used to examine the relation between mean FA and ADC of right and left cingulum bundles and GCS score. Additionally, the TBI group was subdivided into two subgroups based upon having sustained injury as a result of either a high-velocity mechanism of injury (e.g., motor vehicle crash) or a low-velocity injury (e.g., fall), and t-tests were used to determine whether mechanism of injury was related to right and left cingulum bundle FA and ADC. Finally, we used repeated ANCOVA models to examine the effect of right and left cingulum bundle FA and ADC and RT on the each of the working memory load conditions (loads 1, 4, and 6) of the Sternberg Item Recognition Task and the baseline, facilitation, and interference conditions on the Flanker Task.
RESULTS
Demographics
No significant differences were noted in gender composition, SCI score, post-injury interval, or race/ethnicity between the two groups. The OI group was younger than the TBI group (t(86) = −2.14, p = .03); therefore, age at testing/scanning was controlled in subsequent statistical models. As expected, the TBI group was more frequently injured as a result of high-speed mechanisms of injury, such as motor vehicle crashes (χ2(1) = 17.31, p < .0001).
Group Differences in DTI of the Cingulum Bundle
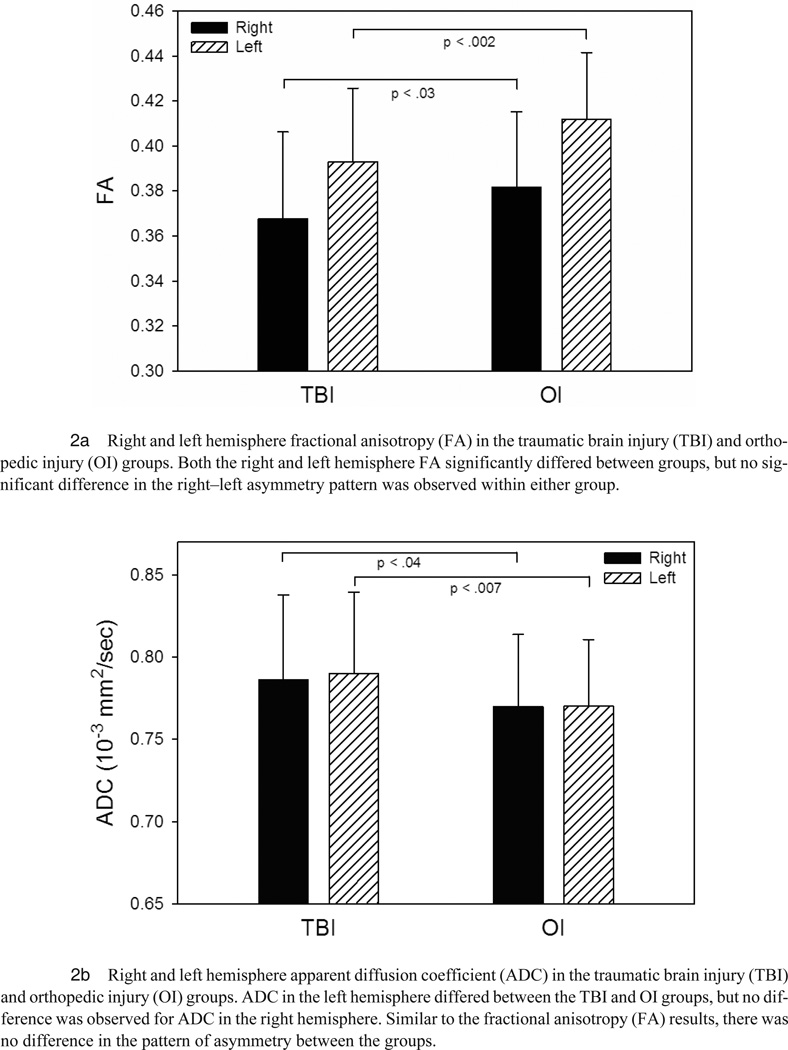
GLM analyses revealed a significant group difference in mean FA for fibers system emanating from the right (F(1, 83) = 5.02, p = .03) and left (F(1, 83) = 11.09, p = .001) cingulate bundles, with higher FA in the OI as compared to the TBI group for both hemispheres. Additionally, analyses revealed significant group differences in the mean ADC for the right (F(1, 83) = 4.58, p = .04) and left (F(1, 83) = 7.70, p = .007) cingulum bundles, with higher ADC in the TBI group.
Hemispheric FA and ADC Differences Within Groups
An analysis of the difference between right and left cingulum bundle mean FA revealed that the left cingulum bundle FA was significantly higher than the right cingulum bundle mean FA in both the OI (t(42) = −8.57, p < .0001) and TBI groups (t(45) = −4.76, p < .0001) (see Figures 2a–b). Groups did not significantly differ on the difference score between right and left cingulum bundle mean FA. Neither group demonstrated significant hemispheric differences for ADC.
FIGURE 2.
Relation of Focal Lesions to DTI Parameters in the Cingulum Bundle
GLM analyses demonstrated no differences in cingulum bundle FA or ADC between TBI patients with and without lesions in brain regions including the right and left (1) frontal, (2) temporal, and (3) parietal lobes. However, for children with TBI who had lesions in a particular brain region, there were significant correlations between lesion volume in the right frontal lobes and right cingulum bundle ADC (number (N) of children in the TBI group with brain lesions in the lobe = 36; r = −0.34, p = .04), left frontal lobe and left cingulum bundle ADC (N = 32; r = −0.34, p = .05), and a marginally significant correlation between left temporal lobe and left cingulum bundle ADC (N = 25; r = −0.37, p = .07). There were no significant correlations between the right temporal lobe lesion volume (N = 24) and right cingulum bundle ADC or between the right or left parietal lobe lesion volume (N = 17) and the ipsilateral cingulum bundle ADC. There were no significant correlations between lesion volume in any lobe and ipsilateral cingulum bundle FA.
Relation Between GCS Scores, Mechanism of Injury, and Cingulum Bundle FA and ADC
Spearman correlation analysis revealed no significant relation with initial GCS score for either right or left cingulum bundle mean FA in the TBI group. However, there was a significant correlation with GCS for the right (r = −0.37, p = .02) and left (r = −0.35, p = .02) cingulum bundle ADC, with lower GCS associated with higher ADC in the TBI group. T-tests examining the difference between subgroups of children and adolescents in the TBI group who were injured as a result of high-speed (N = 29) versus low-speed (N = 17) injury mechanisms revealed a significant group difference in the relation with ADC in the right (t(43) = 2.12, p = .04) and left (t(43) = 2.18, p = .04) cingulum bundles, with higher ADC in the children who were injured as a result of a high-speed injury mechanism.
Relation Between Cingulum Bundle FA and Performance on Measures of Cognitive Control
Individuals in the TBI group generally evidenced slower RT than those in the OI group for both Sternberg and Flanker measures, although the group differences on RT for all variables were not statistically significant. Because the measure of cognitive control is RT and smaller values are indicative of better (or faster) performance, we expected a negative direction of relation between task performance and FA and a positive relation between performance and ADC.
For the Sternberg task, for both groups, there was a significant negative effect of right cingulum bundle FA on the RT for load 1 (F(1,74) = 4.06, p = .048), load 4 (F(1,74) = 5.70, p = .020) and load 6 (F(1,74) = 6.80, p = .011). This effect did not significantly change with load condition (F(2,73) = 1.39, p = .255). For right cingulum bundle ADC, the effect of ADC on Sternberg RT was consistent for both groups and across all conditions. In both groups, there was a significant positive effect of right cingulum bundle ADC on RT on load 1 (F(1,75) = 4.97, p = .029), load 4 (F(1,75) = 7.29, p = .009), and load 6 (F(1,75) = 7.18, p = .009) of the Sternberg task. Again, longer reaction time was associated with increased ADC, and this relationship did not significantly change with load condition (F(2,74) = 1.22, p = .300). For the left cingulum bundle FA and Sternberg RT variables, there was a marginally significant three-way interaction of FA by group by load condition (F(2,71) = 2.44, p = .094), revealing that the group difference tended to differ by load. There was a significant group difference for the lowest condition, load 1 (F(1,72) = 4.78, p = .032), showing that the negative effect of left cingulum bundle FA on performance was much stronger for TBI patients, but there was a consistent negative effect for both groups on the other higher load conditions (i.e., load 4 (F(1,72) = 16.70, p = .0001) and load 6 (F(1,72) = 15.95, p = .0002)). There was a significant positive effect of left cingulum bundle ADC on RT on load 1 (F(1,73) = 5.63, p = .020), 4 (F(1,78) = 6.96, p = .010), and 6 (F(1,73) = 7.27, p = .009) of the Sternberg task. This relationship did not significantly change with load condition (F(2,72) = 0.71, p = .494).
For the Flanker task, a significant negative effect of right cingulum bundle FA on task performance were apparent for the facilitation condition (F(1,67) = 4.00, p = .050), but was marginally significant for baseline (F(1,67) = 3.08, p = .084), and interference (F(1,67) = 2.84, p = .096) conditions. However, the repeated ANCOVA analysis did not show a significant difference of the load for this effect of left cingulum bundle FA on Flanker RT variables on baseline (F(1,67) = 8.17, p = .006), facilitation (F(1,67) = 10.99, p = .002), and interference conditions (F(1, 67) = 6.67, p = .012). This relationship did not significantly differ by condition, as revealed by the repeated ANCOVA analysis (F(2,66) = 0.37, p = .690). No significant effects of ADC for either hemisphere on Flanker task conditions were found.
DISCUSSION
Group Differences in DTI of the Cingulum Bundle
Despite the absence of focal cingulate lesions in the TBI group, their FA and ADC (bilaterally) differed were from the comparison group of orthopedically injured children. As focal pathology within the cingulate cortex or cingulum bundle was not seen on conventional structural MRI, the changes observed on DTI are presumably due to microscopic disruption in the efferent–afferent WM tracts that interconnect the cingulate with the rest of the limbic system and brain in this group of children with TBI. Evidence in support of this comes from animal studies using young animals where post-mortem histological verification can be made. Using a unilateral closed controlled mild impact injury in infant rodents, Dikranian et al. (2008) demonstrated the presence of red blood cells and macrophage/microglia cells in the cingulum region despite the absence of hemorrhage on the cortical surface. Immunohistochemistry revealed prominent apoptosis in the anterior thalamus and posterior cingulate cortex ipsilateral to the site of injury at an early post-injury interval in these animals, and yellow fluorescent protein staining demonstrated frequent axonal swelling in the cingulum bundle, even after mild trauma. In this series of experiments, a three-fold reduction in the number of amyloid precursor protein (APP)–labeled axons and APP immunoreactive profiles were observed predominantly in the cingulum bundle after 48 hours. The authors concluded that early disruption of bundles that pass through the cingulum may result in bidirectional loss of functional synaptic connections between anatomically connected cortical and subcortical areas, which may trigger activation of apoptosis in these neurons and concomitant axonal damage. The vulnerability of the cingulate has also been noted in previous imaging volumetric imaging studies involving adults that showed reductions of gray and white matter volumes in patients with TBI (Gale, Baxter, Roundy, & Johnson, 2005; J. Kim et al., 2008; Levine et al., 2008).
Hemispheric FA Differences Within Groups
Higher left than right cingulum bundle FA was demonstrated in both the TBI and OI groups, and the magnitude of this difference did not differ by group. Previous DTI studies using both voxel-based (Park et al., 2004) and tractography and fiber-based methods (Bonekamp et al., 2007; Gong, Jiang, Zhu, Zang, He et al., 2005; Gong, Jiang, Zhu, Zang, Wang et al., 2005; Wakana et al., 2007; Wilde et al., 2009) have previously demonstrated right–left asymmetry in the cingulum in typically developing children and adults, with the cingulum bundle FA being higher in the left hemisphere of right-handed individuals. Hemispheric differences in cingulate volume have also been previously demonstrated in studies using healthy individuals, with the left cingulate gyrus having a significantly larger volume than the right (Huster, Westerhausen, Kreuder, Schweiger, & Wittling, 2007) in adults. Factors such as the prolonged maturation of the left hemisphere and the stronger cortical folding of the left anterior cingulate as opposed to the right (Huster et al., 2007; Yucel et al., 2001) likely contribute to the asymmetry of cingulate morphology. However, the pathological changes observed in the current study appeared to affect the right and left cingulum bundle equally.
Relation of Focal Lesions to DTI pParameters in the Cingulum Bundle
The cingulate lies adjacent to the falx and its positioning in the brain renders it vulnerable to mechanical forces during acceleration/deceleration injury (Gean, 1994). Interestingly, despite the significant difference in DTI measures between orthopedically injured children and those with TBI, no child with TBI sustained focal lesions visible on conventional imaging in the cingulate cortex or cingulum bundle per se. The cingulate does have extensive connections to other regions of the brain, and we therefore considered subgroups of children in the TBI group with identifiable focal lesions in the frontal, temporal, and parietal, and areas. There were significant correlations (uncorrected for multiple comparisons) between right frontal lobe lesion volumes and right cingulum bundle ADC, and left frontal lobe lesion volume and left cingulum bundle ADC, and amarginally significant correlation between left temporal lobe lesion volume and left cingulum bundle ADC, indicating that although large focal lesions evident on conventional imaging may not be requisite for alteration of microstructural disruption in the cingulum bundle, axonal degeneration or demyelination from areas of focal damage in the frontal and temporal areas may still contribute as potential sources of injury-related dysfunction (Povlishock & Katz, 2005; Rugg-Gunn, Symms, Barker, Greenwood, & Duncan, 2001). As mentioned previously, the most common TBI pathologies are those disrupting the long-coursing WM tracts of the brain (Ashwal, Holshouser, & Tong, 2006; Greenberg, Mikulis, Ng, DeSouza, & Green, 2008; Smith, Meaney, & Shull, 2003). However, the correlations between focal lesion volume and cingulum bundle ADC are modest, and would not be significant when corrected for multiple comparisons (e.g., Bonferroni correction), suggesting that, while focal injury-induced axonal degeneration or demyelination may contribute to changes in the cingulum bundle detected via DTI, they are likely not the only mechanisms of TBI-related injury in this structure. The diffuse TBI-related processes described earlier including axonal injury or disruption within the cingulum bundle itself, immunoreactivity, and apoptosis may also play a significant role in damage to this structure.
Relation Between GCS Scores and Cingulum Bundle FA
In this study, there was a significant correlation with GCS for the right and left cingulum bundles, with lower GCS associated with higher ADC in the TBI group. This is consistent with previous imaging studies that have demonstrated the relation of injury severity to volume loss in the cingulate (Levine et al., 2008; C. L. MacDonald et al., 2008). Not surprisingly, children and adolescents in the TBI group in our study who were injured as a result of high-speed injury mechanisms demonstrated higher ADC in the right and left cingulum bundles than children who were injured as a result of a low-speed mechanism.
Relation Between Cingulum Bundle FA and Performance on Measures of Cognitive Control
Reaction time on measures of cognitive control was related to white matter integrity within the cingulum bundle, including a significant relation between left and right cingulum bundle FA and ADC and RT during the Sternberg task and left cingulum bundle FA and Flanker tasks in children with TBI. This finding is consistent with reports that the cingulate region is involved in various aspects of cognitive control, and in this case, the speed of cognitive processing and response selection. Interestingly, the effect of cingulate DTI metrics was generally stronger on the Sternberg task, a measure of working memory, than on the Flanker task, which contains a condition to measure interference. Additionally, while not significant, there was a tendency for the effect of left cingulum bundle FA on task performance to be greater in the higher load conditions on the Sternberg, but there was no change in the relationship by condition in the Flanker task. This is consistent with reports from a prior fMRI study that found that although there was an overlapping pattern of activation for memory load and interference and that these tasks may rely on some of the same structures, there was a dissociation between the two components, such that memory load was associated with activation in the anterior cingulate, but interference was not (Bunge et al., 2001).
The anterior cingulate gyrus has been said to mediate performance monitoring, response selection, and target identification (Mesulam, Nobre, Kim, Parrish, & Gitelman, 2001), while the posterior region of the cingulate gyrus may be involved in the allocation of spatial attention (Small et al., 2003) and in the modulation of effort during attention demanding tasks (Y. H. Kim et al., 1999). Functional neuroimaging studies have found changes in cingulate metabolism following TBI that relate to these cognitive functions in adults (Cohen Kadosh, Cohen Kadosh, Henik, & Linden, 2008; Scheibel et al., 2009; Soeda et al., 2005). An fMRI study of adults with moderate to severe TBI found that greater injury severity, as assessed by the GCS, was associated with increased anterior cingulate activation during stimulus–response incompatibility (Scheibel et al., 2009). However, in that study, between group comparisons also identified the posterior cingulate as an area with increased activation in patients with severe TBI, relative to OI subjects and individuals with moderate TBI. Soeda et al. (2005) suggested that axonomy of neurons connecting the posterior cingulate gyrus with other brain regions is a frequent mechanism of injury for this structure. The resulting deafferentiation may produce degeneration within the cingulate gyrus (Yount et al., 2002) and, in relation to findings from the current study and (Scheibel et al., 2009), DAI may contribute to decreased neural efficiency within distributed networks that mediate performance during cognitive control.
Limitations and Future Directions
Our study represents the first to specifically examine changes in the cingulum bundle of children with TBI using quantitative DTI tractography and the relation of metrics such as FA and ADC to injury severity and mechanism, and measures of cognitive control. Strengths of the study include its prospective design with imaging performed at a generally uniform post-injury interval, a reasonable size cohort of children with TBI as well as a comparison group of children with orthopedic injury. Reliability of DTI variable measurement is also a strength of this report, with excellent intra- and inter-operator agreement.
Limitations include inter-individual variability in neuroanatomy of the cingulum bundle and the lack of detailed investigation of gender differences given the preponderance of males in our population and the cross-sectional design. Future directions include examining the impact of changes in the cingulate and their relation to additional standardized and functional cognitive outcome measures and emotional functioning measures. Longer-term changes in the cingulate that may be influenced by injury (in TBI) and developmental factors in both TBI and comparison groups will also be explored in future longitudinal studies.
CONCLUSION
DTI has increasingly demonstrated its utility in detecting injury that may not be visible on conventional MRI sequences. This study documents the sensitivity of DTI in revealing injury to structures such as the cingulum bundle where, despite the absence of focal lesions, significant changes in white matter integrity are evident and potentially clinically useful. The cingulate is an essential member of neural networks underlying several important cognitive abilities known to be deleteriously impacted in TBI, and an increased understanding of the disruption of pathways involving the cingulum bundle other related structures using DTI may enable advances in the assessment and treatment of these injuries.
ACKNOWLEDGMENTS
The authors gratefully acknowledge the contribution of Deleene Menefee, Ph.D., Summer Lane, Lori Cook, Sandra B. Chapman, Ph.D., and Gillian Hotz, Ph.D. in data collection, Matthew B. Mors in data analysis, and Stacey K. Martin in manuscript preparation. The authors thank the participants and their families for their willingness to be part of this research.
This work was supported by the National Institute Neurological Disorders and Stroke grant R01-NS21889 (“Neurobehavioral outcome of head injury in children,” Levin, PI). The authors also acknowledge the generous contribution of Mission Connect of the TIRR Foundation.
Contributor Information
Elisabeth A. Wilde, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas, Departments of Radiology and Neurology, Baylor College of Medicine, Houston, Texas, and Michael E. DeBakey VA Medical Center, Houston, Texas
Marco A. Ramos, E.B. Singleton Department of Diagnostic Imaging, Texas Children’s Hospital, Houston, Texas
Ragini Yallampalli, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas.
Erin D. Bigler, Department of Psychology and Neuroscience Center, Brigham Young University, Provo, Utah and Department of Psychiatry and the Utah Brain Institute, University of Utah, Salt Lake City, Utah
Stephen R. McCauley, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas, and Department of Pediatrics, Section of Hematology-Oncology, Baylor College of Medicine, Houston, Texas
Zili Chu, Department of Radiology, Baylor College of Medicine, Houston, Texas and E.B. Singleton Department of Diagnostic Imaging, Texas Children’s Hospital, Houston, Texas.
Trevor C. Wu, Department of Psychology, Brigham Young University, Provo, Utah
Gerri Hanten, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas.
Randall S. Scheibel, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas and Michael E. DeBakey VA Medical Center, Houston, Texas
Xiaoqi Li, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas.
Ana C. Vásquez, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas
Jill V. Hunter, Department of Radiology, Baylor College of Medicine, Houston, Texas E.B. Singleton Department of Diagnostic Imaging, Texas Children’s Hospital, Houston, Texas
Harvey S. Levin, Physical Medicine and Rehabilitation Alliance of Baylor College of Medicine and the University of Texas-Houston Medical School, Houston, Texas and Departments of Neurology, Neurosurgery, and Pediatrics, Baylor College of Medicine, Houston, Texas, and Michael E. DeBakey VA Medical Center, Houston, Texas
REFERENCES
- Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arfanakis K, Haughton VM, Carew JD, Rogers BP, Dempsey RJ, Meyerand ME. Diffusion tensor MR imaging in diffuse axonal injury. American Journal of Neuroradiology. 2002;23(5):794–802. [PMC free article] [PubMed] [Google Scholar]
- Ashwal S, Holshouser BA, Tong KA. Use of advanced neuroimaging techniques in the evaluation of pediatric traumatic brain injury. Developmental Neuroscience. 2006;28(4–5):309–326. doi: 10.1159/000094157. [DOI] [PubMed] [Google Scholar]
- Azouvi P. Neuroimaging correlates of cognitive and functional outcome after traumatic brain injury. Current Opinion of Neurology. 2000;13(6):665–669. doi: 10.1097/00019052-200012000-00009. [DOI] [PubMed] [Google Scholar]
- Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. Journal of Neurotrauma. 2007;24(3):446–459. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Bijur P, Haslum M. Cognitive, behavioral, and motoric sequelae of mild head injury in a national birth cohort. In: Broman SH, Michel ME, editors. Traumatic head injury in children. New York: Oxford University Press; 1995. pp. 147–164. [Google Scholar]
- Bonekamp D, Nagae LM, Degaonkar M, Matson M, Abdalla WM, Barker PB, et al. Diffusion tensor imaging in children and adolescents: Reproducibility, hemispheric, and age-related differences. Neuroimage. 2007;34(2):733–742. doi: 10.1016/j.neuroimage.2006.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botvinick M, Nystrom LE, Fissell K, Carter CS, Cohen JD. Conflict monitoring versus selection-for-action in anterior cingulate cortex. Nature. 1999;402(6758):179–181. doi: 10.1038/46035. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: An update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Dudukovic NM, Thomason ME, Vaidya CJ, Gabrieli JD. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33(2):301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge SA, Ochsner KN, Desmond JE, Glover GH, Gabrieli JD. Prefrontal regions involved in keeping information in and out of mind. Brain. 2001;124(Pt 10):2074–2086. doi: 10.1093/brain/124.10.2074. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: An update of theory and data. Cognitive, Affective, & Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Catroppa C, Anderson V. A prospective study of the recovery of attention from acute to 2 years following pediatric traumatic brain injury. Journal of the International Neuropsychological Society. 2005;11(1):84–98. doi: 10.1017/S1355617705050101. [DOI] [PubMed] [Google Scholar]
- Cohen Kadosh R, Cohen Kadosh K, Henik A, Linden DE. Processing conflicting information: Facilitation, interference, and functional connectivity. Neuropsychologia. 2008;46(12):2872–2879. doi: 10.1016/j.neuropsychologia.2008.05.025. [DOI] [PubMed] [Google Scholar]
- Committee on Injury Scaling. Abbreviated Injury Scale. Des Plaines, IL: Association for the Advancement of Automotive Medicine; 1990. [Google Scholar]
- Dikranian K, Cohen R, Mac Donald C, Pan Y, Brakefield D, Bayly P, et al. Mild traumatic brain injury to the infant mouse causes robust white matter axonal degeneration which precedes apoptotic death of cortical and thalamic neurons. Experimental Neurology. 2008;211(2):551–560. doi: 10.1016/j.expneurol.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a non-search task. Perception and Psychophysics. 1974;16:143–149. [Google Scholar]
- Ewing-Cobbs L, Prasad MR, Swank P, Kramer L, Cox CS, Jr, Fletcher JM, et al. Arrested development and disrupted callosal microstructure following pediatric traumatic brain injury: Relation to neurobehavioral outcomes. Neuroimage. 2008;42(4):1305–1315. doi: 10.1016/j.neuroimage.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontaine A, Azouvi P, Remy P, Bussel B, Samson Y. Functional anatomy of neuropsychological deficits after severe traumatic brain injury. Neurology. 1999;53(9):1963–1968. doi: 10.1212/wnl.53.9.1963. [DOI] [PubMed] [Google Scholar]
- Gale SD, Baxter L, Roundy N, Johnson SC. Traumatic brain injury and grey matter concentration: A preliminary voxel based morphometry study. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76(7):984–988. doi: 10.1136/jnnp.2004.036210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gean A. Imaging of the head. New York: Raven Press; 1994. [Google Scholar]
- Gennarelli TA. Mechanisms of brain injury. The Journal of Emergency Medicine. 1993;11 Suppl 1:5–11. [PubMed] [Google Scholar]
- Goetz P, Blamire A, Rajagopalan B, Cadoux-Hudson T, Young D, Styles P. Increase in apparent diffusion coefficient in normal appearing white matter following human traumatic brain injury correlates with injury severity. Journal of Neurotrauma. 2004;21(6):645–654. doi: 10.1089/0897715041269731. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, He Y, Xie S, et al. Side and handedness effects on the cingulum from diffusion tensor imaging. NeuroReport. 2005;16(15):1701–1705. doi: 10.1097/01.wnr.0000183327.98370.6a. [DOI] [PubMed] [Google Scholar]
- Gong G, Jiang T, Zhu C, Zang Y, Wang F, Xie S, et al. Asymmetry analysis of cingulum based on scale-invariant parameterization by diffusion tensor imaging. Human Brain Mapping. 2005;24(2):92–98. doi: 10.1002/hbm.20072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G, Mikulis DJ, Ng K, DeSouza D, Green RE. Use of diffusion tensor imaging to examine subacute white matter injury progression in moderate to severe traumatic brain injury. Archives of Physical Medicine and Rehabilitation. 2008;89(12 Suppl):S45–S50. doi: 10.1016/j.apmr.2008.08.211. [DOI] [PubMed] [Google Scholar]
- Hauser RM, Warren JR. Raftery A. Sociological methodology. vol 27. Blackwell Publishing; 1999. Socioeconomic indexes for occupations: A review, update, and critique; pp. 177–298. [Google Scholar]
- Hoogenraad F. Multi-center evaluation of in-vivo fibertracking. Philips Medical Systems; 2002. [Google Scholar]
- Huisman TA, Schwamm LH, Schaefer PW, Koroshetz WJ, Shetty-Alva N, Ozsunar Y, et al. Diffusion tensor imaging as potential biomarker of white matter injury in diffuse axonal injury. American Journal of Neuroradiology. 2004;25(3):370–376. [PMC free article] [PubMed] [Google Scholar]
- Huster RJ, Westerhausen R, Kreuder F, Schweiger E, Wittling W. Morphologic asymmetry of the human anterior cingulate cortex. Neuroimage. 2007;34(3):888–895. doi: 10.1016/j.neuroimage.2006.10.023. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: A diffusion tensor imaging study. Journal of Neurosurgery. 2005;103(2):298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kim J, Avants B, Patel S, Whyte J, Coslett BH, Pluta J, et al. Structural consequences of diffuse traumatic brain injury: A large deformation tensor-based morphometry study. Neuroimage. 2008;39(3):1014–1026. doi: 10.1016/j.neuroimage.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Gitelman DR, Nobre AC, Parrish TB, LaBar KS, Mesulam MM. The large-scale neural network for spatial attention displays multifunctional overlap but differential asymmetry. Neuroimage. 1999;9(3):269–277. doi: 10.1006/nimg.1999.0408. [DOI] [PubMed] [Google Scholar]
- Kim YH, Yoo WK, Ko MH, Park CH, Kim ST, Na DL. Plasticity of the attentional network after brain injury and cognitive rehabilitation. Neurorehabilitation and Neural Repair. 2009;23(5):468–477. doi: 10.1177/1545968308328728. [DOI] [PubMed] [Google Scholar]
- Kraus JF, Rock A, Hemyari P. Brain injuries among infants, children, adolescents, and young adults. American Journal of Diseases in Children. 1990;144(6):684–691. doi: 10.1001/archpedi.1990.02150300082022. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: A diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Kumar R, Gupta RK, Husain M, Chaudhry C, Srivastava A, Saksena S, et al. Comparative evaluation of corpus callosum DTI metrics in acute mild and moderate traumatic brain injury: Its correlation with neuropsychometric tests. Brain Injury. 2009;23(7):675–685. doi: 10.1080/02699050903014915. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Kegler SR, Butler JA, Gotsch KE, Johnson RL, Reichard AA, et al. Traumatic brain injury-related hospital discharges. Results from a 14-state surveillance system, 1997. Morbidity and Mortality Weekly Report Surveillance Summaries. 2003;52(4):1–20. [PubMed] [Google Scholar]
- Levin HS, Hanten G, Roberson G, Li X, Ewing-Cobbs L, Dennis M, et al. Prediction of cognitive sequelae based on abnormal computed tomography findings in children following mild traumatic brain injury. Journal of Neurosurgery: Pediatrics. 2008;1:461–470. doi: 10.3171/PED/2008/1/6/461. [DOI] [PubMed] [Google Scholar]
- Levin HS, Wilde EA, Chu Z, Yallampalli R, Hanten GR, Li X, et al. Diffusion tensor imaging in relation to cognitive and functional outcome of traumatic brain injury in children. Journal of Head Trauma Rehabilitation. 2008;23(4):197–208. doi: 10.1097/01.HTR.0000327252.54128.7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine B, Kovacevic N, Nica EI, Cheung G, Gao F, Schwartz ML, et al. The Toronto traumatic brain injury study: Injury severity and quantified MRI. Neurology. 2008;70(10):771–778. doi: 10.1212/01.wnl.0000304108.32283.aa. [DOI] [PubMed] [Google Scholar]
- Lipton ML, Gellella E, Lo C, Gold T, Ardekani BA, Shifteh K, et al. Multifocal white matter ultrastructural abnormalities in mild traumatic brain injury with cognitive disability: A voxel-wise analysis of diffusion tensor imaging. Journal of Neurotrauma. 2008;25(11):1335–1342. doi: 10.1089/neu.2008.0547. [DOI] [PubMed] [Google Scholar]
- MacDonald AW, 3rd, Cohen JD, Stenger VA, Carter CS. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288(5472):1835–1838. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- MacDonald CL, Schwarze N, Vaishnavi SN, Epstein AA, Snyder AZ, Raichle ME, et al. Verbal memory deficit following traumatic brain injury: Assessment using advanced MRI methods. Neurology. 2008;71(15):1199–1201. doi: 10.1212/01.wnl.0000327521.69520.7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateer CA, Sira CS. Cognitive and emotional consequences of TBI: Intervention strategies for vocational rehabilitation. NeuroRehabilitation. 2006;21(4):315–326. [PubMed] [Google Scholar]
- Mesulam MM, Nobre AC, Kim YH, Parrish TB, Gitelman DR. Heterogeneity of cingulate contributions to spatial attention. Neuroimage. 2001;13(6 Pt 1):1065–1072. doi: 10.1006/nimg.2001.0768. [DOI] [PubMed] [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Annals of Neurology. 1999;45(2):265–269. doi: 10.1002/1531-8249(199902)45:2<265::aid-ana21>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Netsch T. Towards real-time multi-modality 3-d medical image registration. Paper presented at the International Conference on Computer Vision; Vancouver. 2001. [Google Scholar]
- Nielson KA, Bryant T. The effects of non-contingent extrinsic and intrinsic rewards on memory consolidation. Neurobiology of Learning and Memory. 2005;84(1):42–48. doi: 10.1016/j.nlm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Park HJ, Westin CF, Kubicki M, Maier SE, Niznikiewicz M, Baer A, et al. White matter hemisphere asymmetries in healthy subjects and in schizophrenia: A diffusion tensor MRI study. Neuroimage. 2004;23(1):213–223. doi: 10.1016/j.neuroimage.2004.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Petrides M, Evans AC, Meyer E. Role of the human anterior cingulate cortex in the control of oculomotor, manual, and speech responses: A positron emission tomography study. Journal of Neurophysiology. 1993;70(2):453–469. doi: 10.1152/jn.1993.70.2.453. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Puybasset L, Tollard E, Lehericy S, Benali H, Galanaud D. Relation between brain lesion location and clinical outcome in patients with severe traumatic brain injury: A diffusion tensor imaging study using voxel-based approaches. Human Brain Mapping. 2009 doi: 10.1002/hbm.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlishock JT, Katz DI. Update of neuropathology and neurological recovery after traumatic brain injury. Journal of Head Trauma Rehabilitation. 2005;20(1):76–94. doi: 10.1097/00001199-200501000-00008. [DOI] [PubMed] [Google Scholar]
- Rugg-Gunn FJ, Symms MR, Barker GJ, Greenwood R, Duncan JS. Diffusion imaging shows abnormalities after blunt head trauma when conventional magnetic resonance imaging is normal. Journal of Neurology, Neurosurgery & Psychiatry. 2001;70(4):530–533. doi: 10.1136/jnnp.70.4.530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheibel RS, Newsome MR, Troyanskaya M, Steinberg JL, Goldstein FC, Mao H, et al. Effects of severity of traumatic brain injury and brain reserve on cognitive-control related brain activation. Journal of Neurotrauma. 2009;26(9):1447–1461. doi: 10.1089/neu.2008.0736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: A longitudinal study. Brain. 2008;131(Pt 2):559–572. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Small DM, Gitelman DR, Gregory MD, Nobre AC, Parrish TB, Mesulam MM. The posterior cingulate and medial prefrontal cortex mediate the anticipatory allocation of spatial attention. Neuroimage. 2003;18(3):633–641. doi: 10.1016/s1053-8119(02)00012-5. [DOI] [PubMed] [Google Scholar]
- Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. Journal of Head Trauma Rehabilitation. 2003;18(4):307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Soeda A, Nakashima T, Okumura A, Kuwata K, Shinoda J, Iwama T. Cognitive impairment after traumatic brain injury: A functional magnetic resonance imaging study using the Stroop task. Neuroradiology. 2005;47(7):501–506. doi: 10.1007/s00234-005-1372-x. [DOI] [PubMed] [Google Scholar]
- Stancin T, Kaugars AS, Thompson GH, Taylor HG, Yeates KO, Wade SL, et al. Child and family functioning 6 and 12 months after a serious pediatric fracture. Journal of Trauma. 2001;51(1):69–76. doi: 10.1097/00005373-200107000-00011. [DOI] [PubMed] [Google Scholar]
- Stancin T, Taylor HG, Thompson GH, Wade S, Drotar D, Yeates KO. Acute psychosocial impact of pediatric orthopedic trauma with and without accompanying brain injuries. Journal of Trauma. 1998;45(6):1031–1038. doi: 10.1097/00005373-199812000-00010. [DOI] [PubMed] [Google Scholar]
- Sternberg S. High-speed scanning in human memory. Science. 1966;153(736):652–654. doi: 10.1126/science.153.3736.652. [DOI] [PubMed] [Google Scholar]
- Suskauer SJ, Huisman TA. Neuroimaging in pediatric traumatic brain injury: Current and future predictors of functional outcome. Developmental Disabilities Research Reviews. 2009;15(2):117–123. doi: 10.1002/ddrr.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974;2(7872):81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Coronado V, Selassie A. The epidemiology of TBI: Implications for public health. In: Zasler ND, Katz RD, Zafonte RD, editors. Brain injury medicine: Principles and practice. New York: Demos Medical Publishing; 2007. [Google Scholar]
- Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde EA, Chu Z, Bigler ED, Hunter JV, Fearing MA, Hanten G, et al. Diffusion tensor imaging in the corpus callosum in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2006;23(10):1412–1426. doi: 10.1089/neu.2006.23.1412. [DOI] [PubMed] [Google Scholar]
- Wilde EA, Hunter JV, Newsome MR, Scheibel RS, Bigler ED, Johnson JL, et al. Frontal and temporal morphometric findings on MRI in children after moderate to severe traumatic brain injury. Journal of Neurotrauma. 2005;22(3):333–344. doi: 10.1089/neu.2005.22.333. [DOI] [PubMed] [Google Scholar]
- Wilde EA, McCauley SR, Chu Z, Hunter JV, Bigler ED, Yallampalli R, et al. Diffusion tensor imaging of hemispheric asymmetries in the developing brain. Journal of Clinical and Experimental Neuropsychology. 2009;31(2):205–218. doi: 10.1080/13803390802098118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak JR, Krach L, Ward E, Mueller BA, Muetzel R, Schnoebelen S, et al. Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: A diffusion tensor imaging (DTI) study. Archives of Clinical Neuropsychology. 2007;22(5):555–568. doi: 10.1016/j.acn.2007.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeates KO, Taylor HG, Drotar D, Wade SL, Klein S, Stancin T, et al. Preinjury family environment as determinant of recovery from traumatic brain injuries in school-age children. Journal of the International Neuropsychological Society. 1997;3(6):617–630. [PubMed] [Google Scholar]
- Yount R, Raschke KA, Biru M, Tate DF, Miller MJ, Abildskov T, et al. Traumatic brain injury and atrophy of the cingulate gyrus. The Journal of Neuropsychiatry and Clinical Neurosciences. 2002;14(4):416–423. doi: 10.1176/jnp.14.4.416. [DOI] [PubMed] [Google Scholar]
- Yuan W, Holland SK, Schmithorst VJ, Walz NC, Cecil KM, Jones BV, et al. Diffusion tensor MR imaging reveals persistent white matter alteration after traumatic brain injury experienced during early childhood. American Journal of Neuroradiology. 2007;28(10):1919–1925. doi: 10.3174/ajnr.A0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yucel M, Stuart GW, Maruff P, Velakoulis D, Crowe SF, Savage G, et al. Hemispheric and gender-related differences in the gross morphology of the anterior cingulate/paracingulate cortex in normal volunteers: An MRI morphometric study. Cerebral Cortex. 2001;11(1):17–25. doi: 10.1093/cercor/11.1.17. [DOI] [PubMed] [Google Scholar]